-
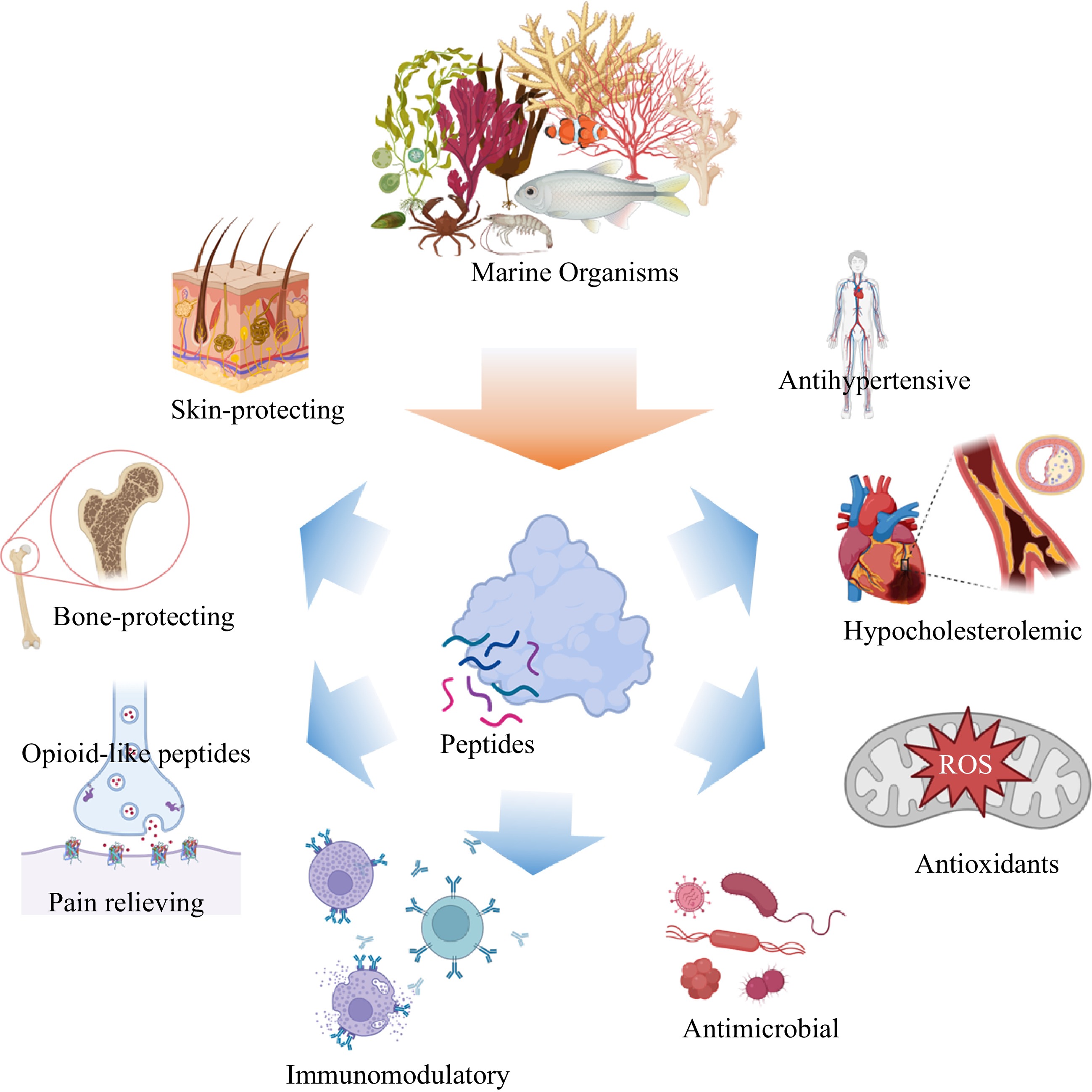
Figure 1.
Spectrum of bioactivities demonstrated by numerous marine derived bioactive peptides.
-
Peptide sequence Bioactivity * (ACE inhibition IC50; μM) Reference Cuttlefish (Sepia officinalis) crude protease (from Bacillus mojavensis A21) hydrolysate Ala-His-Ser-Tyr 11.6 [35] Gly-Asp-Ala-Pro 22.5 Ala-Gly-Ser-Pro 37.2 Asp-Phe-Gly 44.7 Cuttlefish (S. officinalis) muscles hepatopancreas enzyme hydrolysate Ala-Phe-Val-Gly-Tyr-Val-Leu-Pro 25.66 [36] Glu-Lys-Ser-Tyr-Glu-Leu-Pro 14.41 Val-Glu-Leu-Tyr-Pro 5.22 (non-competitive) and lowering of systolic blood pressure in spontaneously hypertensive rats (SHR) Lizard fish (Saurida elongata) muscle protease hydrolysate Gly-Met-Lys-Cys-Ala-Phe 45.7 ± 1.1 [37] Arg-Val-Cys-Leu-Pro 175 [38] Grass carp (Ctenopharyngodon idella) muscle alcalase hydrolysate Val-Ala-Pro 18.6 (competitive) [39] Atlantic salmon (Salmo salar) skin collagen hydrolyzed by Alcalase followed by papain Ala-Pro 0.060 ± 0.001 mg/mL [40] Val-Arg 0.332 ± 0.005 mg/mL Skipjack (Katsuwonus pelamis) roe alcalase hydrolysate Asp-Leu-Asp-Leu-Arg-Lys-Asp-Leu-Tyr-Ala-Asn 67.4 [33] Met-Cys-Tyr-Pro-Ala-Ser-Thr 58.7 Met-Leu-Val-Phe-Ala-Val 3.07 Ser-Pro 0.06 ± 0.01 mg/mL [41] Val-Asp-Arg-Tyr-Phe 0.28 ± 0.03 mg/mL Val-His-Gly-Val-Val 0.90 ± 0.16 mg/mL Tyr-Glu 0.80 ± 0.03 mg/mL Phe-Glu-Met 2.18 ± 0.20 mg/mL Phe-Trp-Arg-Val 0.76 ± 0.10 mg/mL Pacific cod (Gadus macrocephalus) skin gelatin pepsin hydrolysate Gly-Ala-Ser-Ser-Gly-Met-Pro-Gly 6.9 [42] Leu-Ala-Tyr-Ala 14.5 Flounder fish (Paralichthys olivaceus)
muscle pepsinMet-Glu-Val-Phe-Val-Pro 79 (competitive) [43] Val-Ser-Gln-Leu-Arg-Thr 105 (non-competitive) Haruan (Channa striatus) Thermolysin hydrolysate Val-Pro-Ala-Ala-Pro-Pro-Lys 0.45 [44] Asn-Gly-Thr-Trp-Phe-Glu-Pro-Pro 0.63 U. pinnatifida (wakame) peptide from hot water extract Ile-Tyr, Phe-Tyr, Tyr-His, Lys-Tyr Respective IC50 values are 2.7, 3.7, 5.1, 7.7 [45] Jellyfish (Rhopilema esculentum) alcalase hydrolysate Val-Lys-Pro and Val-Lys-Cys-Phe-Arg Respective IC50 values are 1.3 and 34.5 [46] Blue mussel (Mytilus edulis) protein hydrolyzates Ile-Lys, Tyr-Glu-Gly-Asp-Pro, Trp-Phe,
and Ser-Trp-Ile-Ser-SerRespective IC50 values are 0.77, 0.19, 0.40, and 0.32 mg/mL [47] in vitro gastrointestinal digest of Isochrysis zhanjiangensis Glu-Thr-Thr 15.08 (non-competitive) [48] Sonicated Spirulina sp. protease K digest TVLYEH and LQAGGLF 2.88 and 66.83 (competitive) [49] * The IC50 value of ACE inhibitory activity is given in the unit μM if not specified otherwise. Table 1.
ACE inhibitor peptides obtained from marine organisms.
-
Peptide sequence Bioactivity Reference Jumbo squid (Dosidicus gigas) skin gelatin tryptic hydrolysate P1: Phe-Asp-Ser-Gly-Pro-Ala-Gly-Val-Leu
P2: Asn-Gly-Pro-Leu-Gln-Ala-Gly-Gln-Pro-Gly-Glu-ArgLipid peroxidation inhibition is higher than α-tocopherol. Hydroxyl radical scavenging IC50 = 90.90 (P1) and 100.72 μM (P2). Improve the viability of t-Butyl hydroperoxide-induced lung fibroblasts. [60] Alaska pollack skin gelatin serial hydrolyzed
by Alcalase, Pronase E, and collagenase in
a three-step recycling membrane reactor.Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly High antioxidant activity based on TBA assay. Increase viability in tertbutylhydroperoxide (t-BHP)-induced rat liver cells. [67] Giant squid (D. gigas) muscle protein ultrafiltration Asn-Ala-Asp-Phe-Gly-Leu-Asn-Gly-Leu-Glu-Gly-Leu-Ala Improve the viability of cytotoxic embryonic lung fibroblasts by a significant margin at a concentration of 50 g/mL. Suppress radical-induced oxidation of membrane lipids. Radical scavenging activity carbon centered (IC50 396.04 μM), hydroxyl (IC50 497.32 μM), and superoxide radicals (IC50 669.34 μM). [65] Asn-Gly-Leu-Glu-Gly-Leu-Lys Significantly enhance the viability of cytotoxic embryonic lung fibroblasts at 50 μg/mL. Suppress radical-induced oxidation of membrane lipids. Radical scavenging activity carbon centered (IC50 396.04 μM), hydroxyl (IC50 497.32 μM), and superoxide radicals (IC50 669.34 μM). Fermented marine blue mussel
(Mytilus edulis)His-Phe-Gly-Asp-Pro-Phe-His IC50 values for radical scavenging activity, superoxide (21 μM), hydroxyl (34 μM), carbon-centered (52 μM), and DPPH radicals (96 μM). Fe2+ chelating effect. Increase t-BHP-induced WI-38 cell viability. [66] Hard-shelled mussel (Mytilus coruscus)
papain hydrolysateSer-Leu-Pro-Ile-Gly-Leu-Met-Ile-Ala-Met In AAPH administered mice, inhibited malondialdehyde levels per thiobarbituric acid reactive substances assay, and increased superoxide dismutase and glutathione-s-transferase activities. [68] Krill (Euphausia superba) protein pepsin hydrolysate Ala-Met-Val-Asp-Ala-I le-Ala-Arg IC50 value of 0.87 for DPPH radical scavenging assay and ORAC value of 1.56 mM Trolox equivalents/mM peptide. Reduce H2O2-induced oxidative stress in Chang liver cells by increasing superoxide dismutase, glutathione peroxidase, and catalase activities. [69] Table 2.
Antioxidant peptides obtained from marine organisms.
-
Peptide sequence Bioactivity Reference American lobster (Homarus americanus) hemocytes Gln-Tyr-Gly-Asn-Leu-Leu-Ser-Leu-Leu-Asn-Gly-Tyr-Arg Effective against some gram-negative bacteria. Indicate protozoastatic activity against ciliate parasites. [72] Rainbow trout (Oncorhynchuss mykiss) omBD-1 ASFPWACPSLNGVCRKVCLPTELFFGPLGCGKGFLCCVSHFL β-defensin-like peptide sequence. Provide resistance against viral hemorrhagic septicemia rhabdovirus (VHSV) infection in fish cell line Epithelioma papulosum cyprinid. [73] Channel catfish(Ictalurus punctatus) HbβP-1 AANFGPSVFTPEVHETWQKFLNVVVAALGKQYH Homologous to β-chain of hemoglobin. Antiparasitic activity against Ichthyophthirius multifiliis and Aeromonas hydrophila infection in channel catfish. [74] Hemocyte extracts of Spider
crab (Hyas Araneus)MERRTLLVVLLVCSCVVAAAAEASPSRWPSPGRPRPFPGRPKPI Arasin 1, effectively inhibits the growth of Corynebacterium glutamicum, Listonella anguillarum, and E. coli. [71] Table 3.
Antimicrobial peptides derived from marine organisms.
-
Protein source and hydrolysis method Bioactivity Reference Chlorella-derived peptides hot water extract Reduce UVB-induced production of MMP-1 MCP-1, expression of CYR61, c-fos, c-jun, and increase procollagen and TbRII expression, in skin fibroblasts via attenuating AP-1 expression and production of CYR61 and MCP-1. [78] Tuna roe trypsin and alkaline 1:2 hydrolysate. Peptides Ile-Cys-Arg-Asp and Leu-Cys-Gly-Glu-Cys Inhibit UVB-induced apoptosis and alter Keap1/Nrf2-ARE pathway in HaCaT keratinocytes and mice. Inhibit the release of the proinflammatory cytokines. [79] Simulated gastrointestinal digest of grass carp fish scale gelatin hydrolysate (Phe-Thr-Gly-Met-Leu) Tyrosinase inhibition with an IC50 value of 1.89 mmol/L and inhibition of melanin production in the zebrafish model. [80] Skin collagen of Atlantic Codfish (Gadus morhua). Water retaining ability. No toxicity or inflammatory response in HaCaT cells. [77] Tilapia collagen nanofibers Significantly increase HaCaT cell proliferation and accelerates epidermal differentiation through upregulating involucrin, filaggrin, and type I transglutaminase expression. Accelerate wound healing in Sprague–Dawley rats by promoting re-epithelialization. [20] Type I collagen from Magalaspis cordyla and Otolithes ruber bones Accelerate wound healing in Wistar rats [21] Tilapia and grey mullet collagen Enhances cell adhesion, antibacterial activity (Streptococcus mutans, Staphylococcus aureus, E. coli and Bacillus subtills), faster wound healing in Sprague Dawley male rats. [22] Mrigal carp (Cirrhinus cirrhosus) scale collagen Efficient cell growth and proliferation on the collagen sponge. Fibroblast and keratinocyte co-culture indicated the development of a stratified epidermal layer. Faster wound healing, dermal reconstitution, and re-epithelialization in Wistar rats. [81] Low-molecular-weight collagen peptides from Starfish (Asterias pectinifera) Promotes wound healing, bone regeneration, and skin protection. Reduce MMP-1 expression caused by ultraviolet radiation-induced photoaging in CCD-986sk human dermal fibroblasts. [23] Table 4.
The cosmetic potential of marine peptides and protein hydrolysates.
Figures
(1)
Tables
(4)