-
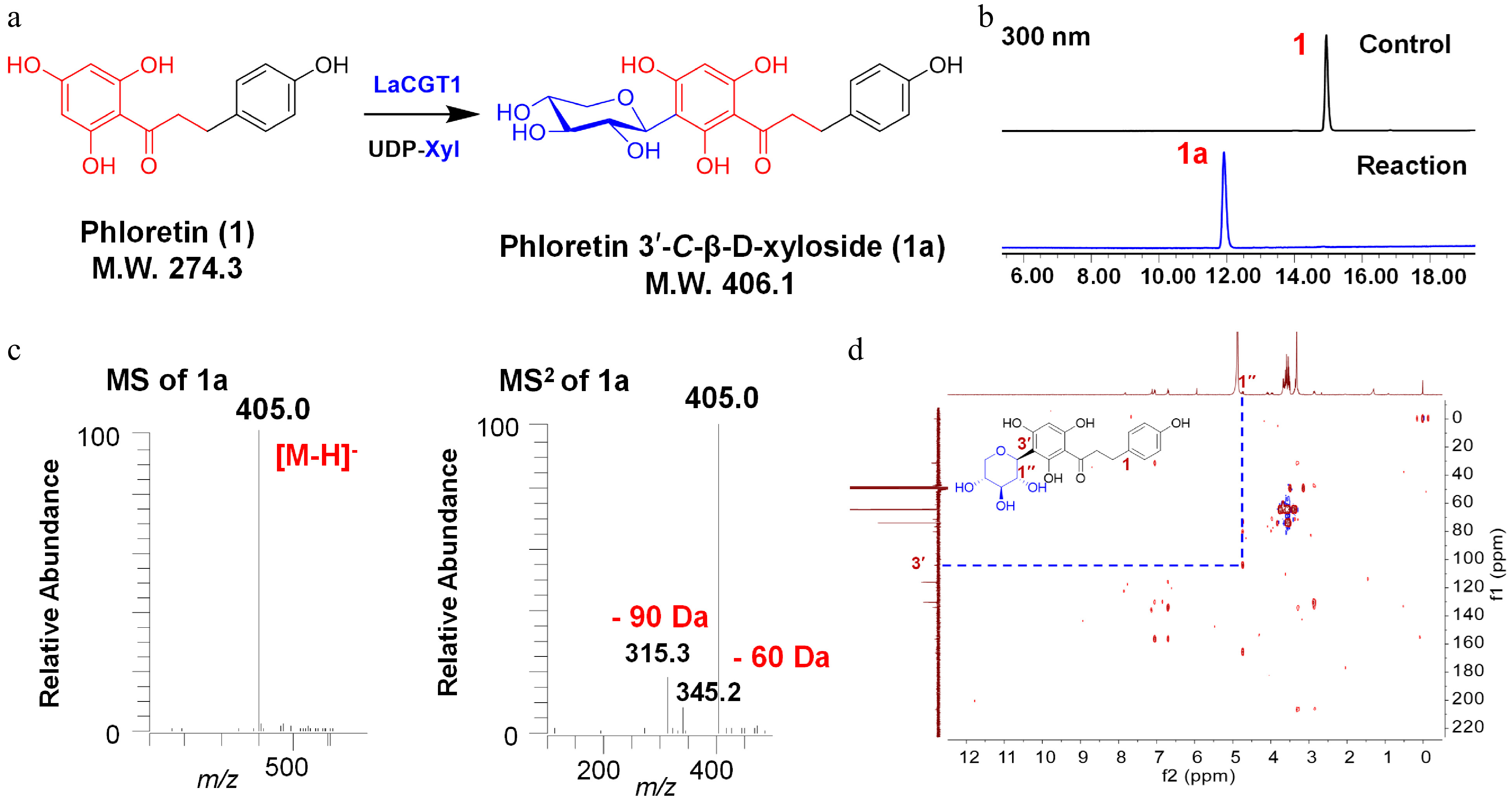
Figure 1.
C-xylosylation of phloretin (1) by LaCGT1. (a) Glycosylation of 1 by recombinant LaCGT1 to produce 1a using UDP-Xyl as sugar donor. (b) HPLC chromatograms of the enzymatic reaction mixture and the control. (c) LC/MS analysis of 1a in the negative ion mode. (d) HMBC spectrum of 1a (methanol-d4, 400 MHz).
-
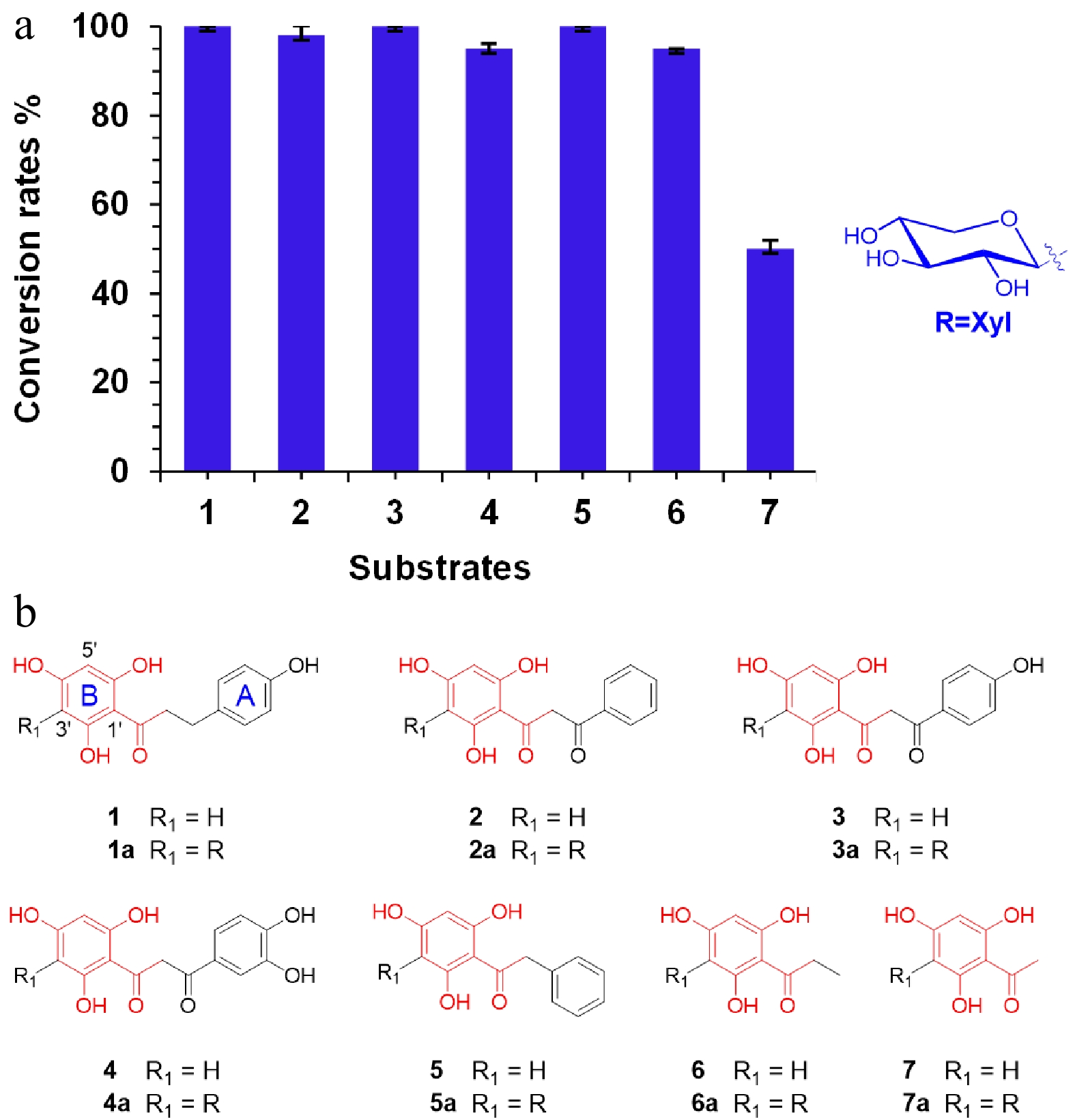
Figure 2.
Substrate specificity of LaCGT1. (a) Conversion rates (%) of glycosylated products for substrates 1−7, using UDP-Xyl as the sugar donor. The conversion rates were calculated by HPLC peak area ratio and the experiments were performed in triplicate. (b) Structures of 1−7.
-
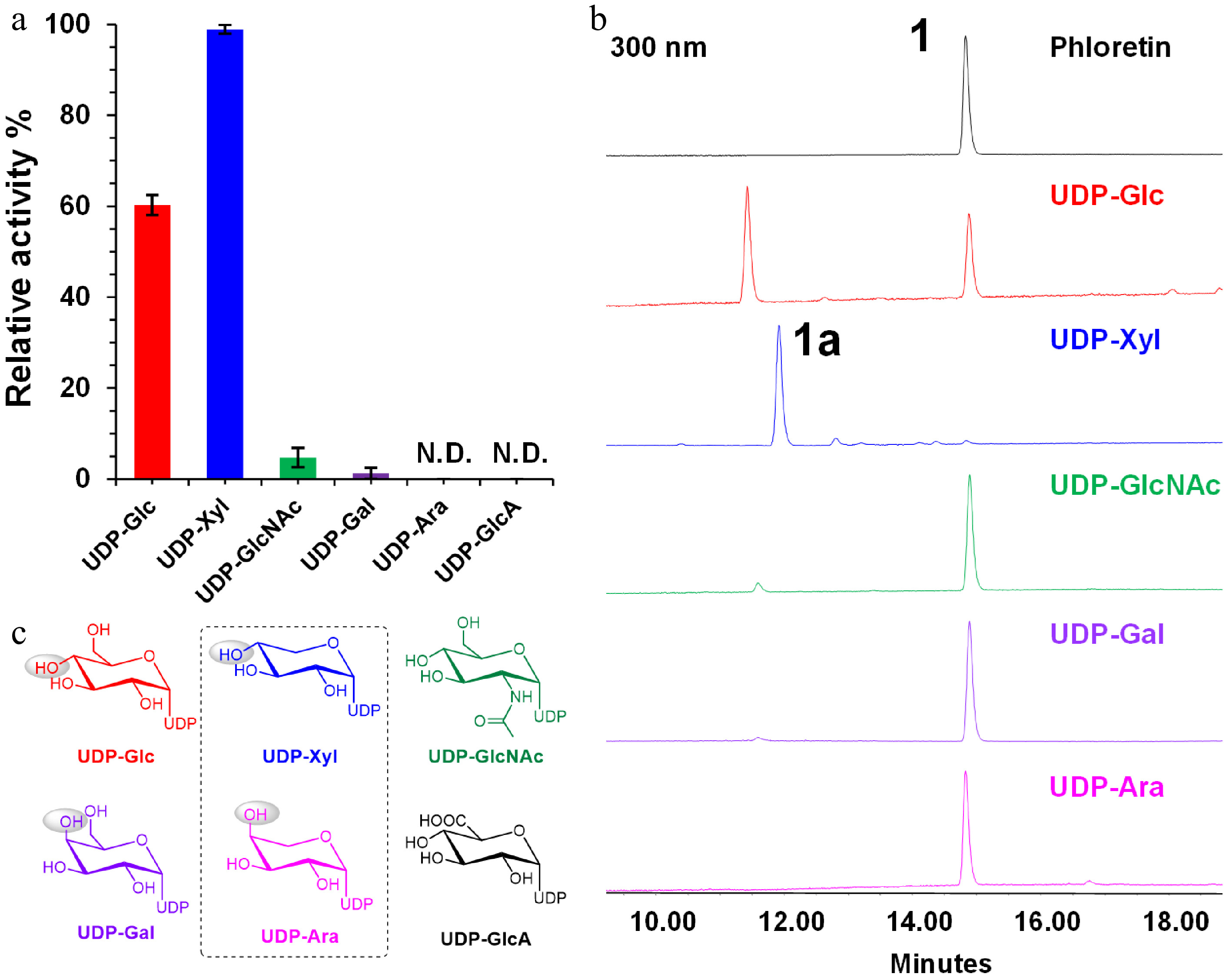
Figure 3.
Sugar donor preference of LaCGT1 at low enzyme concentration. (a) Relative conversion rates of C-glycosylated products using different sugar donors. The experiments were performed in triplicate. (b) HPLC chromatograms of enzyme reactions of LaCGT1 with different sugar donors at low enzyme concentration. (c) Structures of sugar donors.
-
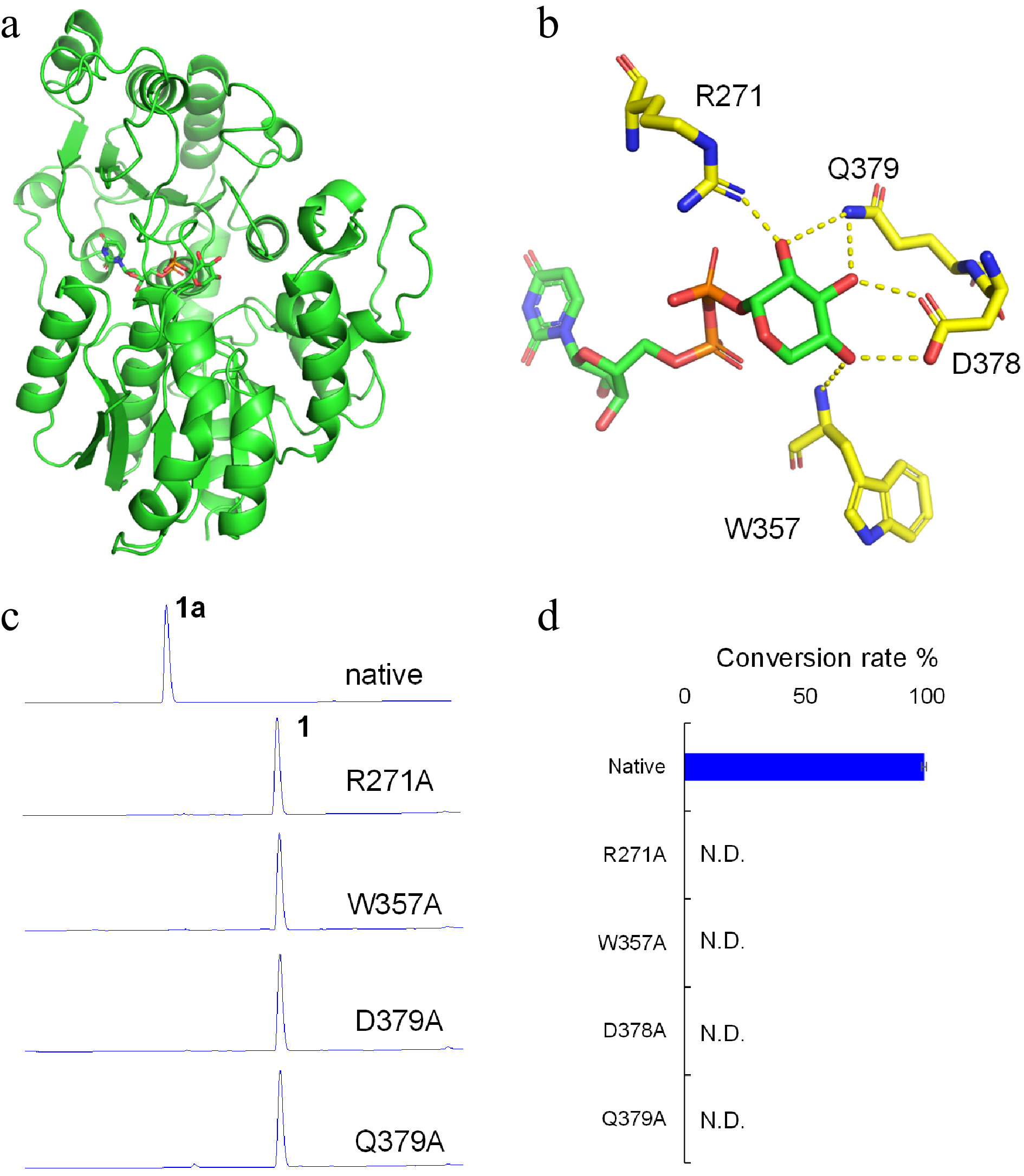
Figure 4.
Molecular modeling and site-directed mutagenesis for LaCGT1. (a) The model of LaCGT1/UDP-Xyl. (b) Key amino acid residues around the xylose residue of UDP-Xyl in LaCGT1. The hydrogen-bond interactions are shown as yellow dashes. (c, d) The glycosylation conversion rates of native and mutants, using phloretin (1) and UDP-Xyl as the sugar acceptor and donor, respectively. The reaction mixtures were incubated at 25 °C for 1 h. Molecular docking was carried out by Autodock and the results were visualized using AutoDocktools v1.5.6 and Pymol. N.D., not detected.
-
Position δH of 1a δH of 5a δH of 6a Aglycone: 1 2 7.06 (2H, d,
J = 8.4 Hz)4.31 (2H, m) 3 6.70 (2H, d,
J = 8.4 Hz)4 5 6.70 (2H, d,
J = 8.4 Hz)5.92 (1H, s) 5.92 (1H, s) 6 7.06 (2H, d,
J = 8.4 Hz)α 3.36 (2H, m) 3.08 (2H, d,
J = 7.3 Hz)β 2.86 (2H, m) 1.13 (3H, t,
J = 7.3 Hz)1′ 2′ 7.18 (2H, m) 3′ 2.00, 1.49 7.09 (2H, m) 4′ 7.20 (2H, m) 5′ 5.95 (1H, s) 5.85 (1H, s) 6′ Xylosyl: 1 4.74 (1H, d,
J = 9.9 Hz)4.65 (1H, d,
J = 9.9 Hz)4.72 (1H, d,
J = 9.9 Hz)2 4.09 (1H, t,
J = 9.3 Hz)4.00 (1H, t,
J = 9.2 Hz)4.05 (1H, t,
J = 9.1 Hz)3 3.38 (1H, m) 3.34 (1H, m) 3.37 (1H, m) 4 3.68 (1H, m) 3.55 (1H, m) 3.63 (1H, m) 5 3.97 (1H, m, H-a);
3.28 (1H, m, H-b)3.88 (1H, t,
J = 5.5 Hz);3.96 (m, H-a);
3.25 (m, H-b)3.19 (1H, m) Table 1.
1H NMR data of compounds 1a, 5a, and 6a (δ in ppm, J in Hz, measured at 400 MHz in methanol-d4).
Figures
(4)
Tables
(1)