-
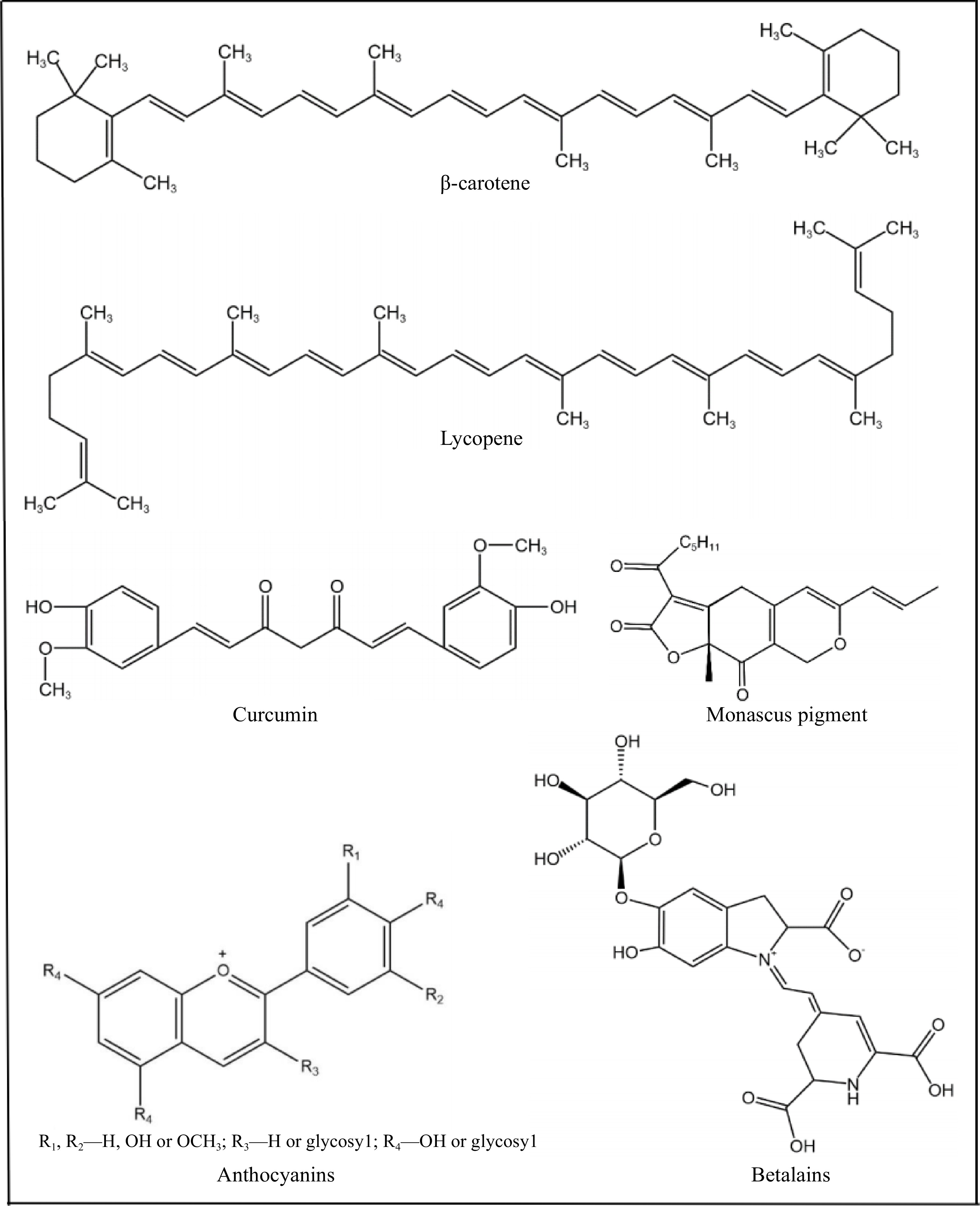
Figure 1.
Natural pigments commonly used in food products.
-
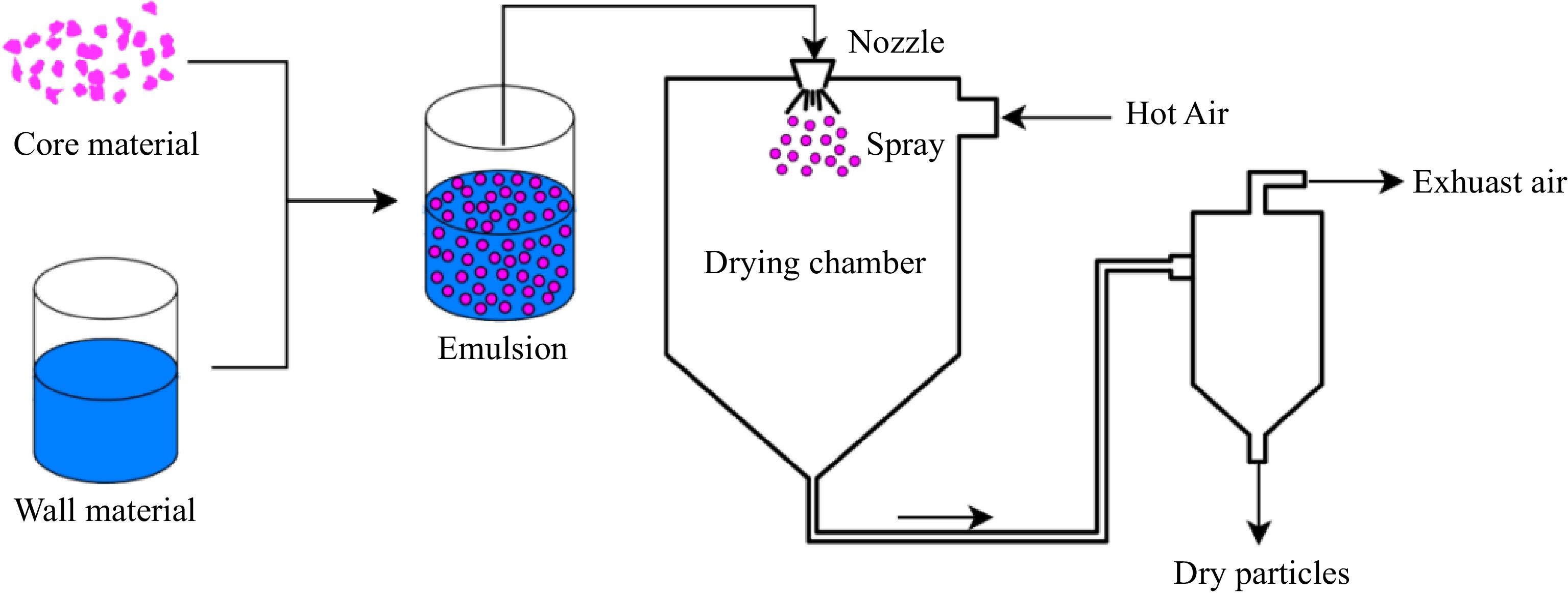
Figure 2.
Schematic representation of the microencapsulation process by spray drying.
-
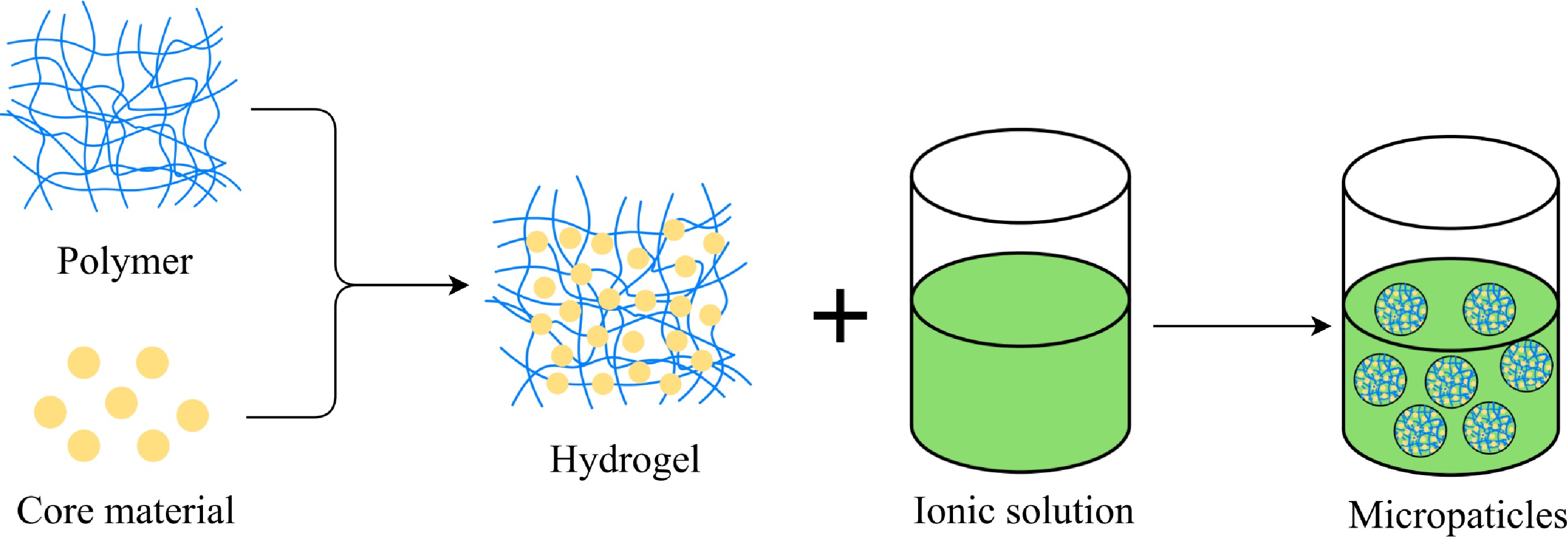
Figure 3.
Schematic representation of the microencapsulation process by ionic gelation.
-
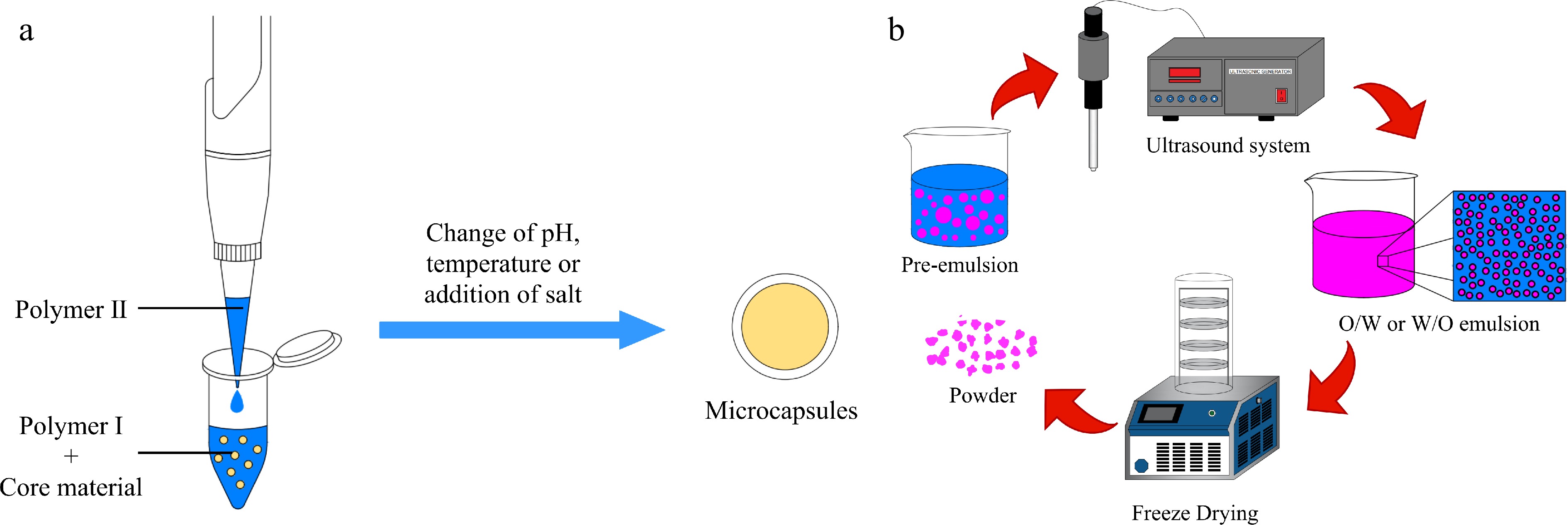
Figure 4.
Schematic representation of the microencapsulation process by (a) complex coacervation and (b) ultrasound.
-
Methods Advantages Disadvantages Spray drying Simple method, low cost, high flexibility, high encapsulation efficiency. High temperatures, the broad size distribution of the particles. Freeze drying Low temperatures, good rehydration properties. Time-consuming, energy-consuming. Supercritical anti-solvent Low cost, mild operation temperatures and simpler steps. The particles are of high purity, small size and uniformity. Use of organic solvents, organic solvent residual. Complex coacervation High encapsulation efficiency (up to 99%), scalability and reproducibility. Tedious, time-consuming, high cost, sensitivity to pH and ionic strength. Ionic gelation Good stability, good hydrogel sustained release, high encapsulation efficiency, low temperatures, no use of organic solvents, low cost. The larger size and lower stability of particles. Easy diffusion and fast release of core material through the ionic gel network. Ultrasound assisted High yield, being rapid and relatively simple without any purification steps. The narrow size distribution of the particles. High temperatures, high pressure. Liposome Good biocompatibility, sustained-releasing potential and targeting properties, biodegradable, non-immunogenic and non-toxic. It is easy to occur in aggregation, fusion, phospholipids hydrolysis and oxidation during storage. Table 1.
Advantages and disadvantages of different encapsulation methods.
-
Stabilization technique Core material Wall material Results Reference Spray drying Beetroot extract Maltodextrin, inulin, whey protein isolate The values of retention was 88.45%~95.69%. The use of whey protein isolate together with inulin achieved high stability and antioxidant activity of beetroot pigments. [51] Spray drying Betacyanine Maltodextrin,
sweet potato starchEncapsulation efficiency reached 90%. The prepared microcapsules had a high degree of functional properties, defined morphology, and modal size distribution. [52] Spray drying Monascus pigments NaAlg, CaCO3 Spray-dried Monascus red pigments microcapsules had lower degradation constant and longest half-life under the treatments of heating, light and in vitro simulated gastrointestinal digestion [48] Spray drying Betalains Maltodextrin,
cladode mucilage from O. ficus-indicaThe addition of cladode mucilage from O. ficus-indica in the formulation increased the encapsulation efficiency, diminished the moisture content, and allowed to obtain more uniform size and spherical particles, with high dietary fiber content. [47] Spray drying Lutein Maltodextrin, gum arabic, modified starch Encapsulation efficiency ranged from 64.79% to 98.82%. The stability of Lutein was improved. [49] Spray drying Curcumin Porous starch,
gelatinThe microencapsules had good encapsulation efficiency. The stability of microencapsulation curcumin against light, heat, and pH was effectively improved and its solubility was increased greatly. [53] Freeze drying Anthocyanins Black glutinous rice maltodextrins Freeze dried anthocyanin powders showed the good properties in terms of bulk density, angle of repose, process yield and anthocyanin retention. [54] Freeze drying Betalains Guar gum, gum arabic, pectin, xanthan gum Encapsulation showed a higher recovery of betalains during freeze drying by 1.3 times than during spray drying. Freeze dried samples has a* (redness) higher than the spray dried samples. [55] Freeze drying Anthocyanins Soy protein isolate, gum arabic The microencapsulation rate reached 93.05%~98.87%, and the thermal stability of all treatment groups was improved in the range of 80~114 °C. [56] Freeze drying Thyme essential oil Whey protein The microcapsules demonstrated strong antibacterial activity against Salmonella ser. Enteritidis and Staphylococcus aureus. The thermal stability of encapsulated oil was effectively enhanced. [57] Ionic gelation Betalains Calcium alginate,
bovine serum albuminThe stability and antioxidant activity of the encapsulated betalains during processing and storage were improved in a lower humidity environment. [58] Ionic gelation Anthocyanins Curdlan, pectin and sodium alginate Encapsulation efficiency ranged from 80.3% to 96.7%. The release curves followed first order kinetics, with a strong burst effect,
80% to 100% of the anthocyanins released in solution at
pH 1 after 20 min.[59] Ionic gelation Epigallocatechin gallate (EGCG) Debranched starch, carboxymethyl debranched starch The highest encapsulation efficiency was 84.4%. The nanoparticles provided a controlled release of EGCG. [60] Complex coacervation Astaxanthin Gelatin, gum arabic Encapsulation efficiency reached 93.5%, Encapsulation reduced the characteristic odor, improved the coloring capacity effectively. [61] Complex coacervation Anthocyanins Gelatin, gum arabic The selected method significantly increased the stability of anthocyanins up to 23.66% after 2 months of storage at 37 ± 2 °C. The selected optimal microcapsules revealed intense red color over the time of storage. [62] Complex coacervation Carotenoids Whey proteins isolate, gum acacia Encapsulation efficiency reached 56%, the water solubility of the carotenoid was improved, and the encapsulated carotenoid showed good stability and microbiological properties during accelerated storage. [63] Complex coacervation Betacyanin Maltodextrin, gum arabic, xanthan gum, and gelatin were mixed with sodium alginate Encapsulation efficiency ranged from 80.53% to 92.27%. All of the wall material compositions provided protection against high temperatures and pH variations. [64] Complex coacervation Betanin Amaranth protein isolate, carboxymethylcellulose (CMC) The encapsulation efficiency of betanin was high, ranging from
61% to 87%. The microcapsules protected betanin from thermal degradation (50 °C), increasing its half-life by approximately
2.92-fold. After gastrointestinal digestion, the bioaccessibility of encapsulated betanin was approximately 84%.[65] Supercritical
anti-solventAstaxanthin Polylactic acid (PLLA) Encapsulation efficiency reached 91.5%. Astaxanthin/PLLA microspheres greatly enhanced the stability of astaxanthin during storage, and the levels of residual solvents were far lower than the ICH limits. [66] Supercritical
anti-solventAstaxanthin Polyhydroxy-butyrate-co-valerate Encapsulation efficiency reached 48.25%. Encapsulation enhanced the stability of astaxanthin during storage. [67] Supercritical
anti-solventLutein Hydrogenated phosphatidylcholine Encapsulation efficiency reached 90.0%. Encapsulation improved solubility, stability and bioavailability of lutein. [68] Ultrasonic Curcumin Zein, sodium caseinate With an encapsulation efficiency of 90.19 ± 0.33%, the sensitivity of curcumin to temperature was reduced, and the treatment improved the stability of storage. [69] Ultrasonic Betalains Maltodextrin The encapsulation efficiency of the betalains were above 79%. Therefore, modest ultrasound treatment can be used for microcapsulation to improve the stability of betalains. [70] Ultrasonic Anthocyanins Soy protein isolate (SPI), gum arabic (GA) Encapsulation efficiency ranged from 93.05% to 98.87% for all microcapsules. All microcapsules enhanced the thermal stability of anthocyanin in the temperature range 80~114 °C. In addition, SPI and GA combination presented good release behavior under simulated gastrointestinal conditions compared with unencapsulated anthocyanin. [56, 71] Self-assembly Lycopene β-lactoglobulin The results suggested that nanomicelles effectively improved the stability and bioavailability of hydrophobic bioactives lycopene. [72] Self-assembly Curcumin Hydroxypropylated debranched starch (HPDS) After encapsulation, the water solubility and physical stability of curcumin could be increased up to 226-fold and 6-fold, respectively. The HPDS nanospheres also exhibited good safety (including hemolysis and cytotoxicity) and sustainable release of curcumin. [73] Self-assembly Curcumin Lactoferrin peptides The encapsulation efficiency was 93.44%. Nano-micelles have been shown to improve thermal stability, dilution stability, storage stability, the transformation rate and bioaccessibility of curcumin. [74] Self-assembly Curcumin Acylated ovalbumin (AOVA) Curcumin encapsulated in AOVA nanogels displayed higher encapsulation efficiency (93.64%) and slower sustained release under simulated gastrointestinal conditions. [75] Self-assembly β-carotene Rapeseed peptides Encapsulation efficiency > 80%, encapsulation improved solubility, stability, flavor and bioavailability of β-carotene. [76] Liposome Vitamin C,
β-caroteneYolk lecithin, cholesterol The co-encapsulation of Vitamin C and β-carotene could significantly improve the storage stability of β-carotene. Liposomes could protect bioactives from damage in the stomach and release them in the small intestine, where they can be absorbed. [77] Liposome β-carotene Hydrogenated phosphatidylcholine and sucrose The liposome was highly solubility, and capable of preserving more than 90% of the incorporated beta-carotene for 60 d of refrigerated storage under vacuum. After 60 d, the color of the dispersions was preserved. [78] Liposome Epigallocatechin gallate (EGCG),
quercetinLecithin, cholesterol,
Tween 80The liposomes were homogeneous with a narrow size distribution and both the encapsulation efficiency for EGCG and quercetin were higher than 60%. High stability of the system was exhibited during the 30 d storage. [79] Liposome Betalains Lecithin The results showed that the color and stability of betalains increased to 76%. [80] Table 2.
Encapsulation of bioactives with different techniques and wall materials.
Figures
(4)
Tables
(2)