-
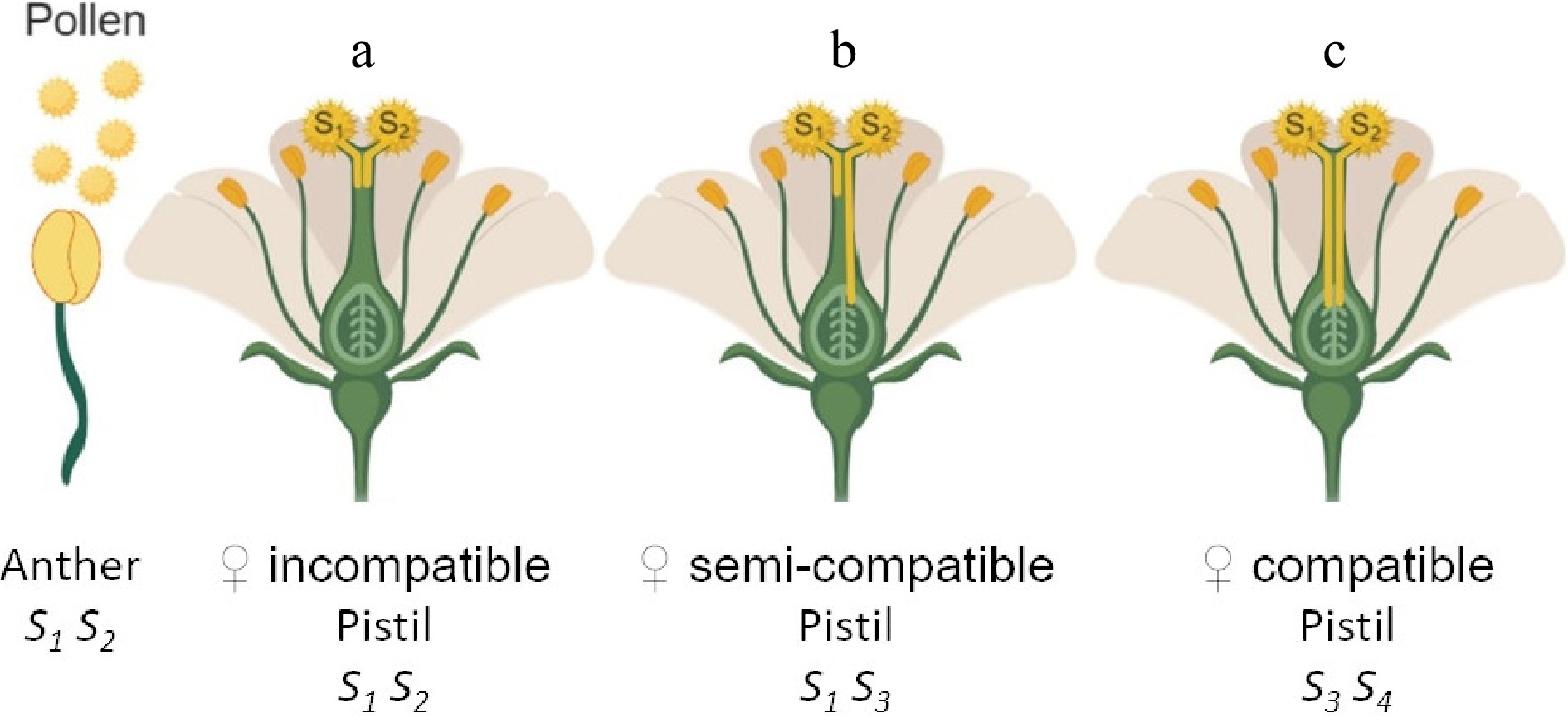
Figure 1. Genetic control of gametophytic self-incompatibility (GSI) in Malus. The S-locus is composed of two tightly linked components, found in the pollen and pistil respectively. In GSI, the pollen self-incompatibility phenotype is controlled gametophytically, i.e., the genotype of the haploid pollen itself (gametophyte) determines its incompatibility type. For example, the pollen composition of a certain pollen donor plant is phenotypically half S1 and half S2. In the female parent, two alleles are co-dominant and both are expressed in the pistil. Pollen inhibition occurs when there is a match between the donor pollen S-haplotype and either of the two haplotypes present in the pistil, producing an incompatible reaction that inhibits the growth of the 'self' pollen tube growth. Three types of reactions can occur during a cross: (a) incompatible; neither of the two gametes will germinate, (b) semi-compatible; half the donor pollen will be inhibited and the other half will germinate and grow normally, (c) compatible; all pollen will germinate and grow normally.
-

Figure 2. High resolution melting (HRM) curve profiles of seven S-allele-specific markers. Amplification curves of real-time PCR marker assays (left panels), HRM difference plots, where the derivative fluorescence signal (dF/dT) is plotted as a function of temperature (right panels). Each colour represents a specific S-genotype as shown by the legends. Light grey represents samples that were not amplified in the real-time PCR.
-

Figure 3. Frequency of S-alleles and S-genotypes of the 183 apple advanced selections of the PFR’s breeding programme. Inner plot shows the percentage frequency distribution of S-alleles from the total 366 alleles observed among the 183 genotypes tested. Outer plot represents the absolute frequency of each S-genotype. All outer slices not showing a percentage value in the figure represent 0.3% respectively.
-
Marker name Type Primer sequences '5 - 3' Physical location Genbank locus S-Rnase alleles and product sizes (bp) S1_apple_PFR HRM Forward ACAGGCCACTGGTGGA not found in reference genome MG598487.1:1981−1996 S1, S20, S24 (38) Reverse ATTGCGTATGGCATTTTCAAT Chr17:30844510−30844530 MG598487.1:1998−2018 S2_apple_PFR HRM Forward TTGAACAAATATTATTCAATGGGGA Chr17:31240988−31240964 MG598488.1:860−884 S2 (54) Reverse CATCGTAACTATATATACCATCCGCGTA Chr17:31240964−31240943 MG598488.1:886−913 S5_apple_PFR HRM Forward AATTTATAAAACACGTGATCA not found in reference genome MG598491.1:326−346 S5 (43) Reverse GCTCCTATTGATCGATCAT not found in reference genome MG598491.1:350−370 S8_apple_PFR HRM Forward TTCGATTATTTTCAATTTACGCTT Chr17:31240889−31240871 MG598494.1 :1159−1182 S8 (162) Reverse ATTTAAGGTTGTTTCTTTGCAATAC not found in reference genome MG598494.1 :1296−1320 S9_apple_PFR HRM Forward GCTCAGGAAATGACCCAATATAC not found in reference genome MG598495.1:1284−1306 S9 (61) Reverse AATATTACCTTAGTAGAATTCATGGTTGT not found in reference genome MG598495.1:1315−1344 S23_apple_PFR HRM Forward TTTATGGCCTTCAAACTGGAA not found in reference genome MG598501.1:1065−1085 S23 (42) Reverse CAGAAGATTGGGTCGGGT not found in reference genome MG598501.1:1089−1106 S28_apple_PFR HRM Forward TGCCTCGCTCTTGAACAAA not found in reference genome MG598505.1:782−800 S28 (47) Reverse CCCCGTAATTCCCATTGAATAATA not found in reference genome MG598505.1:805−828 Myb110a1_PFR SSR Forward TCTCCCTCATCCCAGAACA Chr17:32151473−32151491 S1 (166), S3 (184), S5 (180), S7 (170), S20 (158), S24 (176), S25 (188) Reverse CGAGCCAAACAAAATTGGA Chr17:32151642−32151624 Myb110a2_PFR SSR Forward CTCTCCCTCATCCCAGAACA Chr17:32151472−32151491 S1 (325), S3 (343), S5 (339), S7 (314), S20 (317), S23 (309), S24 (320), S25 (347) Reverse TCCTACTCGGCTCGACAATC Chr17:32151800−32151781 Myb110b_PFR SSR Forward CTTCGGGCTTATTTGGGTTT Chr17:32187809−32187790 S1 (202), S2 (233), S3 (214), S5 (209), S7 (191), S9 (247), S10 (216), S23 (239), S24 (217), S25 (238) Reverse TTTGCCCCTTCAAAGATCAG Chr17:32187616−32187635 GSI_SSR_PFR SSR Forward GCCCCTTACATTCCTTTTCTTT Chr17:31704109−31704130 S1 (314), S3 (338), S5 (335), S7 (324), S10 (328), S20 (317), S23 (322), S24 (217), S25 (329), S28 (352) Reverse CAATCTTGAGTTGTCGTTGGAG Chr17:31704430−31704409 Table 1. Primer sequences of quantitative real-time PCR and SSR-based markers.
Figures
(3)
Tables
(1)