-
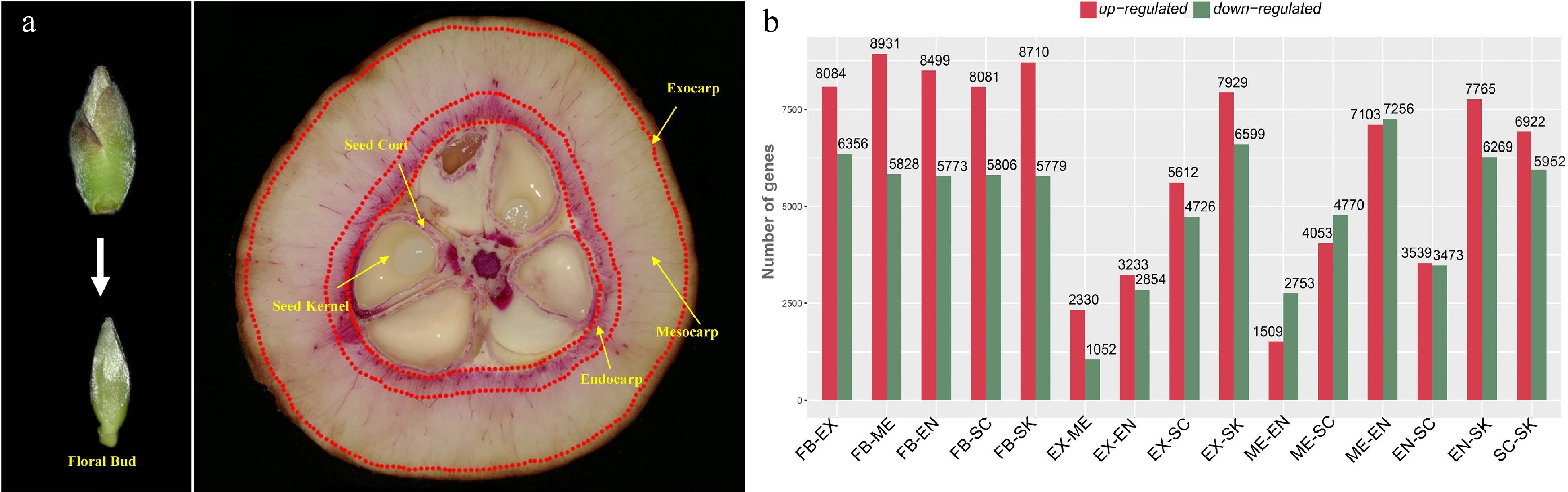
Figure 1.
Tissue-specific transcriptomics in fruit of Camellia chekiangoleosa. (a) Morphology of tissues used for RNA sequencing. On the left is the incipient floral bud; its outside scale leaves are removed before a sample’s preparation. On the right are fruit tissue types at the stage of fruit enlargement; for each, three biological replicates were used for independent library construction and sequencing. FB, floral bud; EX, exocarp; ME, mesocarp; EN, endocarp; SC, seed coat; SK, seed kernel. The red-stained areas indicate the lignified tissues stained by phloroglucinol-hydrochloric acid. Yellow arrows indicate the tissues that were collected for sampling. Three biological replicates were used for library preparation and sequencing analysis. (b) Numbers of differentially expressed transcripts between tissue types. Red and green colors indicate the up-regulated and down-regulated genes, respectively, in each comparison.
-
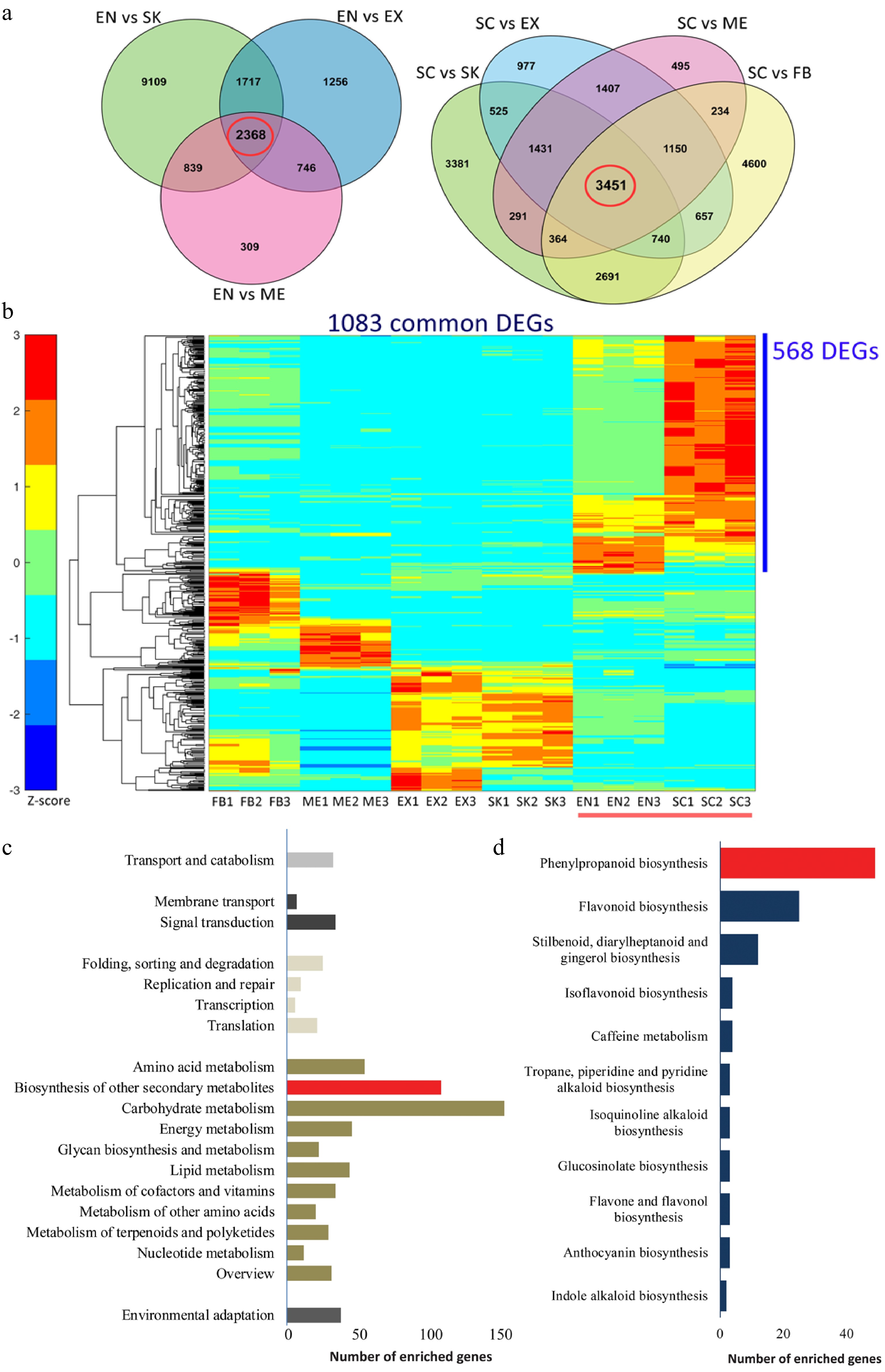
Figure 2.
Functional characterization of differentially expressed genes (DEGs) that are involved in the lignification of the endocarp and seed coat of Camellia chekiangoleosa. (a) Venn diagrams of the DEGs in comparison to EN (left) and SC (right), which revealed 2348 and 3451 unigenes in the EN-group (left red circle) and SC-group (right red circle), respectively. (b) The EN-group and SC-group analysis yielded 1083 DEGs for gene expression analysis. The heatmap analysis of these 1083 genes identified clusters of them highly expressed in various tissue types. The red bar indicates the highly lignified EN and SC tissues. The blue bar indicates those genes highly expressed in EN and SC (568 DEGs); C, KEGG enrichment analysis of 568 DEGs that were highly expressed in EN and SC. D, Distribution of the number of genes that are enriched in 'Biosynthesis of other secondary metabolites'.
-

Figure 3.
Tissue-specific expression analysis of lignin biosynthesis and transcriptional regulation gene in Camellia chekiangoleosa. (a) Heatmap of gene expression patterns for lignin-related genes that were identified based on sequence similarity. The gene symbols from Arabidopsis are used to indicate the potential homologs in C. chekiangoleosa. The red arrow indicates the NST homolog. Mean expression levels of transcripts are used for the expression analysis. (b) The key genes participating in lignin biosynthesis and its transcriptional regulation are presented according to known pathways identified in Arabidopsis. The master switch of secondary cell wall formation as regulated by NAC family TFs are highlighted in the red-dashed square.
-
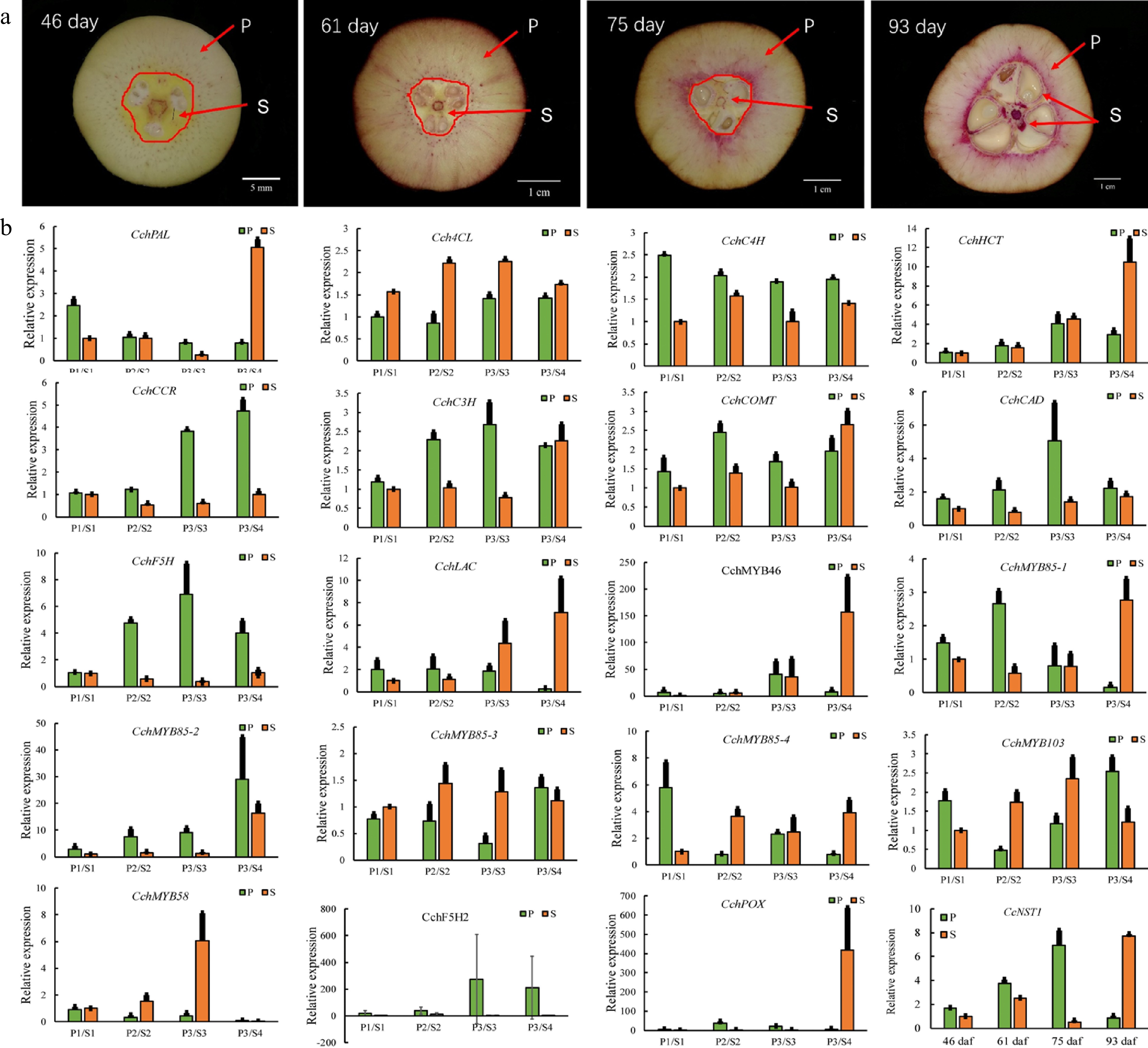
Figure 4.
Expression analysis of genes involved in the regulation of lignin biosynthesis in pericarp and seed tissues during the fruit development of Camellia chekiangoleosa. (a) Staining of the vertical section of C. chekiangoleosa fruit in different periods. The purple stain signals from phloroglucinol-HCl indicate the lignified cells; the red arrowheads indicate the respective pericarp and seed tissue portions sampled at different stages of fruit development. The selection of the sampling is based on the developmental curves of C. chekiangoleosa fruits. P denotes mixed pericarp tissues; S denotes the mixed seed tissues. The arrows point to the areas of mixed tissues sampled at different developmental stages. (b) The qRT-PCR analysis of expression patterns of lignin biosynthesis genes at the four critical stages of P and S tissues. The expression of CchNST1 was significantly up-regulated at 75 to 93 d post-fertilization in the P and S samples, corresponding to the lignification of the endocarp and seed coat. Values are means ± s.d. of three biological replicates.
-
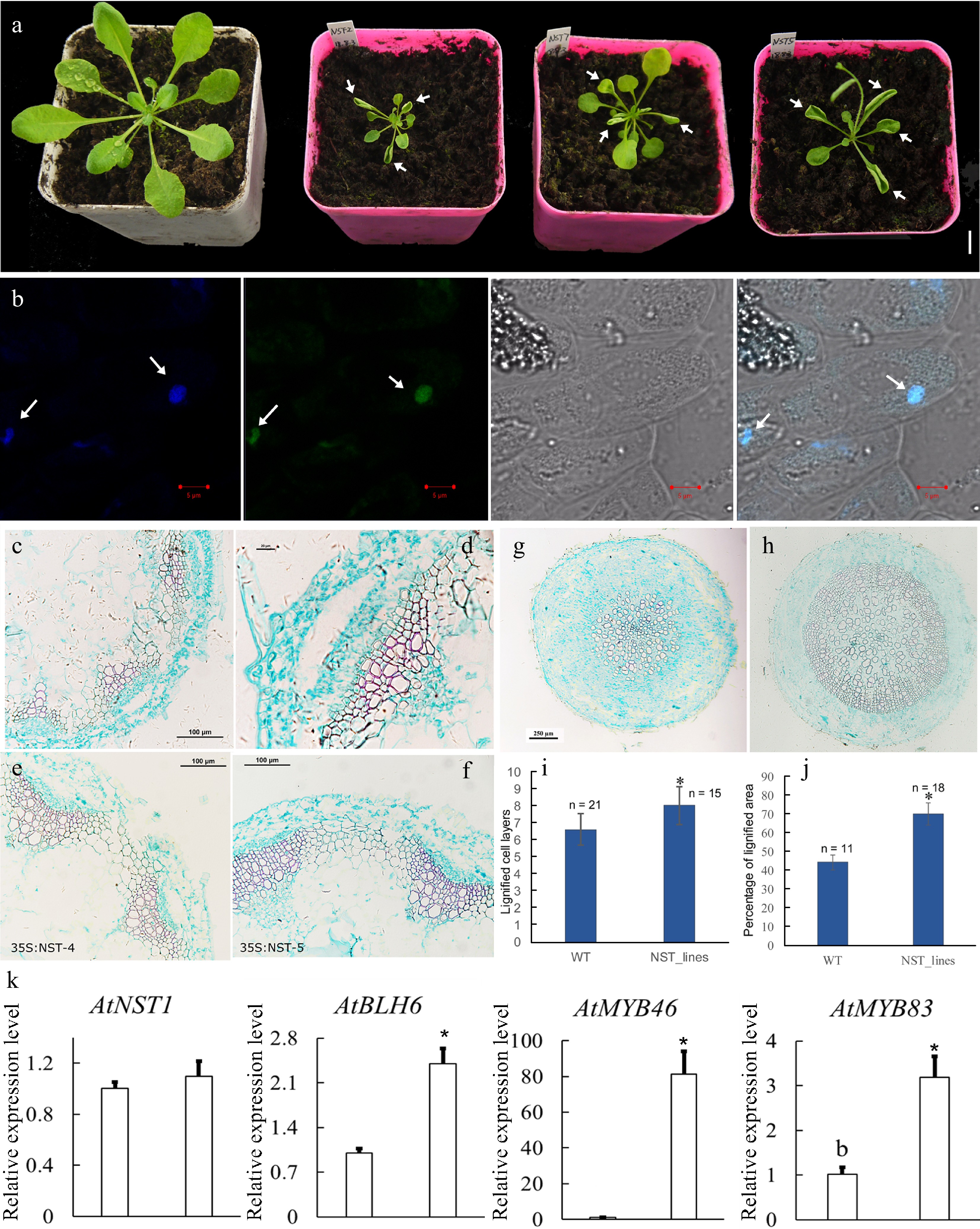
Figure 5.
Overexpression of CcNST1 in Arabidopsis. (a) Overexpression lines displayed various growth defects. Scale bar = 1 cm. The arrows indicate the striking curling leaves. (b) Subcellular localization analysis of the CcNST1:GFP fusion protein by confocal microscopy. Arrows indicate the signals in the nucleus. From left to right, the panels depict the DAPI signal, GFP signal, bright field, and superimposed images; scale bars = 5 µm. (c) & (d) Histological analysis of stem morphology in wild-type stems. (e) & (f) Histological analysis of stem morphology in the transgenic lines; scale bars = 100 µm. Histological analysis of root morphology in the transgenic lines (g) and (h) wild type; scale bars = 100 µm. (i) Statistical analysis of lignified cells in stem and root tissues. n indicated the independent measurements; values are means ± s.d.. (j) Statistical analysis of lignified areas in root tissues. The number of samples used for each statistical analysis is indicated by n. (k) Relative expression levels of Arabidopsis NST1, BLH6, MYB46 and MYB83 genes between the wild type and transgenic lines. Three independent transgenic lines were used for gene expression analysis. The expression of endogenous AtNST1 was not significantly changed. Asterisks indicate significant p-values (< 0.05) for the Student’s t-test.
-
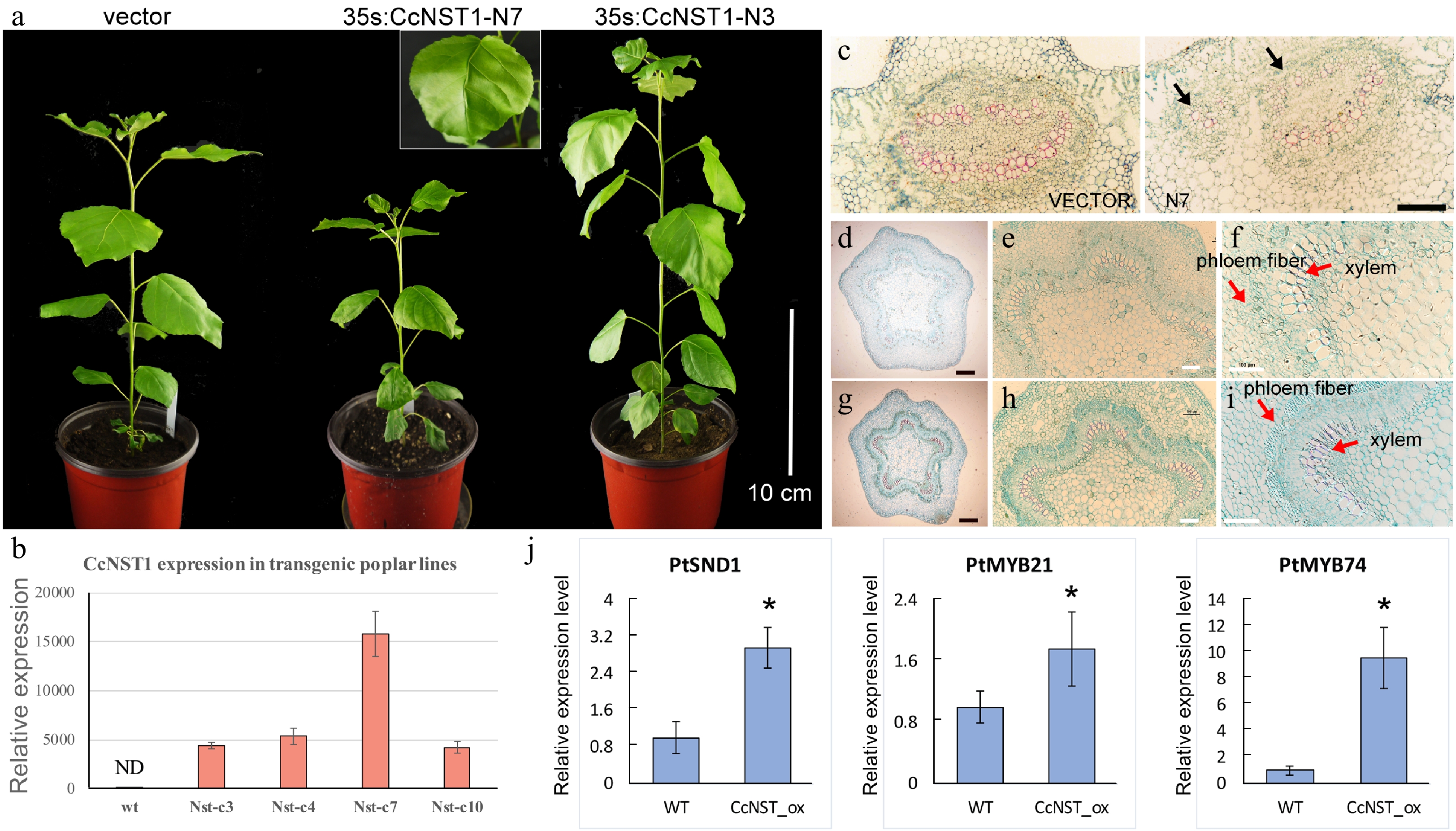
Figure 6.
Ectopic expression of CchNST1 in hybrid poplar (‘Nanlin 895’). (a) Comparison of overall morphology between the control and transgenic poplar plants. On the left is a transgenic plant of the empty vector as a control; middle and right are plants of independent lines of 35s:CcNST1. The inset shows a close-up view of the distorted leaf midvein in the transgenic lines. Scale bar = 10 cm. (b) Expression level of CcNST1 in different independent transgenic lines. ND, not detected. (c) Vertical sections of the midrib in control (left) and 35s:CcNST1 (right) lines. (d)-(f) Cross sections of the fourth internode of control lines. Red arrows indicate the phloem fiber cells and xylem cells. Black scale bars = 250 µm, white scale bars = 100 µm. (g)-(i) Cross sections of the fourth internode of the 35s:CcNST1 lines. The enhanced secondary cell wall in phloem fiber cells and xylem cells are shown. Black scale bars = 250 µm, white bars 100 = µm. (j) Expression of downstream genes PtSND1, PtMYB21, and PtMYB74 in the control and transgenic CcNST1 lines. Three independent transgenic lines were used for gene expression analysis. Asterisks indicate a significant Student’s t-test (p < 0.05). In (b) and (j) values are means ± s.d. of three biological replicates.
Figures
(6)
Tables
(0)