-
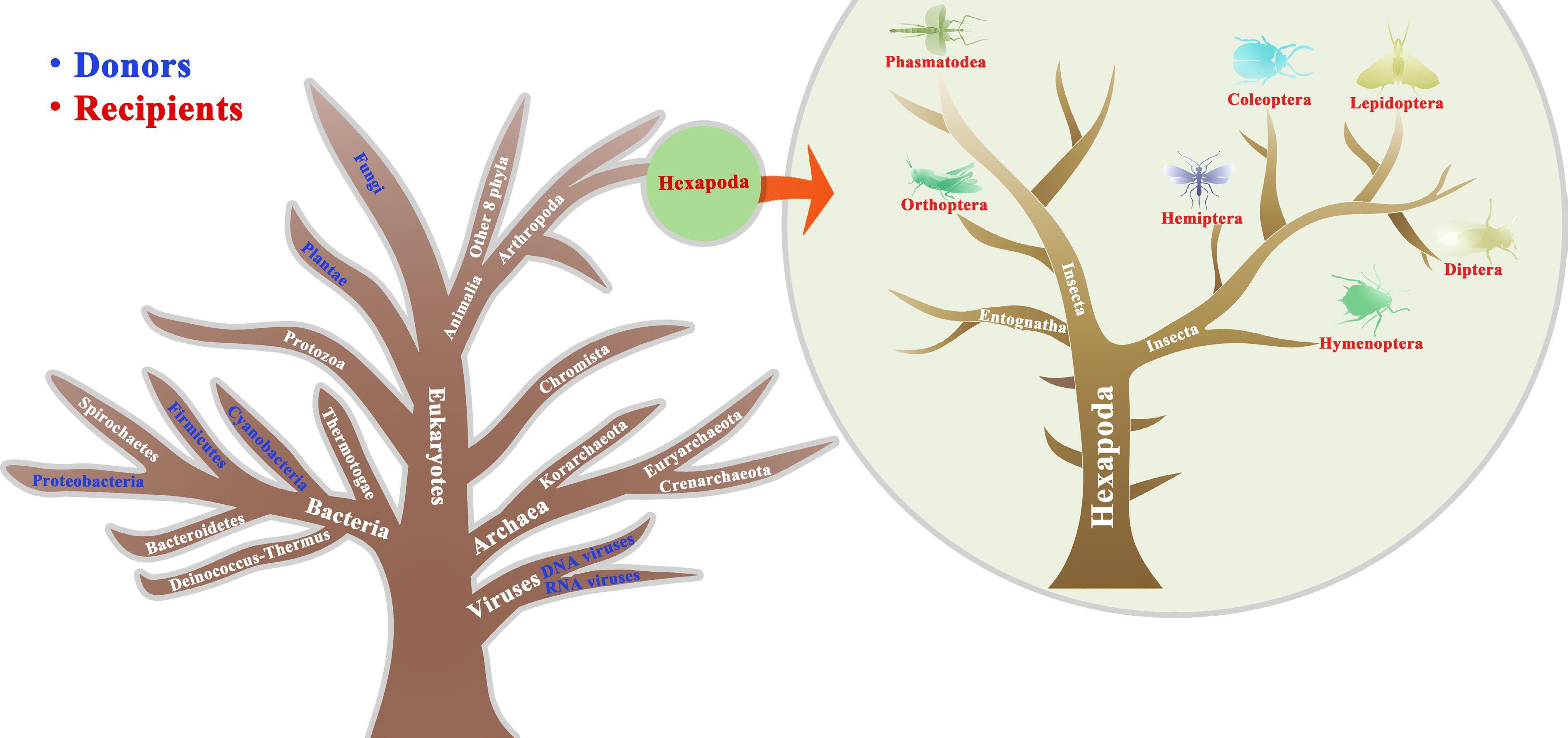
Figure 1.
Illustration of HGTs from other organisms to insects described in the main text. Blue and red represents the donors and recipients, respectively. The tree is designed only for illustration purposes merely explaining the donors and recipient insects that have undergone HGTs and is unable to accurately reflect a true phylogeny, which is modified from previous studies[1,4].
-

Figure 2.
An overview of HGTs in insects and mainly available examples associated with their functions. Part I: 'Donors' represent several groups providing the candidate horizontally transferred genes. 'Recipients' represent the nine insect groups that have received the horizontally transferred genes from donors. Part II: The molecular process of horizontal gene transfer. Part III: The most obvious examples of insect HGT events that have acquired novel functions.
-
Donor Recipient Gene name Functional category Reference Ascomycetes: undefined species Apriona japonica, Callosobruchus maculatus, Chrysomela tremula, Dendroctonus ponderosae, Diabrotica virgifera, Gastrophysa viridula, Leptinotarsa decemlineata, Phaedon cochleariae, Pissodes strobe, Sitophilus oryzae Coleoptera Pectin-degrading polygalacturonase (PG) Carbohydrate metabolism [72] Bacteria and fungi: undefined species Diabrotica virgifera Glycosyl hydrolase (GH45,
GH48, and GH28)[65] γ-proteobacteria: Cellvibrio, Teredinibacter Phaedon cochleariae Glycosyl hydrolase (GH11) [62] Bacilli: Bacillus Hypothenemus hampei Mannanase (HhMAN1) [13] Bacilli: Citrobacter, Enterobacter, Klebsiella Agrilus planipennis β-fructofuranosidase [64] Bacilli: Cedecea, Shigella, Yersinia Sphenophorus levis [63] α-proteobacteria: Wolbachia Callosobruchus chinensis wsp Undefined [25] 57 genes [36] Monochamus alternatus 31 genes [37] Zygomycetes: Blakeslea, Mucor, Phycomyces Asteromyia carbonifera, Chaitophorus populeti, Mayetiola destructor Diptera Phytoene
synthase and desaturaseFormation of body color [71] Cyanobacteria: Calothrix, Tolypothrix Aedes aegypti, Culex quinquefasciatus Ribosome inactivating gene Antimicrobic defense [68,69] Bacteriophage: APSE-2 Drosophila ananassae, D. biarmipes, D. bipectinata, D. primaeva, Myzus cerasi, M. persicae, Scaptomyza flava, S. nr. nigrita, S. pallida Cytolethal distending toxin B (cdtB) Defense against enemies [82] α-proteobacteria: Wolbachia Aedes aegypti, A. mascarensis, Culex pipiens quinquefasciatus AAEL004181, AAEL004188 Undefined [40] Drosophila ananassae nuwt [27] Glossina morsitans morsitans 16S rRNA, wsp, fbpA [26,41] Culex pipiens quinquefasciatus, Drosophila ananassae, D. sechellia, D. simulans Undefined [8,15] α-proteobacteria: Wolbachia; γ-proteobacteria: Buchnera Acyrthosiphon pisum Hemiptera ldcA, rlpA, AmiD, bLys, DnaE, AtpH, RlpA4 Amino acid metabolism, transport, and defense response [44, 45,
48−50]Saccharomycetes: Xanthophyllomyces; Zygomycetes: Phycomyces Carotenoid desaturase and synthase Formation of body color [32] Zygomycetes: Blakeslea, Mucor, Phycomyces Phytoene
synthase and desaturase[71] Plant: undefined species Bemisia tabaci Glucoside malonyltrasferase (BtPMaT) Phenolic glycoside detoxification [11] Ribosome inactivating gene Antimicrobic defense [75] β-proteobacteria: Candidatus; γ-proteobacteria: Carsonella Diaphorina citri ribC Riboflavin biosynthesis [51] γ-proteobacteria: Carsonella Pachypsylla venusta argH, cm, rsmJ, ribC, ydcJ Amino acid metabolism, transport, and defense response [52] α-proteobacteria: Wolbachia; γ-proteobacteria: Carsonella, Serratia, Sodalis Planococcus citri ≥ 22 genes Carbohydrate metabolism [54] Plant: undefined species Trialeurodes vaporariorum Ribosome inactivating gene Antimicrobic defense [75] Zygomycetes: Phycomyces, Rhizopus 34 Aphids Carotenoid desaturase and synthase Formation of body color [70] Chytridiomycetes: Rozella Copidosoma oridanum, Melittobia spp., Muscidifurax raptor, M. raptorellus, M. uniraptor, Nasonia longicornis, N. giraulti, N. Oneida, N. vitripennis, Spalangia cameroni, S. endius, Tachinaephagus zealandicus, Trichogramma pretiosum, Trichomalopsis sarcophagae, Urolepis rupes Hymenoptera Chitinase (GH19) Antifungal defense [73] α-proteobacteria: Wolbachia Nasonia giraulti, N. longicornis, N. vitripennis, Melittobia digitata Ankyrin repeat-containing gene Embryogenesis [8,38,39] Bracoviruses: Cotesia vestalis bracovirus Cotesia vestalis Helitron Undefined [80] Bacilli: Bacillus;
γ-proteobacteria: Cedecea, Rahnella, YersiniaAmyelois transitella, Bicyclus anynana, Bombyx mori, Danaus plexippus, Junoni coenia, Heliconius melpomene, Papilio glaucus, P. machaon, P. xuthus, Plodia interpunctella, Spodoptera frugiperda Lepidoptera β-fructofuranosidase Carbohydrate metabolism and alkaloid detoxification [56,61] Bacilli: Bacillus;
γ-proteobacteria: Serratia;
Baculovirus: Autographa californica nucleopolyhedrovirusBombyx mori Chitinase (GH19) Antifungal defense [55] Bacilli: Bacillus, Enterococcus, Lactococcus, Listeria, Streptococcus
α-proteobacteria: Methylobacterium, Wolbachia;
γ-proteobacteria: Edwardsiella, Photorhabdus, Providencia, Pseudomonas, Salmonella, Serratia;
Ascomycetes: Talaromyces24 genes Metabolic detoxification [58−60] Chlorophyceae: Chlamydomonas Dioxygenase Undefined [57] Bacilli: Bacillus, Enterococcus, Listeria, Streptococcus;
α-proteobacteria: Methylobacterium, Rickettsia;
γ-proteobacteria: Erwinia, Pseudomonas, Serratia, Yersinia;
Ascomycetes: TalaromycesDanaus plexippus 22 genes Carbohydrate metabolism and detoxification [59,60] Bacilli: Alicyclobacillus, Bacillus, Enterococcus, Lactobacillus, Listeria;
α-proteobacteria: Methylobacterium;
γ-proteobacteria: Pseudomonas, Serratia
Ascomycetes: TalaromycesHeliconius melpomene 20 genes [60] Ascovirus, baculovirus, poxvirus: undefined species Helicoverpa, Heliothis, Spodoptera Parasitoid killing factor (PKF) Defense against parasitoids [79] Bacilli: Enterococcus Plutella xylostella Glycosyl hydrolase (GH31) Metabolic detoxification [59] Bacilli: Listeria Alcohol dehydrogenase (LOC105383139) Courtship behavior [10] Bracoviruses: Cotesia kariyai bracovirus Spodoptera littoralis Gasmin Antimicrobic defense [78] α-proteobacteria: Wolbachia Chorthippus parallelus Orthoptera Minor capsid gene Undefined [28] γ-proteobacteria: Frateuria, Pantoea Aretaon asperrimus, Extatosoma tiaratum, Medauroidea extradentata, Peruphasma schultei, Ramulus artemis, Sipyloidea sipylus Phasmatodea Glycosyl hydrolase (pectinase) Carbohydrate metabolism [66,67] Table 1.
A list of horizontally transferred genes in insects.
Figures
(2)
Tables
(1)