-
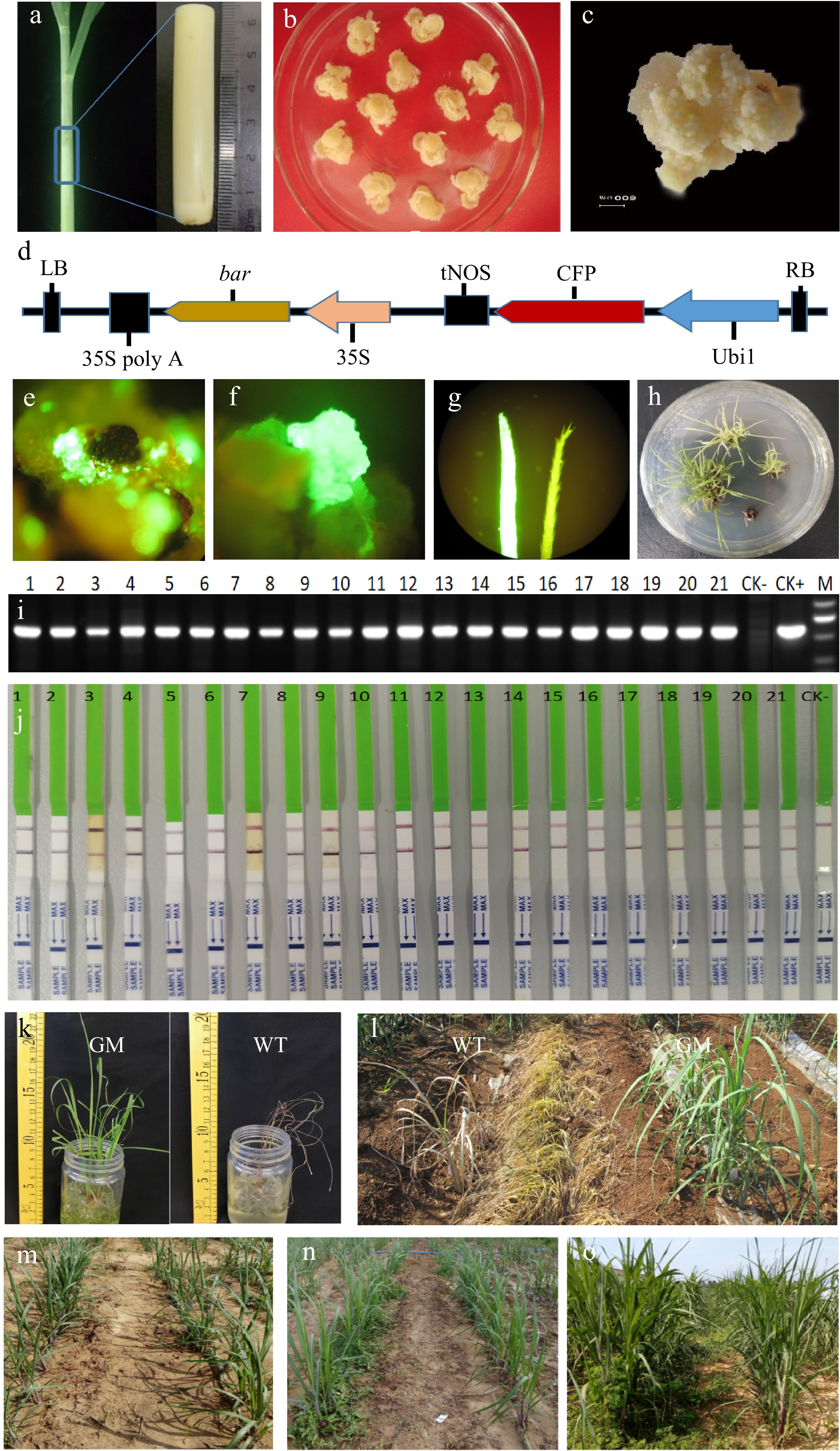
Figure 1.
Efficient sugarcane transgenic system based on herbicide screening. (a) Original explant from sugarcane tillers for callus induction. (b) Two weeks after first induction through the addition of a high concentration (2 mg/L) of 2,4-D to the medium. (c) Two to three weeks after third induction through the addition of a low concentration (1 mg/L) of 2,4-D and a very low concentration (0.1 mg/L) of 6-BA to the medium. (d) Schematic of the pCAMBIA3300-CFP plant expression vector. RB: Right border of pCAMBIA3300; LB: Left border of pCAMBIA3300; Ubi1: maize Ubi 1 promoter; CFP: CFP visible marker gene; tNOS: nopaline synthase terminator; 35S: cauliflower mosaic virus 35S promoter; bar: bar selective marker gene; 35S poly A: cauliflower mosaic virus 35S poly A tail. (e) Fluorescence observation of infected calluses after 7 d of resting cultivation. (f) Fluorescence observation of resistant calluses after 30 d of callus screening. (g) Fluorescence observation of leaves after 14 d of regeneration, Left: leaf of transgenic shoot, Right: leaf of wild-type shoot. (h) Resistant shoots on the rooting medium. (i) Molecular assay for the bar selective marker gene by traditional PCR; CK−: nontransformation shoots; CK+: plant expression vector; and M: DNA marker ladder. (j) PAT/bar protein assay by QuickStix Strips; 1–21: Resistant shoots; CK−: nontransformation shoots. (k) Herbicide tolerance testing of transgenic shoots. GM: transgenic shoots (2.0 mg/mL Basta); WT: Wild-type shoots (0.5 mg/mL Basta). (l) Tenth day after herbicide spraying; GM: transgenic shoots; WT: Wild-type shoots. (m) First day after weed control in the field; Left: Weed control of wild-type shoots by hoeing; Right: Weed control of transgenic shoots by herbicide. (n) Two weeks after weed control in the field; Left: Weed control of wild-type shoots by hoeing; Right: Weed control of transgenic shoots by herbicide. (o) Two months after weed control; Left: Weed control of wild-type shoots by hoeing; Right: Weed control of transgenic shoots by herbicide.
-
Name of
vector/geneCalluses
used (g)Transgenic shoots provided (lines) Target of genetic transformation Strategy of genetic modification Institutes serviced Date Cry2A 2 17 Pest-resistant genes OE Huazhong Agricultural University 2017.5 Cry1C 2 20 OE 2017.5 Hc-Pro 2 20 Functional gene of yje SCSMV virus OE Yangzhou University 2019.12 ScD27 2 20 Tiller-associated genes OE Sugarcane Research Institute, Yunnan Academy of Agriculture Science 2018.5 ScD10 2 25 RNAi 2019.6 INV 2 15 Sucrose invertase gene OE Institute of Nanfan & Seed Industry, Guangdong Academy of Science 2021.10 FUG 2 15 Haploidy induction gene GE 2019.12 SsWRKY1- OE 2 15 Drought resistance-associated genes OE Yunnan Agricultural University 2020.8 SsWRKY1-RNAi 2 20 RNAi 2020.8 DREB 2 12 Drought resistance-associated genes OE South Subtropical Crops Research Institute, Chinese Academy of Tropical Agriculture Science 2020.9 REMO 2 14 OE 2020.9 MYB8i 2 15 MYB transcription factors RNAi Fujian Agriculture and Forestry University 2020.11 MYB11i 2 12 RNAi 2020.11 ERF99 2 13 Ethylene-responsive factors OE 2020.11 Z6 3 45 JAZ transcription factors OE 2021.9 Z10 3 50 OE 2021.9 VSR 3 80 Vacuolar sorting receptors OE Yulin Normal University 2022.6 R1 3 35 Plant activator polypeptide receptor OE 2022.10 RK1 3 30 Ratoon stunting disease-responsive factors RNAi Sugarcane Research Institute, Guangxi Academy of Agriculture Science 2022.10 * OE: Target gene overexpression; RNAi: Target gene suppression by RNAi; GE: Gene mutation by genomic editing. Table 1.
Technical service using the efficient transgenic system.
Figures
(1)
Tables
(1)