-
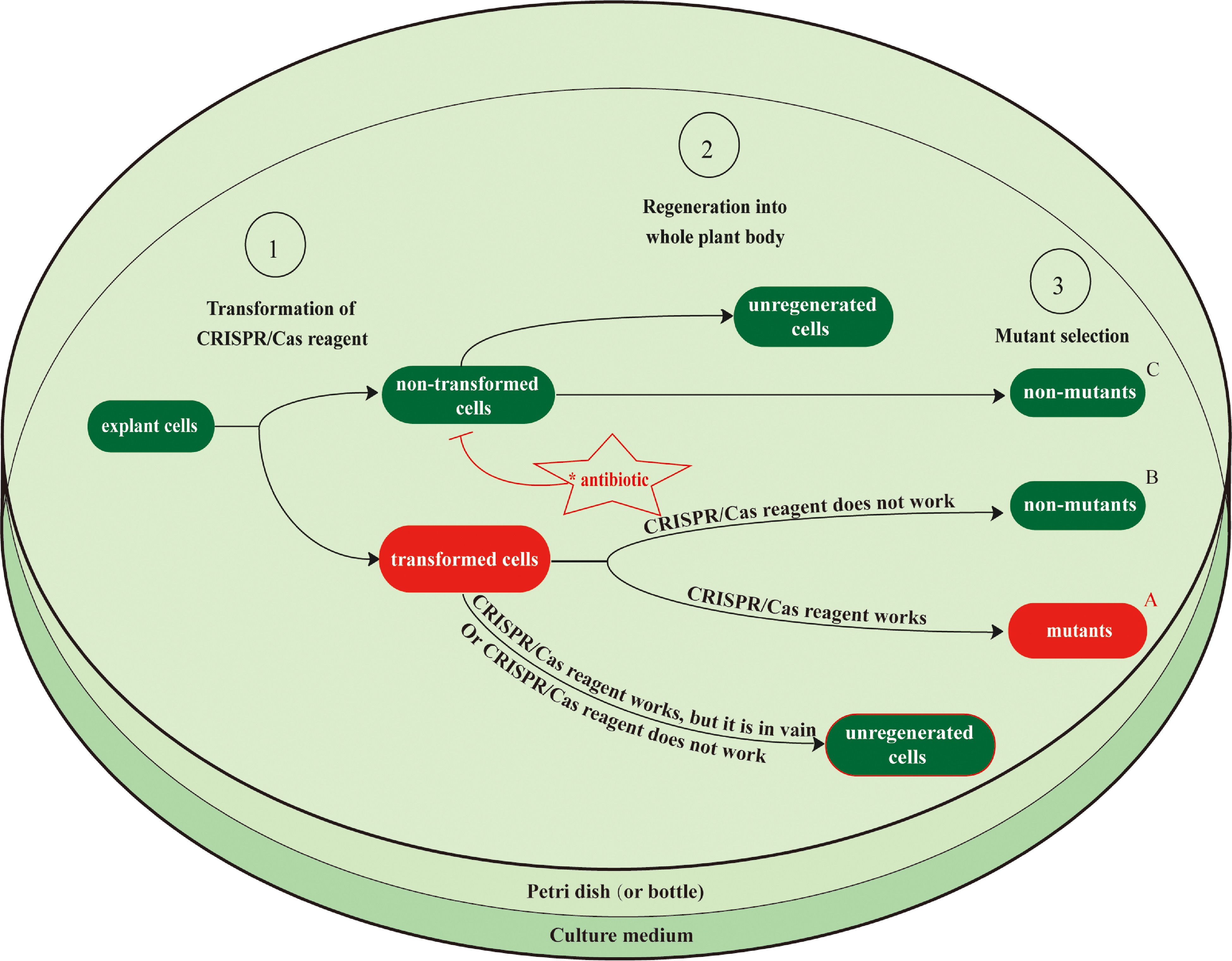
Figure 1.
Schematic diagram for the wet-lab workflow of CRISPR/Cas-based genome editing, showing the limitations of current transformation and regeneration protocols. After CRISPR/Cas reagents, such as a plasmid DNA vector or RNP for the genes of interest, are transformed into explant cells, these cells must then be regenerated into mutant transgenic plants. Each step of the tissue culture process reduces its efficiency owing to the regeneration of non-transformed plants and the regeneration of transformed plants that lack the desired edit. Antibiotics are typically added to the culture medium to increase the proportion of transformed cells by inhibiting the growth of non-transformed cells (only in the Agrobacterium T-DNA transfer method). The transformation efficiency, regeneration rate, and in vivo activity of CRISPR/Cas reagents all impact the total genome editing efficiency during this process. However, genome editing efficiency in most tree genome editing practices (Table 1) has not been accurately measured. Efficiency is typically calculated as A/(A+B+C), where A indicates the number of mutant transgenic plants, and B and C indicate the numbers of non-mutant transgenic plants obtained from transformed and non-transformed cells. This does not account for the number of explant cells that were transformed but not regenerated. Most tree genome editing studies have focused more on whether the CRISPR/Cas reagents function than on their efficiency. Conventional protocols for transformation and regeneration are laborious and time-consuming, and their low efficiencies are major obstacles to tree genome editing using the CRISPR/Cas system. Possible solutions to these problems are discussed in Section 4.
-
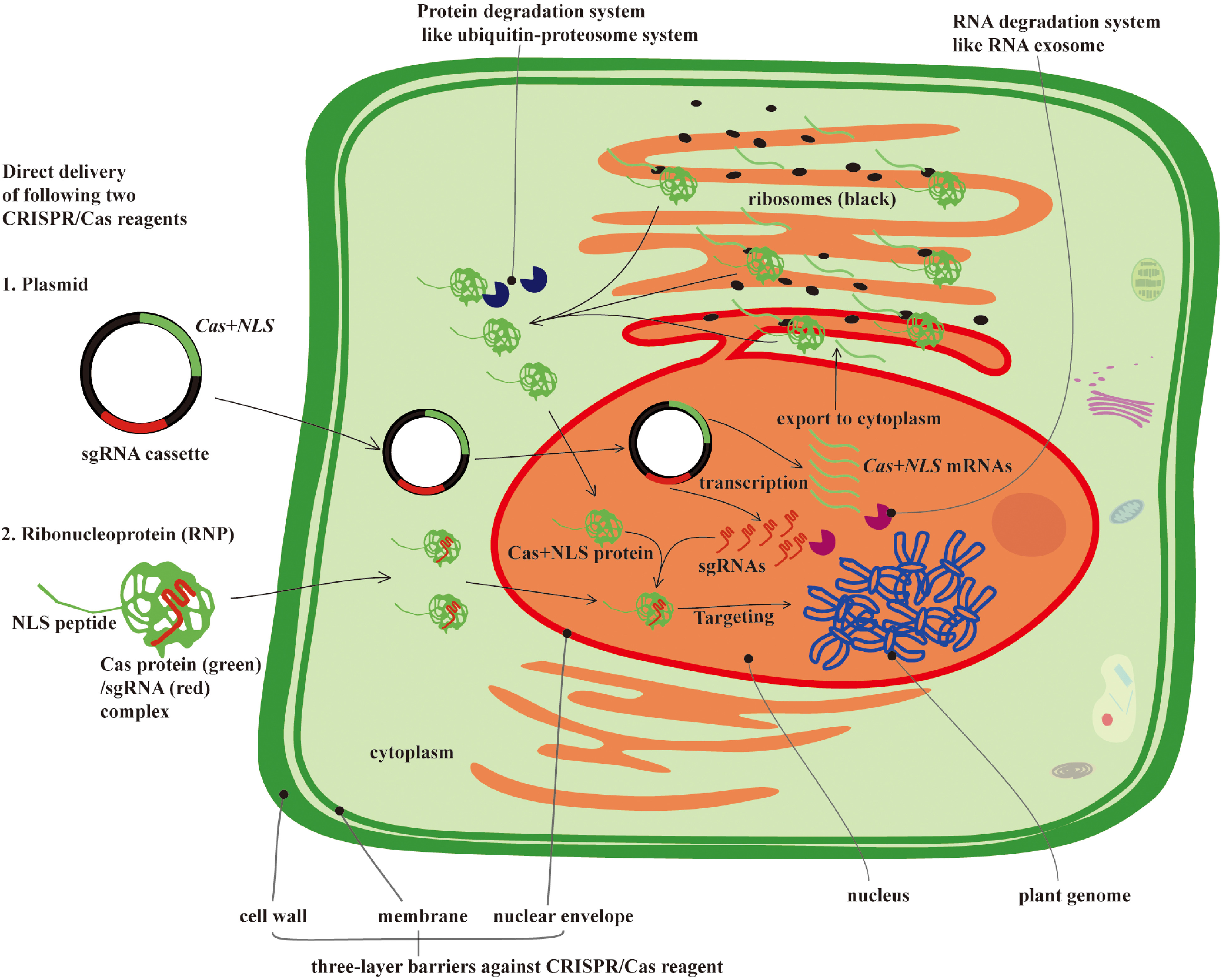
Figure 2.
Direct delivery of CRISPR/Cas reagents (plasmid or RNP) and potential barriers affecting their delivery efficiency and intranuclear genome editing activities. The active form of CRISPR/Cas reagents is the RNP, which is generated from transcription and translation of the CRISPR/Cas and sgRNA sequences. Because transcription only takes place in the nucleus, these plasmids must therefore gain entry to this cellular compartment. In the nucleus, Cas+NLS and sgRNAs are transcribed into RNAs, and the Cas + NLS mRNA must be exported into the cytoplasm to be translated into the Cas + NLS protein, which then re-enters the nucleus to form the RNP complex with sgRNAs. Therefore, the plasmid delivery process involves a total of three passes through the nuclear envelope. Although this process has been studied extensively, it still remains unclear how the nuclear envelope regulates the import of plasmid DNA, RNA, or RNP complexes into the nucleus, and the low efficiency of direct delivery systems may be due to the negative regulatory role of the nuclear envelope during the nucleocytoplasmic transfer of CRISPR/Cas reagents into the nucleus. Furthermore, intracellular protein and RNA degradation systems, such as the Ubiquitin-Proteosome and RNA exosome, may be potential obstacles for the RNP complex. These “degradosomes” may render the activity of RNPs more transient, resulting in a much lower editing efficiency.
-
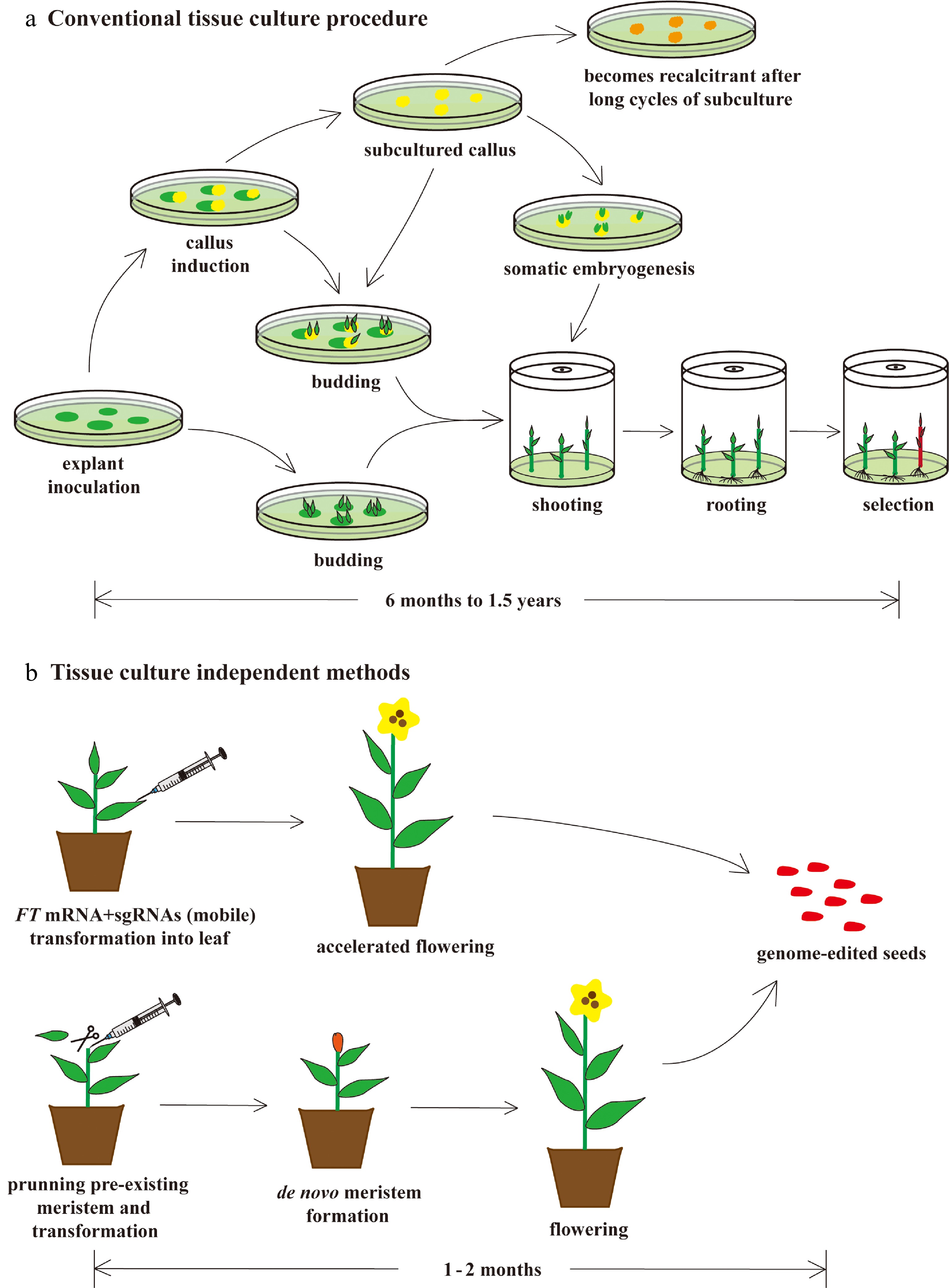
Figure 3.
Faster and easier regeneration of genome-edited plants by tissue culture–independent protocols. (a) Conventional tissue culture is both tedious and laborious. This process normally takes anywhere from six to eighteen months and requires a sterile environment and a large amount of tissue culture medium, dishes, bottles, and chemical reagents. Its regeneration efficiency is relatively low, and recalcitrancy limits its utility. (b) Recently, novel technologies, such as mobilization of sgRNAs by FT mRNA fusion and de novo meristem induction, have been developed, enabling researchers to overcome some of the problems of conventional tissue culture. In the FT mRNA/sgRNAs protocol, FT mRNA encodes the mobile florigen essential for induction of flowering, which is fused to sgRNAs to facilitate their movement from the leaf to the shoot apical meristem. This causes genome editing of the floral meristem, which results in genome-edited seed production. In the de novo meristem induction protocol, genome editing and meristem induction are performed simultaneously to generate genome-edited seeds. These in planta transformation protocols require only one or two months to generate genome-edited plants. In addition, these protocols do not require laborious processes of sterilization and sterile tissue culture.
-
Tree species sgRNA design
toolCas delivery enhancer Promoter
(sgRNA)Promoter
(Cas)Multiplex targeting Transformation protocol/explant Regeneration protocol/time Mutagenesis efficiency; mutation; mutants Potential off-targets (Number; activity) Reference Actinidia chinensis
cv. HongyangCas-Designer NLS AtU6 35S PTG Agrobacterium/
leaf discCI-S; — 7.14%–91.67%; indel; biallelic, chimeric 4; N [32] A. chinensis
cv. Hort16AGeneious NLS AtU3, AtU6 Ubi, 35S PTG Agrobacterium/
leaf stripCI-S-R; — 30%–75%; indel; biallelic, heterozygous — [33] Bambusa oldhamii — NLS OsU3 2× 35S — PEG (DNA)/protoplast CI-S; 3 mon 12.5% (5/40); del&subs; — — [14] Citrus sinensis cv. Valencia — NLS 35S 35S — Agroinfiltration/leaf — 3.2%–3.9%; del; — 46; N [10] C. sinensis Osbeck — — AtU6 35S — Agrobacterium/epicotyl S-G; — 34.5% (38/110); del; biallelic, homozygous, heterozygous 11; 1-bp point mutations (5–10%) [20] C. paradisi — NLS 35S 35S — Agrobacterium/epicotyl S-G; — —; indel; — 85; N [21] 35S — Agrobacterium/leaf & epicotyl S-G; — 23.8%–89.36%; indel; — 7; N [22] Yao, 35S — Agrobacterium/epicotyl S-G; — 42.8% (3/7); del; — 0 [23] Dendrocalamus latiflorus Munro — — OsU6 Ubi — PEG (DNA)/protoplast CI-S-R; — 83.3%–100%; indel; homozygous, biallelic, heterozygous, chimeric — [15] Hevea brasiliensis — — — — — PEG (RNP)/protoplast — 3.74%–20.11%; indel; — — [55] Malus × domestica Bork. CRISPOR NLS MdU3, MdU6 PcUbi4-2 Gateway cloning Agrobacterium/young leaves B; 6 mon 90% (27/30); indel&subs; biallelic chimeric 4; N [11] Malus prunifolia cv. Golden Delicious CRISPR RGEN NLS — — — PEG (RNP)/protoplast — 6.9%; indel; — — [12] M. prunifolia Borkh. 'Seishi'× M. — NLS AtU6 2× 35S — Agrobacterium/leaf disc S-R; 8 mon 10.9% (18/164); indel; homozygous, heterozygous, chimeric 0 [13] Coffea canephora
clone 197— NLS AtU6 35S Restriction enzyme ligation Agrobacterium/
embryonic calliSE; 18 mon 30.4% (28/92); indel; homozygous, heterozygous 0 [27] Manihot esculenta cv. 60444; cv. TME 204 CRISPR-P NLS AtU6 35S — Agrobacterium/
embryonic calliSE; — 19.1%–46.6%; indel&subs; homozygous, biallelic, heterozygous — [17] M. esculenta
cv. 60444CRISPR-P — AtU6 2× 35S Gibson assembly Agrobacterium/
embryonic calliSE; — 91%; indel; homozygous, biallelic, heterozygous, chimeric 5; single mutation for one off-target was detected [18] Jatropha curcas CRISPR-P NLS AtU3 35S — Agrobacterium/cotyledon CI-S; 4 mon —; indel; homozygous — [54] Parasponia
andersoniiGPP sgRNA designer NLS AtU6 35S — Agrobacterium/
stem, petioleCI-S-R; 4 mon 37.9%–88.9%; indel; biallelic, heterozygous — [16] Poncirus. trifoliata L. Raf. × C. sinensis L.
Osb— NLS AtU6 Yao — Agrobacterium/epicotyl S; 4 mon 85% (17/20); indel&subs; homozygous, monoallelic heterozygous 2; N [24] 35S, AtU6 35S — Agrobacterium/epicotyl S-G; — 15.55%–79.67%; indel; homozygous 3; N [25] Populus alba ZiFiT NLS AtU3, AtU6 35S Golden Gate cloning Agrobacterium/young leaves CI-S-R; 3 mon 89%; del; — — [35] 84K poplar (P. alba ×
P. glandulosa)CRISPR-P 2.0 NLS AtU6 PcUbi4-2 — Agrobacterium/leaf disc — —; indel; — — [36] — NLS AtU6 2× 35S — Agrobacterium/leaf disc CI-S-R; — 6.7%–70%; del; biallelic, homozygous, heterozygous — [53] Populus tomentosa Carr. clone 741 — NLS AtU3, AtU6 35S Golden Gate cloning Agrobacterium/leaf disc CI-S-R; 3 mon 51.7% (30/59); indel&invers; biallelic homozygous, heterozygous — [37] — Golden Gate cloning Agrobacterium/leaf disc CI-S-R; — 93.33%–100%; indel; — — [38] 35S Golden Gate cloning Agrobacterium/leaf disc CI-S-R; — 48% (12/25); indel; — — [39] 35S Golden Gate cloning Agrobacterium/leaf disc CI-S-R; — —; indel; — — [40] 35S — Agrobacterium/leaf disc CI-S-R; — —; indel; — — [41] 35S Golden Gate cloning Agrobacterium/leaf disc CI-S-R; — —; indel; — — [42] Populus tremula × P. alba clone 717 ZiFiT NLS AtU6 2× 35S Restriction enzyme ligation Agrobacterium/leaf, petiole, stem CI-S-R; 18 wk 81.8% (479/585); indel&subs&invers; homozygous, biallelic, heterozygous, chimeric 5; N [43] Geneious NLS MtU6 2× 35S — Agrobacterium/
leaf, stemCI-S-R; — 100%; indel; biallelic — [44] Geneious NLS AtU6 PcUbi4-2 — Agrobacterium/leaf disc CI-S-R; 4–8 mon —; indel; — — [45] Populus tremula × P. alba INRA clone 717-1B4 — — MtU6 35S — Agrobacterium/ — CI; — — — [46] Geneious NLS MtU6 2× 35S — Agrobacterium/
hairy rootHR; — 40%; indel; — 5; N [47] CRISPR-P 2.0 NLS AtU6 Atact2 Golden Gate MoClo system assembly Agrobacterium/leaf HR; — 87.5% (14/16); indel; homozygous, biallelic, heterozygous — [48] Populus tremula × tremuloides clone 353 ZiFiT NLS AtU6 2× 35S Restriction enzyme ligation Agrobacterium/leaf, petiole, stem CI-S-R; 18 wk 88.8% (88/99); indel&subs&invers; homozygous, biallelic, heterozygous, chimeric 5; N [43] Populus tremula × tremuloides clone T89 CRISPR-P 2.0 NLS — 35S Golden Gate cloning Agrobacterium/ — — — — [49] Populus trichocarpa Nisqually-1 CRISPR-P 2.0 3x NLS AtU6 2× 35S, PUbi4 Golden Gate cloning Agrobacterium/leaf disc CI-S; — —; indel; — — [50] — — AtU6 2× 35S — Agrobacterium/stem S-R; 17 wk —; indel; — — [51] CRISPRdirect — AtU6 2× 35S Golden Gate cloning Agrobacterium/stem S-R; 14 wk 75%–100%; indel; homozygous, biallelic, heterozygous, chimeric 8; N [52] Punica granatum L. Cas-Designer NLS AtU6 AtUbi Restriction enzyme ligation Agrobacterium/
hairy rootHR; — —; indel&subs; homozygous, biallelic, chimeric — [34] Pyrus communis L. cv. Conference CRISPOR NLS MdU3, MdU6 PcUbi4-2 Gateway cloning Agrobacterium/young leaves B; 7 mon 9% (5/54); indel&subs; biallelic chimerism 4; N [11] Theobroma cacao Geneious — AtU6 35S Golden Gate cloning Agrobacterium/leaf, primary SE cotyledon SE; — 27%; indel; — 9; 0.29–1.9% (off-target rate) [26] Vitis vinifera L. cv. Chardonnay CRISPR-P NLS AtU6 35S Golden Gate cloning Agrobacterium/callus S; — 100% (3/3); indel; heterozygous, chimeric 4; N [28] CRISPR RGEN NLS — — — PEG (RNP)/protoplast — 0.1%; indel; — — [12] V. vinifera cv. Thompson CRISPR-P, CRISPR RGEN NLS AtU3, AtU6 2× 35S Golden Gate cloning Agrobacterium/
embryonic callusSE; 12 mon 31% (22/72); large del; biallelic, monoallelic 6; N [29] V. vinifera L. cv. Neo Muscat — NLS AtU6 PcUbi — Agrobacterium/
embryonic callusCI-SE; 19–21 mon 2.7%–72.2%; indel; biallelic 3; N [30] V. vinifera cv. Chasselas × V. berlandieri CRISPR-P — AtU6 35S — Agrobacterium/
embryonic cellSE; — 66.6% (4/6); indel; biallelic, heterozygous, chimeric 2; N [31] Abbreviations: NLS, nuclear localization signal; AtU3/AtU6, Arabidopsis promoters for small nuclear RNA transcription; MtU3/MtU6, Medicago truncatula U3/6 promoters; MdU3/MdU6, Malus domestica U3/6 promoters; Cas, Cas nucleases; 35S, cauliflower mosaic virus (CaMV) 35S promoter; Ubi, ubiquitin promoter; PcUbi, Petroselinum crispum ubiquitin promoter; PTG, Polycistronic tRNA process system; GFP, green fluorescent protein; Agrobacterium, Agrobacterium-mediated T-DNA transfer; PEG (DNA), Polyethylene glycol–mediated DNA transfection; PEG (RNP), Polyethylene glycol–mediated ribonucleoprotein transfection; mon, month; wk, week; B, budding; CI, callus induction; S, shooting; R, rooting; G, grafting; SE, somatic embryogenesis; indel, insertion and deletion mutations; del, deletion mutation; subs, substitution mutation; invers, sequence inversion mutation; N, no activity detected; —, not mentioned. Table 1.
Application of the CRISPR/Cas system to tree genome editing.
Figures
(3)
Tables
(1)