-
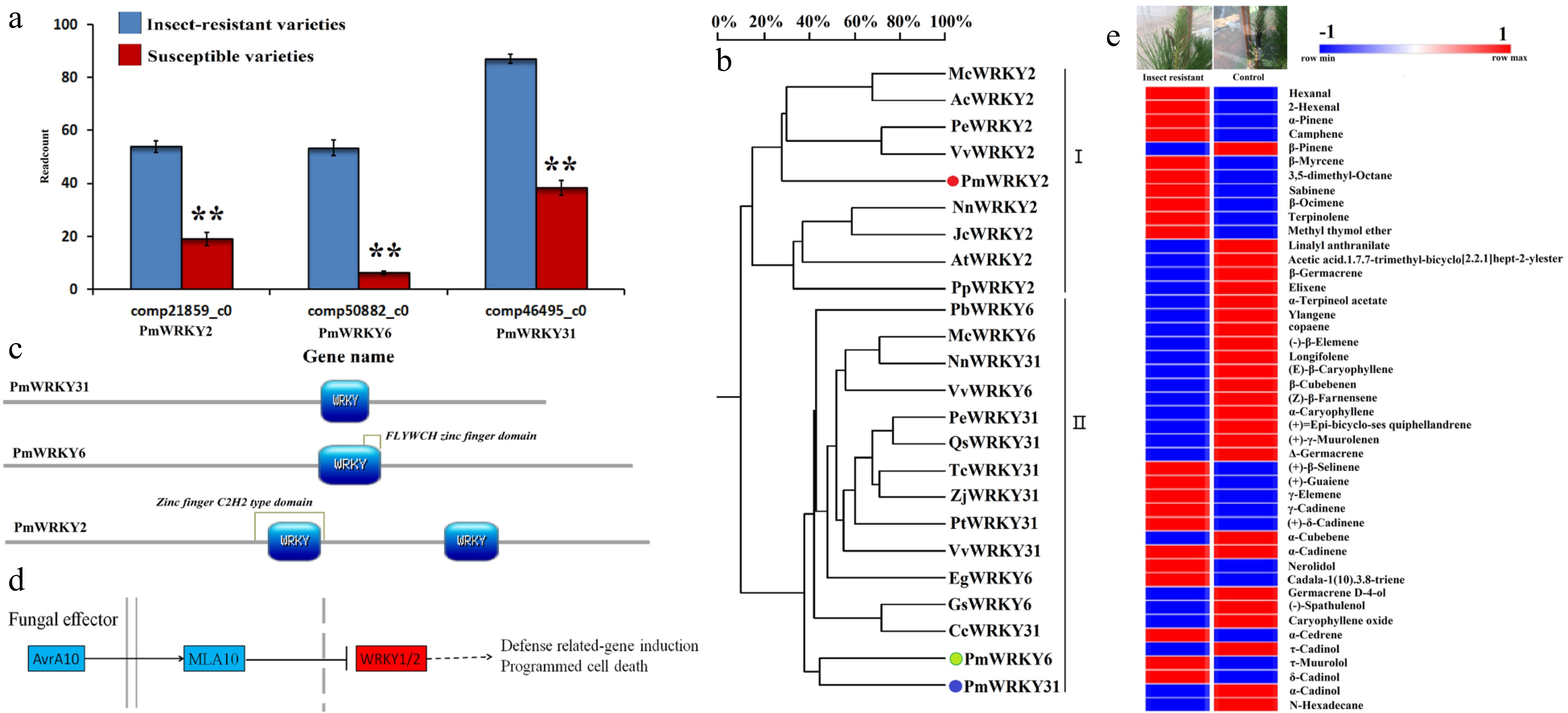
Figure 1. WRKY gene discovery and bioinformatics analysis. (a) Three WRKY transcriptomes obtained from 511 differentially expressed genes in high throughput sequencing analysis of the transcriptome of insect-resistant varieties vs susceptible varieties. The data were read counts of insect-resistant varieties and susceptible varieties, and each sample transcriptome was replicated three times. (b) The analysis of the WRKY polygenetic tree. Pm, Pinus massoniana L.; Nn, Nelumbo nucifera; Vv, Vitis vinifera; Qs, Quercus suber; Pt, Populus trichocarpa; Zj, Ziziphus jujube; Cc, Cajanus cajan; Jr, Juglans regia; Tc, Theobroma cacao; Mc, Macleaya cordata; Ac, Aquilegia coerulea; Pp, Physcomitrella patens; At, Amborella trichopoda; Jc, Jatropha curcas; Pe, Populus euphratica; Gs, Glycine soja; Pb, Pyrus x bretschneideri; Eg, Elaeis guineensis. The different WRKYs include NnWRKY31 (XP_010252466.1); VvWRKY31 (XP_002269696.2); QsWRKY31 (XP_023921697.1); PtWRKY31 (XP_002321134.3); ZjWRKY31 (XP_015877768.1); CcWRKY31 (XP_020234210.1); JrWRKY31 (XP_018811738.1); TcWRKY31 (EOX93243.1); NnWRKY2 (XP_010270167.1); PpWRKY2 (XP_024368161.1); AtWRKY2 (XP_006836767.1); VvWRKY2 (CBI39865.3); JcWRKY2 (XP_012070967.1); ZjWRKY6 (XP_015877768.1); QsWRKY6 (XP_023921697.1); GsWRKY6 (KHN36523.1); NnWRKY6 (XP_010252466.1); PbWRKY6 (XP_018502314.1); McWRKY6 (OVA03405.1); EgWRKY6 (XP_010926185.1); PeWRKY6 (XP_011047241.1); VvWRKY6 (XP_002263115.1). (c) NCBI blasts were prediction and SMART and Motif Scan online software were used for functional domain analysis of four genes PmWRLY2, PmWRKY6, PmWRKY31, and PmLp8, which were mapped with PROSITE software (https://prosite.expasy.org/mydomains). Colored sections are the main functional domains of the genes. (d) The annotation and signaling pathways of the WRKY2 gene using the KEGG database. (e) Results for the needle volatile substances of insect resistant varieties and susceptible varieties in May. The results were analyzed by GC and GC-MS (HP6890 gas chromatograph ((Hewlett-Packard Company, USA), GCMS-QP5050A gas chromatography-mass spectrometry (Shimadzu Corporation, Japan)). The sample was repeated three times.
-

Figure 2. Interaction between PmWRKY31 and PmLp8 in vitro and in vivo. (a) Pull down experiments of PmWRKY31 and PmLp8 in vitro. A GST prokaryotic expression vector for the bait PmLp8 protein and a prokaryotic expression vector of the prey PmWRKY31 protein were constructed. Western blotting was performed after adding loading buffer to GST-PmLp8 and His-PmWRKY31 fusion proteins to verify the normal expression of the fusion proteins. The GST protein and GST-PmLp8 protein with GST resin were incubated with His-PmWRKY31 protein overnight and eluted with reduced glutathione the next day. The following day, elution was performed with reduced glutathione. Western blotting was performed after an appropriate amount of the eluate was treated with loading buffer. (b) BiFC-validated interactions of PmLp8 and PmWRKY31. Constructed of pSPYNE-35S-PmLp8 and pSPYCE-35S-PmWRKY31 vectors were used for BiFC with Arabidopsis thaliana. From left to right, the pictures show the yellow fluorescence channel, red fluorescence channel, bright field, and superimposed map. (c) Localization of the PmWRKY31 gene subcellularly. Constructed vector plasmids were transferred into Agrobacterium, and tobacco plants in good growth conditions were selected. A 1-mL syringe without a gun tip used to inject solution into the lower epidermis of tobacco leaves for labeling; plants were incubated in weak light for 2 d after injection. Tobacco leaves were taken, observed and photographed with a confocal laser microscope (Olympus FV1000, excitation light: 480, emitting light: 510). From left to right, the pictures show bright light.
-

Figure 3. Effects of hormone and Ca2+ treatments on D. punctatus behavior. The seedlings were treated with different hormones and calcium according to the experimental design. Fresh needles were used to feed the 3rd instar D. punctatus with needles sprayed with ddH2O water as the control. On the 5th day of feeding, the feeding amount and weight of D. punctatus were weighed and calculated according to the corresponding equation. (a) Effects of different treatments on D. punctatus feeding; (b) Effects of different treatments on D. punctatus weight. Each sample was repeated three times. ** P < 0.01, Student's t-tests.
-
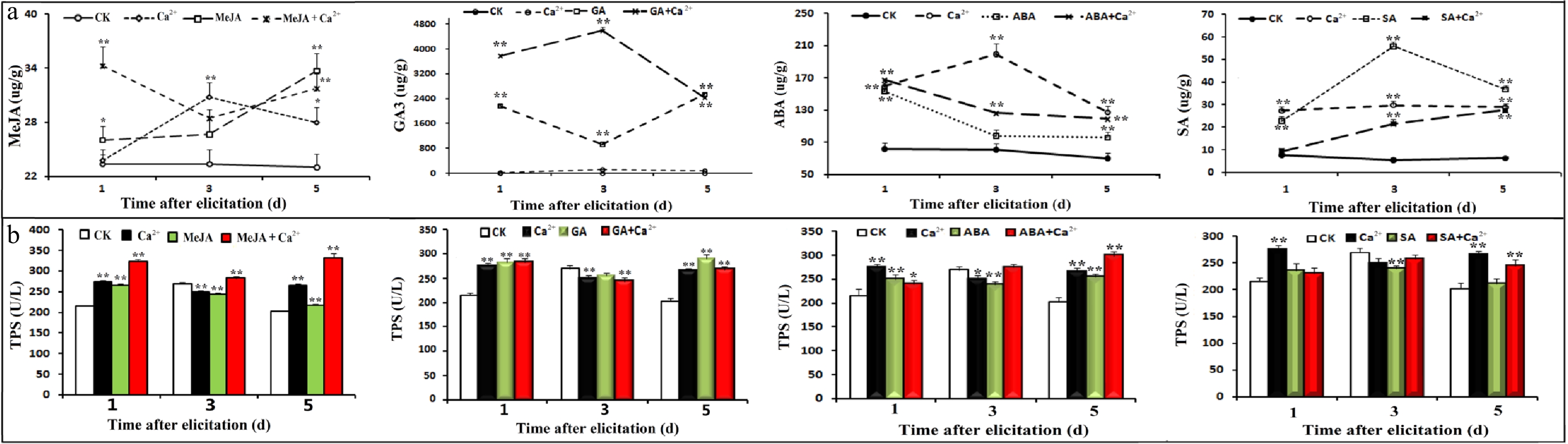
Figure 4. Effects of hormones and Ca2+ treatments on hormone and TPS contents. Seedlings were treated with different hormones and calcium according to the experimental design, and samples were collected on days 1, 3 and 5. Four hormones were determined by UPLC-MS-MS. A plant TPS ELISA kit (Shanghai, China) was used to detect of TPSs in different samples according to the manufacturer's instructions. (a) Effects of different treatments on hormones; (b) Effects of different treatments on TPSs.
-

Figure 5. Effects of hormone and Ca2+ treatments on volatile substances. Seedlings were treated with different hormones and calcium according to the experimental design. Samples were collected on days 1, 3 and 5. Volatile substances were determined by a SCION SQ and TQ (GC-MS) system. The extract was subjected to GC-MS analysis. (a)−(d) The effects of MeJA, GA, ABA, SA, and Ca2+ treatments on volatile substances. Each sample was repeated three times, ** P < 0.01, Student's t-tests.
-
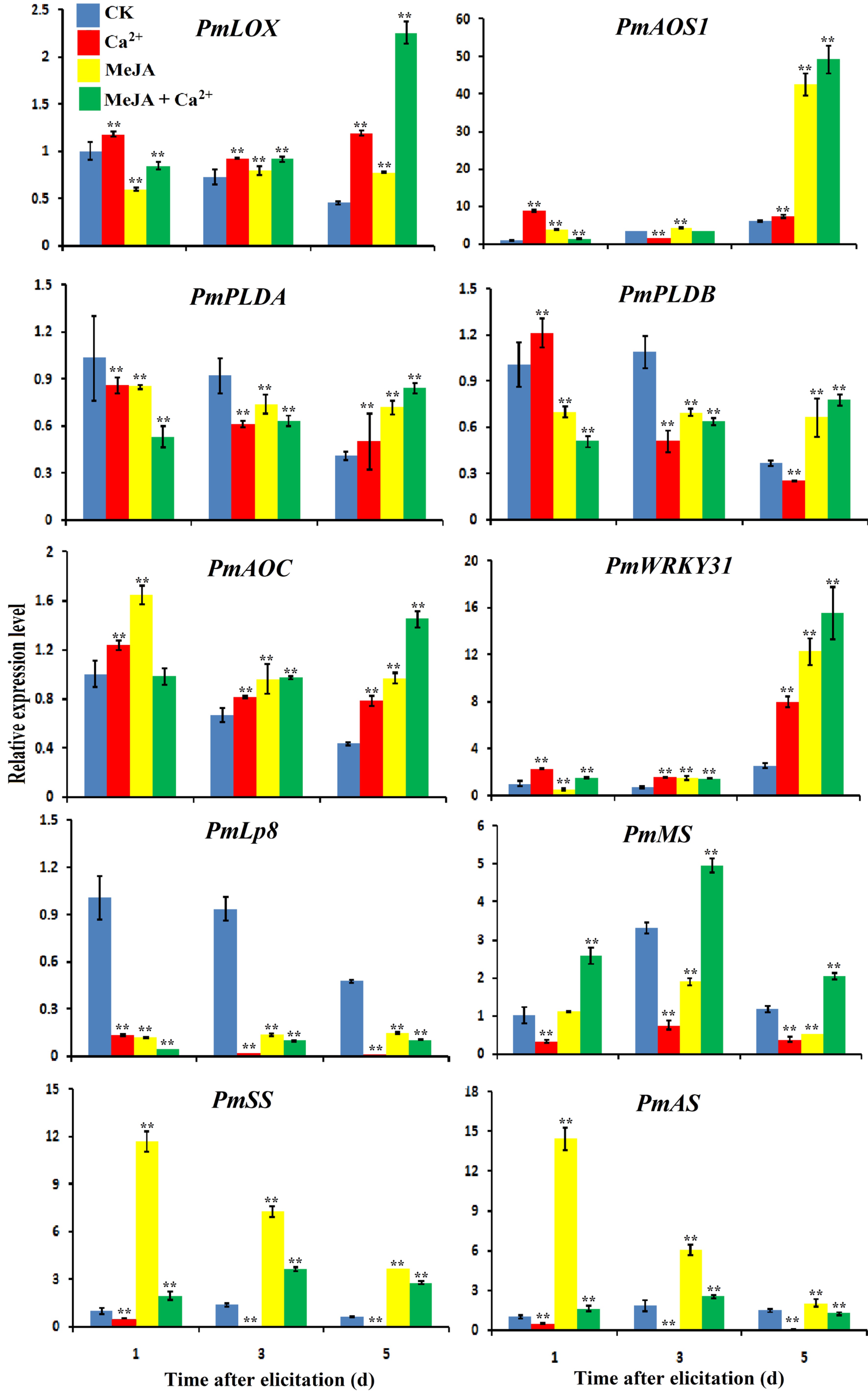
Figure 6. Effects of MeJA and Ca2+ treatments on the expression of related genes. Samples were collected on days 1, 3 and 5 of the different treatments according to the experimental design. RNA was extracted using a polyphenol polysaccharide plant RNA extraction kit, cDNA reverse transcription was performed by M-MLV reverse transcriptase, and gene expression analysis was performed using real-time fluorescence quantitative PCR. The gene names were PmLOX (Lipoxygenase), PmAOS1 (Allene oxide synthase), PmPLDB (Phospholipase D beta), PmPLDA (Phospholipase D alpha), PmAOC (Allene oxide cyclase), PmAS (Diterpene synthase), PmSS (Sesquiterpene synthase), and PmMS (Monoterpene synthase). Each sample was repeated three times. ** P < 0.01, Student's t-tests.
-
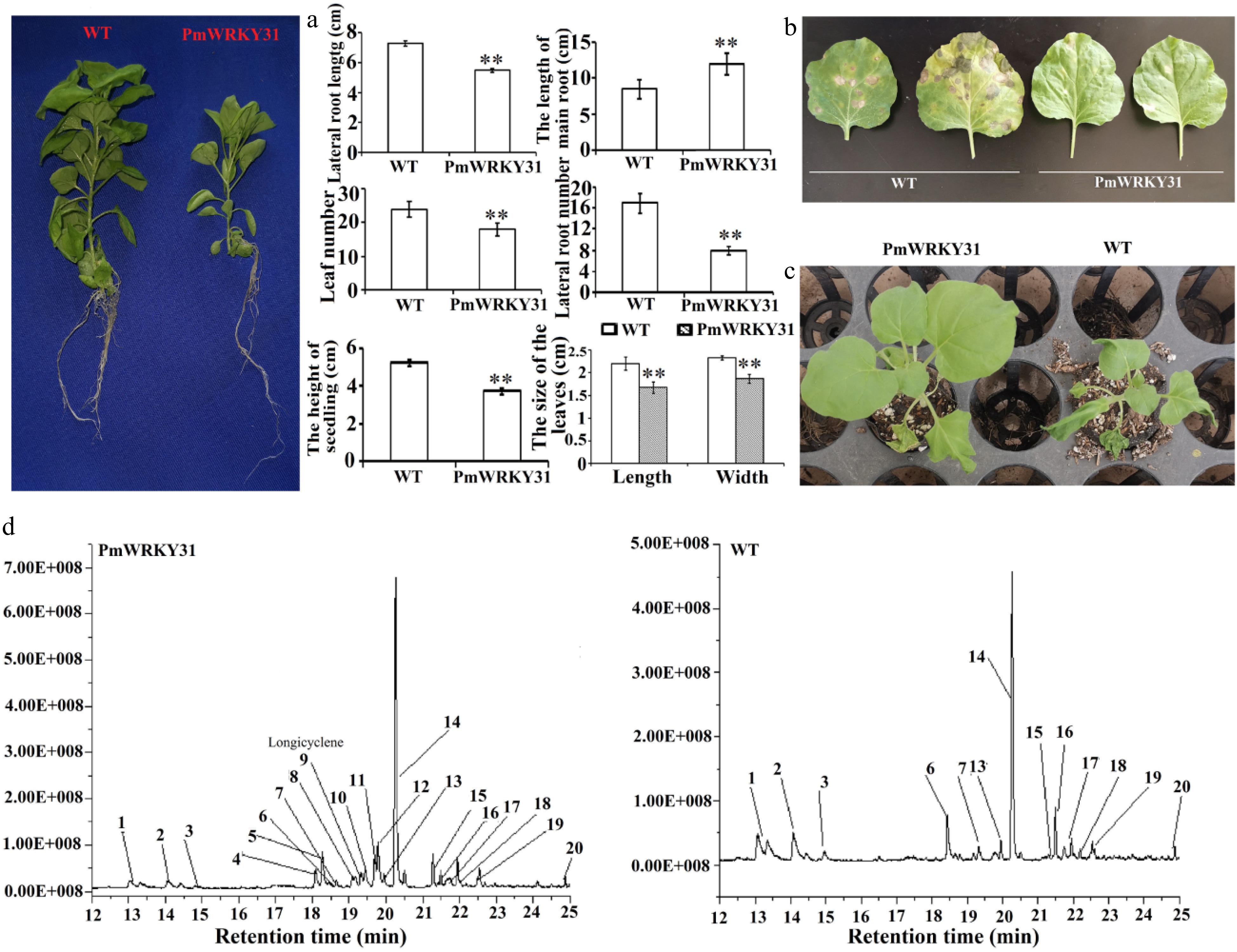
Figure 7. Function verification of PmWRKY31. (a) Effects of a transgene on tobacco plants. (b) Effects of a transgene on disease resistance. (c) Effects of a transgene on drought tolerance. (d) Effects of a transgene on volatiles. Each sample was repeated three times. ** P < 0.01, Student's t-tests.
-

Figure 8. Preliminary model of improving the PmWRKY31 gene and PmLp8 gene interaction to regulate hormone and calcium signaling pathways to enhance resistance to D. punctatus. The application of exogenous signaling substances was able to rapidly initiate the expression of PmWRKY31, and increase the downstream hormone signals and key gene responses of the terpene synthesis pathway by regulating the PmLp8 gene, thereby increasing the content of endogenous MeJA, GA, SA, ABA and terpene synthase and volatiles in P. massoniana to promote the ability to resist D. punctatus. All treatments strongly induced PmWRKY31 expression. MeJA, MeJA+Ca2+, GA, ABA, and SA positively regulated PmLp8 and terpene synthase genes, while treatment with GA, ABA, and SA with Ca2+, and Ca2+ negatively regulated these genes. Arrows represent negative or regulation; barred lines represent negative regulation; arrows with "+" represent positive regulation, and arrows with "−" represent negative regulation.
Figures
(8)
Tables
(0)