-
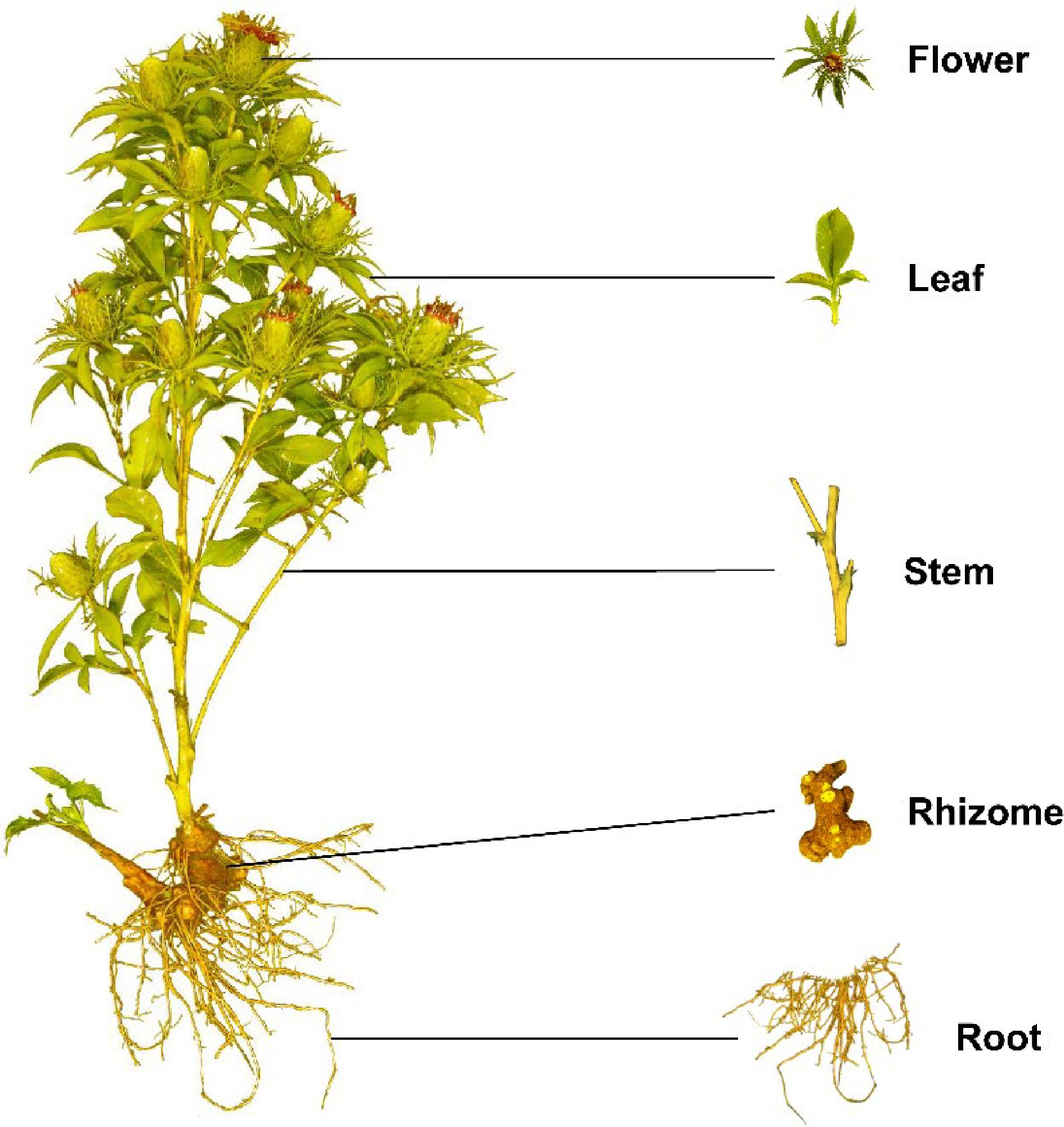
Figure 1.
Plant morphology of A. macrocephala.
-

Figure 2.
Current progress of A. macrocephala.
-
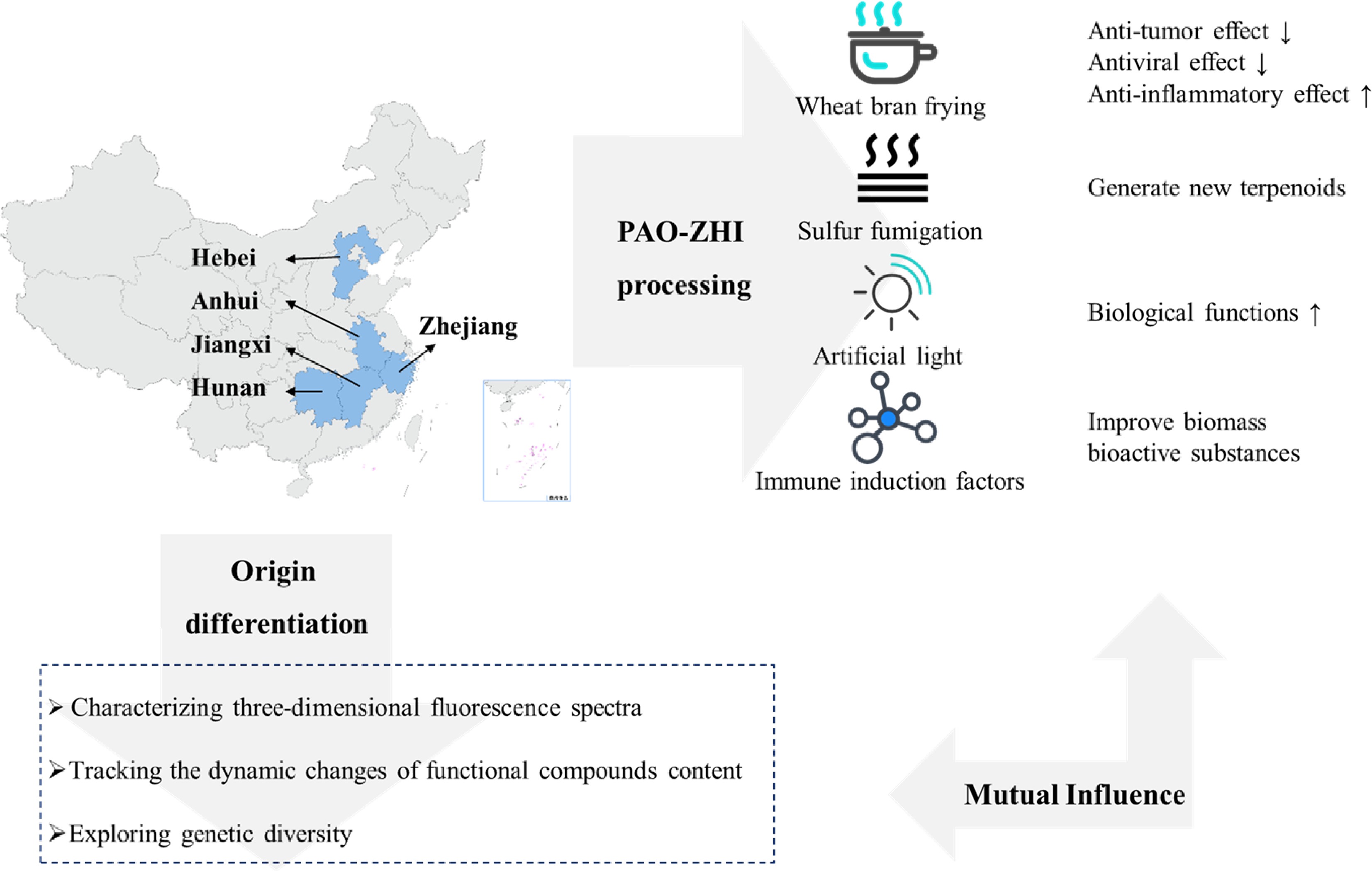
Figure 3.
Origin, distribution and processing of A. macrocephala.
-

Figure 4.
Structure of small molecule compounds with bioactivities from AMR. Atractylenolide I (1); Atractylenolide II (2); Atractylenolide III (3); 3β-acetoxyl atractylenolide I (4); 4R,5R,8S,9S-diepoxylatractylenolide II (5); 8S,9S-epoxyla-tractylenolide II (6); Atractylmacrols A (7); Atractylmacrols B (8); Atractylmacrols C (9); Atractylmacrols D (10); Atractylmacrols E (11); 2-[(2E)-3,7-dimethyl-2,6-octadienyl]-6-methyl-2,5-cyclohexadiene-1,4-dione (12); 8-epiasterolid (13); (3S,4E,6E,12E)-1-acetoxy-tetradeca-4,6,12-triene-8,10-diyne-3,14-diol (14); (4E,6E,12E)-tetradeca-4,6,12-triene-8,10-diyne-13,14-triol (15); 1-acetoxy-tetradeca-6E,12E-diene-8, 10-diyne-3-ol (16); 1,3-diacetoxy-tetradeca-6E, 12E-diene-8,10-diyne (17); Biatractylenolide II (18); Biepiasterolid (19); Biatractylolide (20).
-
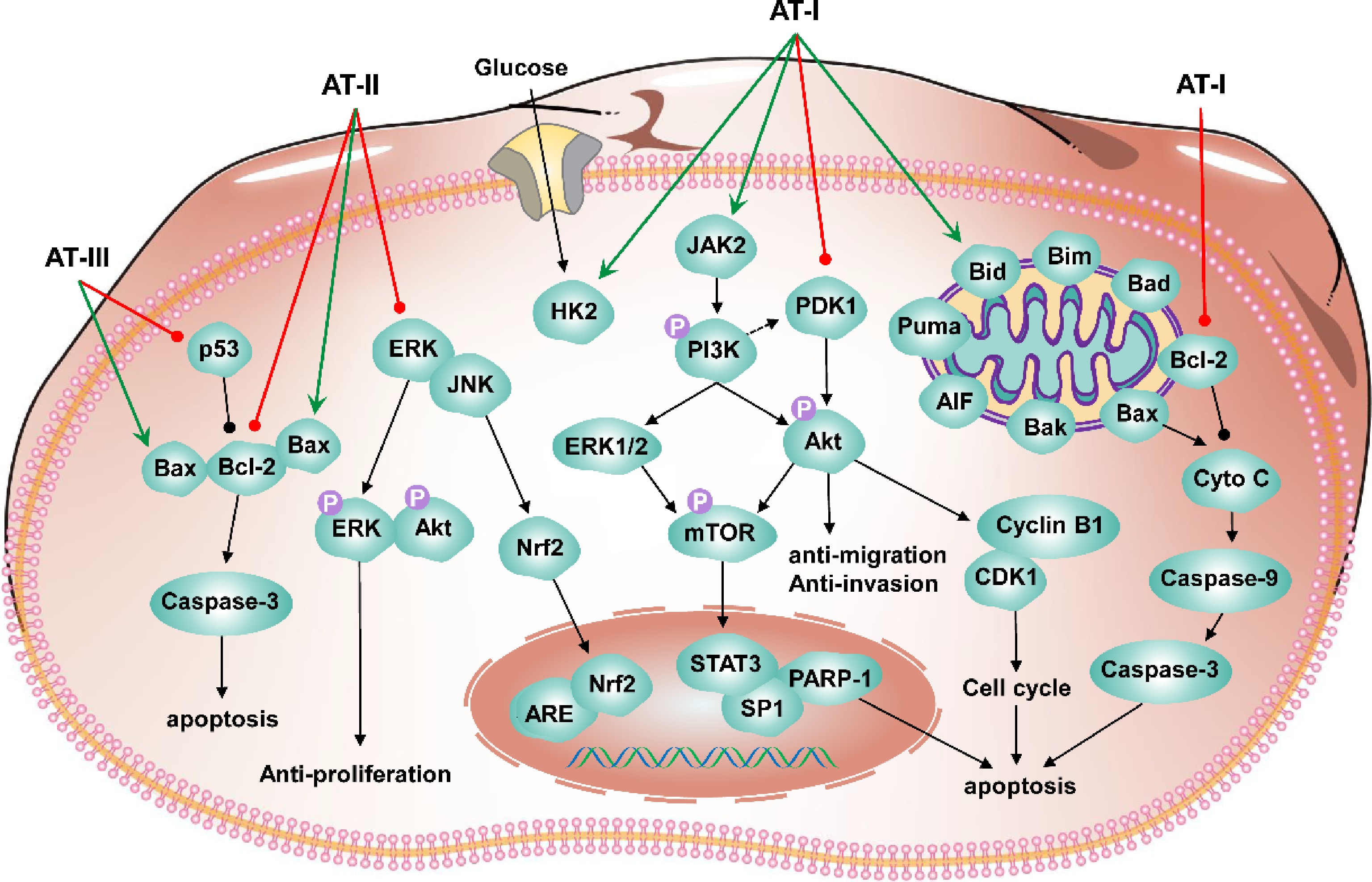
Figure 5.
Schematic diagram for the anti-tumor mechanism of atractylenolides.
-

Figure 6.
Biosynthetic pathways for bioactive compounds of A. macrocephala.
-
Pharmacological activities Detailed function Polysaccharides information Model Dose Test index Results Ref. Immunomodulatory effects Restore immune
function/ Chicken models
(HS-induced)200 mg/kg Oxidative index;
Activities of mitochondrial complexes and ATPases;
Ultrastructure in chicken spleens;
Expression levels of cytokines, Mitochondrial dynamics- and apoptosis-related genesAlleviated
the expression of
IL-1 ↑,TNF-α ↑, IL-2 ↓, IFN- γ ↓; mitochondrial dynamics- and anti-apoptosis-related genes ↓; pro-apoptosis-related genes ↑;
the activities of mitochondrial complexes and ATPases ↓ caused by HS[35] Regulate the immune function / Chicken models
(HS-induced)200 mg/kg iNOS–NO activities;
ER stress-related genes;
Apoptosis-related genes;
Apoptosis levelsAlleviated NO content ↑; activity of iNOS ↑ in the chicken spleen; GRP78, GRP94, ATF4, ATF6, IRE ↑; caspase3 ↑; Bcl-2 ↓ caused by HS [36] Relieve immunosuppression Commercial AMR powder (purity 70%) Geese models
(CTX-induced)400 mg/kg Spleen development;
Percentages of leukocytes in peripheral bloodAlleviated the spleen damage;
T and B cell proliferation ↓; imbalance of leukocytes; disturbances of humoral; cellular immunity caused by CTX[37] Active the lymphocytes Commercial AMR powder (purity 95%) Geese models
(CTX-induced)400 mg/kg Thymus morphology;
The level of serum GMC-SF, IL-1b, IL-3, IL-5;
mRNA expression of CD25, novel_mir2, CTLA4 and CD28 signal pathwayMaintain normal cell morphology of thymus;
Alleviated GMC-SF ↓, IL-1b ↓, IL-5↓, IL-6↓, TGF-b↓; IL-4 ↑, IL-10 ↑; novel_mir2 ↓, CD25↓, CD28↓ in thymus and lymphocytes caused by CTX[38] Alleviate immunosuppression Commercial AMR powder (purity 70%) Geese models
(CTX-induced)400 mg/kg Thymus development;
T cell proliferation rate;
The level of CD28, CD96, MHC-II;
IL-2 levels in serum;
differentially expressed miRNAsAlleviated thymus damage;
T lymphocyte proliferation rate ↓; T cell activation ↓; IL-2 levels ↓ caused by CTX;
Promoted novel_mir2 ↑; CTLA4 ↓; TCR-NFAT signaling pathway[39] Alleviates T cell activation decline Commercial AMR powder (purity 95%) BALB/c female mice (CTX-induced) 200 mg/kg Spleen index;
Morphology, death, cytokine concentration of splenocytes;
Th1/Th2 ratio, activating factors of lymphocytes;
T cell activating factors;
mRNA expression level in CD28 signal pathwayImproved the spleen index;
Alleviated abnormal splenocytes morphology and death; Balance Th1/Th2 ratio; IL-2 ↑, IL-6 ↑, TNF-α ↑, IFN-γ ↑; mRNA levels of CD28, PLCγ-1, IP3R, NFAT, AP-1 ↑[40] Immunoregulation and Immunopotentiation Commercial AMR powder (purity 80%) BMDCs (LPS-induced);
Female BALB/c mice (ovalbumin as a model antigen)/ Surface molecule expression of BMDCs;
Cytokines secreted by dendritic cell supernatants;
OVA-specific antibodies in serum;
Cytokines in serum;
Lymphocyte immunophenotypeExpression of CD80 and CD86 ↑; IL-1β ↑, IL-12 ↑, TNF-α↑ and IFN-γ ↑; OVA-specific antibodies in serum ↑; Secretion of cytokines ↑; Proliferation rate of spleen lymphocytes ↑; Activation of CD3+CD4+ and CD3+CD8+ lymphocytes [46] Increase immune-response capacity of the spleen in mice Commercial AMR powder (purity 70%) BALB/c female mice 100, 200, 400 mg/kg Spleen index;
Concentrations of cytokines;
mRNA and protein expression levels in TLR4 signalingIn the medium-PAMK group:
IL-2, IL-4, IFN-c, TNF-a ↑; mRNA and protein expression of TLR4, MyD88, TRAF6, TRAF3, NF-κB in the spleen ↑[41] Immunological activity Commercial AMR powder (purity 80%) Murine splenic lymphocytes (LPS or PHA-induced) 13, 26, 52, 104, 208 μg/mL T lymphocyte surface markers Lymphocyte proliferation ↑;
Ratio of CD4+/CD8+ T cells ↑[47] Immunomodulatory activity Total carbohydrates content 95.66 % Mouse splenocytes
(Con A or LPS-induced)25, 50, 100 μg/mL Splenocyte proliferation;
NK cytotoxicity;
Productions of NO and cytokines;
Transcription factor activity;
Signal pathways and receptorPromoted splenocyte proliferation; Cells enter S and G2/M phases; Ratios of T/B cells ↑; NK cytotoxicity ↑; Transcriptional activities of NFAT ↑; NF-κB, AP-1 ↑; NO, IgG, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-6, IL-10, IL-12p40, IL-12p70, IL-13, IFN-γ, TNF-α, G-CSF, GM-CSF, KC, MIP-1α, MIP-1β, RANTES, Eotaxin ↑ [42] Promote the proliferation of thymic epithelial cells Contents of fucrhaara, galactose, glucose, fructose,
and xylitol: 0.98%, 0.40%, 88.67%, 4.47%, and 5.47%MTEC1 cells 50 μg/mL Cell viability and proliferation;
lncRNAs, miRNAs, and mRNAs expression profiles in MTEC1 cellsThe differential genes were 225 lncRNAs, 29 miRNAs, and 800 mRNAs; Genes enriched in cell cycle, cell division, NF-κB signaling, apoptotic process, and MAPK signaling pathway [44] Immunomodulatory activity MW: 4.354 × 103 Da;
Composed of mannose, galacturonic acid, glucose, galactose and arabinose;
The main linkages are →3-β-glcp-(1→, →3,6-β-glcp-(1→, →6-β-glcp-(1→, T-β-glcp-(1→,
→4-α-galpA-(1→, →4-α-galpA-6-OMe-(1→, →5-α-araf-(1→, →4,6-β-manp-(1→ and →4-β-galp-(1→CD4+ T cell 50, 100, 200 μg/mL Molecular weight;
Monosaccharide composition;
Secondary structure;
Surface topography;
Effect on Treg cellsTreg cells percentage ↑; mRNA expressions of Foxp3, IL-10 and IL-2 ↑; STAT5 phosphorylation levels ↑; IL-2/STAT5 pathway [28] Immunostimulatory activity MW of AMAP-1, AMAP-2, and AMAP-3 were 13.8×104 Da, 16.2×104 Da and 8.5×104 Da;
HG region consists of α-(1→4)-linked GalpA residuesRAW264.7 cells (LPS-induced) 80, 40, 200 μg/mL Molecular weight;
Total carbohydrate;
Uronic acid contents;
Secondary structure;
Monosaccharide composition;
Immunostimulatory activityRG-I-rich AMAP-1 and AMAP-2 improved the release of NO [29] Immunomodulatory effect MW: 1.867×103 Da;
Contents of glucose, mannose, rhamnose,
arabinose and galactose: 60.67%, 14.99%, 10.61%, 8.83% and 4.90%SMLN lymphocytes 25
μg/mlMolecular weight;
Monosaccharide composition;
Ultrastructure;
Intracellular Ca2+concentration;
Target genes;
Cell cycle distribution[Ca2+]i ↑; More cells in S and G2/M phases; IFN-γ ↑, IL-17A ↑; mRNA expressions of IL-4 ↓ [30] Macrophage activation Total carbohydrates content 95.66 % RAW264.7 macrophages (LPS-induced) 25, 50, 100 μg/mL Pinocytic activity;
Phagocytic uptake;
Phenotypic characterization;
Cytokine production;
Bioinformatics analysis;
Transcription factor inhibitionIL-6, IL-10 and TNF-α ↑; CCL2 and CCL5 ↑; Pinocytic and phagocytic activity ↑; CD40, CD80, CD86, MHC-I, MHC-II ↑; NF-κB and Jak-STAT pathway [43] Immunomodulatory effect Total carbohydrates content 95.66 % SMLN lymphocytes 25, 50, 100 μg/mL Cytokine production;
CD4+ and CD8+ lymphocytes;
Target genes;
Bioinformatics analysis;
T and B lymphocyte proliferation;
Receptor binding and blockingIFN-γ, IL-1α, IL-21, IFN-α, CCL4, CXCL9, CXCL10 ↑; CD4+ and CD8+subpopulations proportions ↑;
c-JUN, NFAT4, STAT1, STAT3 ↑;
67 differentially expressed miRNAs (55 ↑ and
12 ↓), associated with immune system pathways; Affect T and B lymphocytes[45] Improving gastrointestinal function Relieve enteritis and improve intestinal
flora disorderCommercial AMR powder (purity 70%);
Contents of fucrhaara, galactose, glucose, xylitol, and fructose: 0.98%, 0.40%, 88.67%, 4.47%, and 5.47%Goslings (LPS-induced) 400 mg/kg Serum CRP, IL-1β, IL-6, and TNF-α levels;
Positive rate of IgA;
TLR4, occludin, ZO-1, cytokines, and immunoglobulin mRNA expression;
Intestinal flora of gosling excrementRelieved IL-1β, IL-6, TNF-α levels in serum ↑; the number of IgA-secreting cells ↑; TLR4 ↑; tight junction occludin and ZO-1 ↓; IL-1β mRNA expression in the small intestine ↑; Romboutsia ↓ caused by LPS [48] Ameliorate ulcerative colitis MW: 2.391 × 104 Da;
Composed of mannose, glucuronic acid, glucose and arabinose in a molar ratio of 12.05:6.02:72.29:9.64Male C57BL/6J mice (DDS-induced) 10, 20, 40 mg/kg bw Histopathological evaluation;
Inflammatory mediator;
Composition of gut microbiota;
Feces and plasma for global metabolites profilingButyricicoccus, Lactobacillus ↑;
Actinobacteria, Akkermansia, Anaeroplasma, Bifidobacterium, Erysipelatoclostridium, Faecalibaculum, Parasutterella,
Parvibacter, Tenericutes, Verrucomicrobia ↓;
Changed 23 metabolites in fecal content; 21 metabolites in plasma content[49] Attenuate ulcerative colitis / Male SD rats (TNBS-induced);
Co-culture BMSCs and IEC-6 cells540 mg/kg
(for rats);
400 μg/mL (for cell)Histopathological analysis;
Cell migration;
Levels of cytokinesPotentiated BMSCs’ effect on preventing colitis and homing the injured tissue, regulated cytokines;
BMSCs and AMP promoted the migration of IEC[52] Against intestinal mucosal injury MW: 3.714 × 103 Da;
Composed of glucose, arabinose, galactose, galacturonic acid, rhamnose
and mannose with molar ratios of 59.09:23.22:9.32:4.70:2.07:1.59Male C57BL/6 mice (DDS-induced) 100 mg/kg Intestinal morphology;
IL-6, TNF-α and IL-1β in serum;
mRNA expression;
Intestinal microbiotaAlleviated body weight ↓; colon length ↓; colonic damage caused by DSS;
Over-expression of TNF-α, IL-1β, IL-6 ↓; Infiltration of neutrophils in colon ↓; Mucin 2 ↑;
Tight junction protein Claudin-1 ↑;
Harmful bacteria content ↓;
Beneficial bacteria content ↑[50] Against intestinal injury Total carbohydrates 95.66 % IECs (DDS-induced) 5, 25, 50 μg/mL Cell proliferation and apoptosis;
Expression levels of intercellular TJ proteins;
lncRNA screeningProliferation and survival of IECs ↑;
Novel lncRNA ITSN1-OT1 ↑;
Blocked the nuclear import of phosphorylated STAT2[51] Anti-tumor activity Induce apoptosis in transplanted H22 cells in mice MW: 4.1× 103 Da;
Neutral heteropolysaccharide composed of galactose, arabinose, and glucose with α-configuration (molar ratio, 1:1.5:5)Female Kunming mice 100 and 200 mg/kg (for rats) Secondary structure;
Molecular weight;
Molecular weight;
Thymus index and Spleen index;
Lymphocyte Subpopulation in peripheral blood;
Cell cycle distributionIn tumor-bearing mice CD3+, CD4+, CD8+ ↓;
B cells ↑[31] Regulate the innate immunity of colorectal cancer cells Commercial AMR powder (purity 70%) C57BL/6J mice (MC38 cells xenograft model) 500 mg/kg Expression of pro-inflammatory cytokines and secretion IL-6, IFN-λ, TNF-α, NO ↑ through MyD88/TLR4-dependent signaling pathway;
Survival duration of mice with tumors ↑;
Prevent tumorigenesis in mice[54] Induce apoptosis of Eca-109 cells MW: 2.1× 103 Da;
Neutral hetero polysaccharide composed
of arabinose and glucose (molar ratio, 1:4.57) with pyranose rings and α-type and β-type glycosidic linkagesEca-109 cells 0.25, 0.5, 1, 1.5, 2.00 mg/mL Cell morphology;
Cell cycle arrest;
Induction of apoptosisAccelerate the apoptosis of Eca109 cells [53] '/' denotes no useful information found in the study. Table 1.
Components and bioactivity of polysaccharides from Atractylodes macrocephala Koidz. Rhizome.
-
Types Substances Model Index Dose Signal pathway Results Ref. Human colorectal cancer AT-III HCT-116 cell;
HCT-116 tumor xenografts bearing in nude miceCell viability;
Cell apoptotic;
mRNAs and protein
expressions of Bax, Bcl-2, caspase-9 and caspase-325, 50, 100, 200 μM (for cell);
50, 100,
200 mg/kg (for rats)Bax/Bcl-2 signaling pathway Promoting the expression of proapoptotic related gene/proteins; Inhibiting the expression of antiapoptotic related gene/protein; Bax↑; Caspase-3↓; p53↓; Bcl-2↓ [55] Human gastric carcinoma AT-II HGC-27 and AGS cell Cell viability;
Morphological changes;
Flow cytometry;
Wound healing;
Cell proliferation, apoptosis, and motility50, 100, 200, 400 μM Akt/ERK signaling pathway Cell proliferation, motility↓; Cell apoptosis↑; Bax↑;
Bcl-2↓; p-Akt↓; p-ERK↓[56] Mammary
tumorigenesisAT-II MCF 10A cell;
Female SD rats (NMU-induced)Nrf2 expression and nuclear accumulation;
Cytoprotective effects;
Tumor progression;
mRNA and protein levels of Nrf2;
Downstream detoxifying enzymes20, 50, 100 μM (for cell);
100 and 200 mg/kg (for rats)JNK/ERK-Nrf2-ARE signaling pathway;
Nrf2-ARE signaling pathwayNrf2 expressing↑; Nuclear translocation↑; Downstream detoxifying enzymes↓; 17β-Estradiol↓; Induced malignant transformation [57] Human colon adenocarcinoma AT-I HT-29 cell Cell viability;
TUNEL and Annexin V-FITC/PI double stain;
Detection of initiator and
executioner caspases level10, 20, 40, 80, 100 μM Mitochondria-dependent pathway Pro-survival Bcl-2↓; Bax↑; Bak↑; Bad↑; Bim↑; Bid↑; Puma↑ [58] Sensitize triple-negative
TNBC cells to paclitaxelAT-I MDA-MB-231 cell;
HS578T cell;
Balb/c mice (MDA-MB-231 cells-implanted)Cell viability
Transwell migration
CTGF expression25, 50, 100 μM (for cell);
50 mg/kg (for rats)/ Expression and secretion of CTGF↓; CAF markers↓; Blocking CTGF expression and fibroblast activation [59] Human ovarian cancer AT-I A2780 cell Cell cycle;
Cell apoptosis;
Cyclin B1 and CDK1 level12.5, 25, 50, 100 and 200 μM PI3K/Akt/mTOR
signaling pathwayCyclin B1, CDK1↓; Bax↑;
Caspase-9↓; Cleaved caspase-3↓; Cytochrome c↑; AIF↑; Bcl-2↓; Phosphorylation level of PI3K, Akt, mTOR↓[60] Impaired metastatic properties transfer of CSCs AT-I LoVo-CSCs; HT29-CSCs Cell migration
and invasion;
miR-200c expression;
Cell apoptosis200 μM PI3K/Akt/mTOR signaling pathway Suppressing miR-200c activity; Disrupting EV uptake by non-CSCs [61] Colorectal cancer AT-I HCT116 cell;
SW480 cell;
male BALB/c nude mice (HCT116-implanted)Cell viability;
Cell apoptosis;
Glucose uptake;
Lactate Production;
STAT3 expression;
Immunohistological analysis25, 50, 100, 150, 200 μM (for cell);
50 mg/kg (for rats)JAK2/STAT3 signaling Caspase-3↑; PARP-1↓;
Bax↑; Bcl-2↓; Rate-limiting glycolytic
enzyme HK2↓; STAT3 phosphorylation↓[62] Human lung cancer AT-I NSCLC cells (A549 and H1299);
female nude mice (A549-Luc cells- implanted)Cell viability;
Cell cycle;
Phosphorylation and protein expression of
ERK1/2, Stat3,
PDK1, transcription factor SP1;
mRNA levels of PDK1 gene12.5, 25, 50, 100, 150 μM (for cell);
25 and 75 mg/kg (for rats)/ ERK1/2↑; Stat3↓; SP1↓;
PDK1↓[63] '/' denotes no useful information found in the study. Table 2.
Anti-tumor activity of atractylenolides.
-
Activities Substances Model Index Dose Signal pathway Results Ref. Establish a PD model AT-II; AT-I;
Biepiasterolid;
Isoatractylenolide I;
AT-III; 3β-acetoxyl atractylenolide I;
(4E,6E,12E)- tetradeca-4,6,12-triene-8,10-diyne-13,14-triol;
(3S,4E,6E,12E)-1-acetoxy-tetradeca-4,6,12-triene-8,10-diyne-3,14-diolSH-SY5Y cell (MPP+-induced) Cell viability 10, 1, 0.1 μM / All compounds have inhibitory activity on MPP+-
induced SH-SY5Y cell[64] / 4R,5R,8S,9S-diepoxylatractylenolide II;
8S,9S-epoxyla-tractylenolide IIBV-2 microglia cells (LPS-induced) Cell viability;
NO synthase
inhibitor;
IL-6 levels6.25, 12.5, 25, 50, 100 μM NF-κB signaling
pathwayNO inhibition with IC50 values
of 15.8, and 17.8 μМ, respectively;
IL-6 ↓[65] Protecting Alzheimer’s disease Biatractylolide PC12 cell (Aβ25-35-induced);
Healthy male Wistar rats (Aβ25-35-induced)Cell viability;
Morris water maze model;
TNF-α, IL-6, and IL-1β20, 40, 80 μM (for cells);
0.1, 0.3, 0.9 mg/kg (for rats)NF-κB signaling
pathwayReduce apoptosis; Prevent cognitive decline; Reduce the activation of NF-κB signal pathway [66] / Biatractylolide PC12 and SH-SY5Y cell (glutamate-induced) Cell viability;
Cell apoptosis;
LDA;
Protein expression10, 15, 20 μM PI3K-Akt-GSK3β-Dependent
PathwaysGSK3β protein expression ↓;
p-Akt protein expression ↑[67] Parkinson's Disease AT-I BV-2 cells (LPS-induced);
Male C57BL6/J mice (MPTP-intoxicated)mRNA and protein levels;
Immunocytochemistry; Immunohistochemistry;25, 50, 100 μM (for cells);
3, 10, 30 mg/kg/mL (for rats)/ NF-κB ↓; HO-1 ↑; MnSOD ↑; TH-immunoreactive neurons ↑; Microglial activation ↓ [68] Anti depressant like effect AT-I Male ICR mice (CUMS induced depressive like behaviors) Hippocampal neurotransmitter levels;
Hippocampal pro inflammatory cytokine levels;
NLRP3 inflammasome in the hippocampi5, 10, 20 mg/kg / Serotonin ↓;
Norepinephrine ↓; NLRP3 inflammasome ↓; (IL)-1β ↓[69] Alzheimer's disease Biatractylenolide II / AChE inhibitory activities;
Molecular docking/ / Biatractylenolide II can interact with PAS and CAS of AChE [70] Cerebral ischemic injury and
neuroinflammationAT-III Male C57BL/6J mice (MCAO- induced);
Primary microglia (OGDR
stimulation)Brain infarct size;
Cerebral blood flow;
Brain edema;
Neurological deficits;
Protein expressions of proinflammatory;
Anti-inflammatory
cytokines0.01, 0.1, 1, 10, 100 μM (for cells);
0.1–10 mg/kg
(for rats)JAK2/STAT3/Drp1-dependent mitochondrial fission Brain infarct size ↓;
Restored CBF;
ameliorated brain edema; Improved neurological deficits;
IL-1β ↓; TNF-α ↓; IL-6 ↓;
Drp1 phosphorylation ↓[71] Reduces depressive- and anxiogenic-like behaviors AT-III Male SD rats (LPS-induced and CUMS rat model) Forced swimming test;
Open field test;
Sucrose preference test;
Novelty-suppressed feeding test;
Proinflammatory cytokines levels3, 10, 30 mg/kg / 30 mg/kg AT-III produced an anxiolytic-like effect; Prevented depressive- and anxiety-like behaviors; Proinflammatory cytokines levels ↓ [72] Alleviates
injury in rat
hippocampal neuronsAT-III Male SD rats (isoflurane-induced) Apoptosis and autophagy in the hippocampal neurons;
Inflammatory factors;
Levels of p-PI3K,
p-Akt, p-mTOR1.2, 2.4, 4.8 mg/kg PI3K/Akt/mTOR signaling pathway TNF-α ↓; IL-1β ↓; IL-6 ↓; p-PI3K ↑; p-Akt ↑; p-mTOR ↑ [73] ''/' denotes no useful information found in the study. Table 3.
Neuroprotective effects of esters and sesquiterpenoids.
-
Activities Substance Model Index Dose Signal pathway Result Ref. Against LPS-induced NO production Atractylmacrols A-E RAW264.7 macrophages (LPS-induced) Isolation;
Structural identification;
Inhibition activity of
NO production25 μM / Have effects on LPS-induced NO production [74] Anti-inflammatory 2-[(2E)-3,7-dimethyl-2,6-octadienyl]-6-methyl-2,5-cyclohexadiene-1,
4-dione;
1-acetoxy-tetradeca-6E,12E-diene-8, 10-diyne-3-ol;
1,3-diacetoxy-tetradeca-6E, 12E-diene-8,
10-diyneRAW 264.7
macrophages (LPS-induced)Level of NO and PGE2;
Level of iNOS, COX-2;
Levels of pro-inflammatory cytokines;
Phosphorylation of MAPK(p38, JNK, and ERK1/2)2 and 10 μM NF-κB signaling pathway IL-1β ↓; IL-6 ↓; TNF-α ↓;
p38 ↓; JNK ↓; ERK1/2 ↓[75] Anti-inflammatory AT-I; AT-II;
8-epiasterolidRAW264.7 macrophages;
BV2 microglial cells (LPS-
induced)Structure identification;
NO, PGE2 production;
Protein expression of iNOS, COX-2, and cytokines40 and 80 μM NF-κB signaling pathway. NO ↓; PGE2 ↓; iNOS ↓;
COX-2 ↓; IL-1β ↓; IL-6 ↓; TNF-α ↓[76] Intestinal inflammation AT-III Male C57BL/6 mice (TNBS-induced) Levels of myeloperoxidase;
Inflammatory factors;
Levels of the prooxidant markers, reactive oxygen species, and malondialdehyde;
Antioxidant-related enzymes;
Intestinal flora5, 10, 20 mg/kg FPR1 and Nrf2 pathways Disease activity index score ↓; Myeloperoxidase ↓; Inflammatory factors interleukin-1β ↓; Tumor necrosis factor-α ↓; Antioxidant enzymes catalase ↓; Superoxide dismutase ↓; Glutathione peroxidase ↓; FPR1 and Nrf2 ↑; Lactobacilli ↓ [77] Anti-inflammatory AT-III MG6 cells (LPS-
induced)mRNA and protein levels of TLR4,
TNF-α, IL-1β, IL-6, iNOS, COX-2;
Phosphorylation of p38 MAPK and JNK100 μM p38 MAPK and JNK signaling pathways TNF-α ↓; IL-1β ↓; IL-6 ↓;
iNOS ↓; COX-2 ↓[78] Ameliorates spinal cord injury AT-III BV2 microglial (LPS-
induced);
Female SD rats (Infinite Horizon impactor)Spinal cord lesion area;
Myelin integrity;
Surviving neurons;
Locomotor function;
Microglia/macrophages;
Inflammatory factors1, 10, 100 μM (for cell);
5 mg/kg (for rats)NF-κB,
JNK MAPK, p38 MAPK, and Akt pathwaysActive microglia/macrophages;
Inflammatory mediators ↓[79] Ulcerative colitis AT-III IEC-6 (LPS-induced);
C57BL/6J male mice (DSS-induced)MDA,GSH content;
SOD activity;
Intestinal permeability;
Mitochondrial membrane potential;
Complex I and complex IV activity40 and 80 μM (for cell);
5 and 10 mg/kg (for rats)AMPK/
SIRT1/PGC-1α signaling pathwayDisease activity index ↓;
p-AMPK ↑; SIRT1 ↑;
PGC-1α ↑;
Acetylated PGC-1α ↑[80] '/' denotes no useful information found in the study. Table 4.
Immunomodulatory and anti-inflammatory activities of esters and sesquiterpenoids.
Figures
(6)
Tables
(4)