-
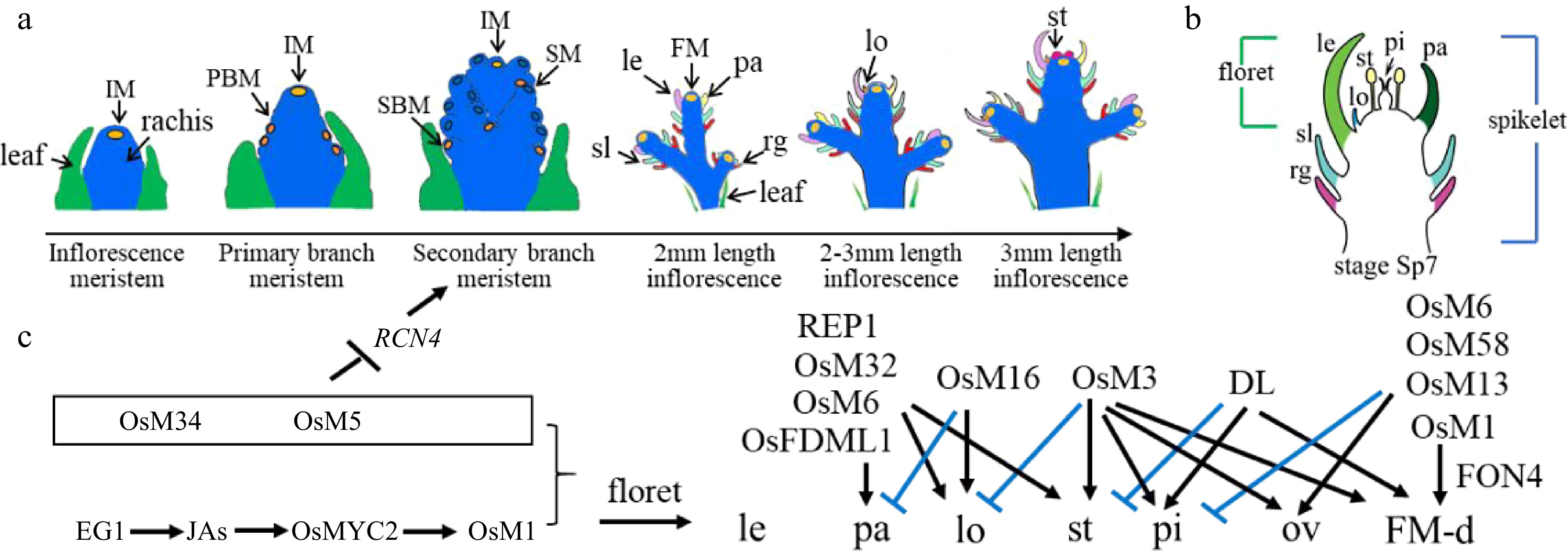
Figure 1.
Rice inflorescence and spikelet development. (a) Schematic diagram of rice inflorescence development from the inflorescence meristem to stamen stages, showing leaf cells (green), rachis cells (blue), meristem cells (yellow), and other colors as annotated: FM, floret meristem; IM, inflorescence meristem; le, lemma; lo, lodicule; pa, palea; PBM, primary branch meristem; rg, rudimentary glume; SBM, secondary branch meristem; sl, sterile lemma; SM, spikelet meristem; st, stamen. (b) Schematic diagram of a rice spikelet, comprising two sterile lemmas and rudimentary glumes, and one floret. pi: pistil. (c) Genes that were cloned and studied by Prof. Zhang's group. Black color represents positive regulation or activation, while blue color represents negative regulation or repression. JA, jasmonic acid; OsM, OsMADS; ov: ovule; FM-d: flower meristem determinacy.
-
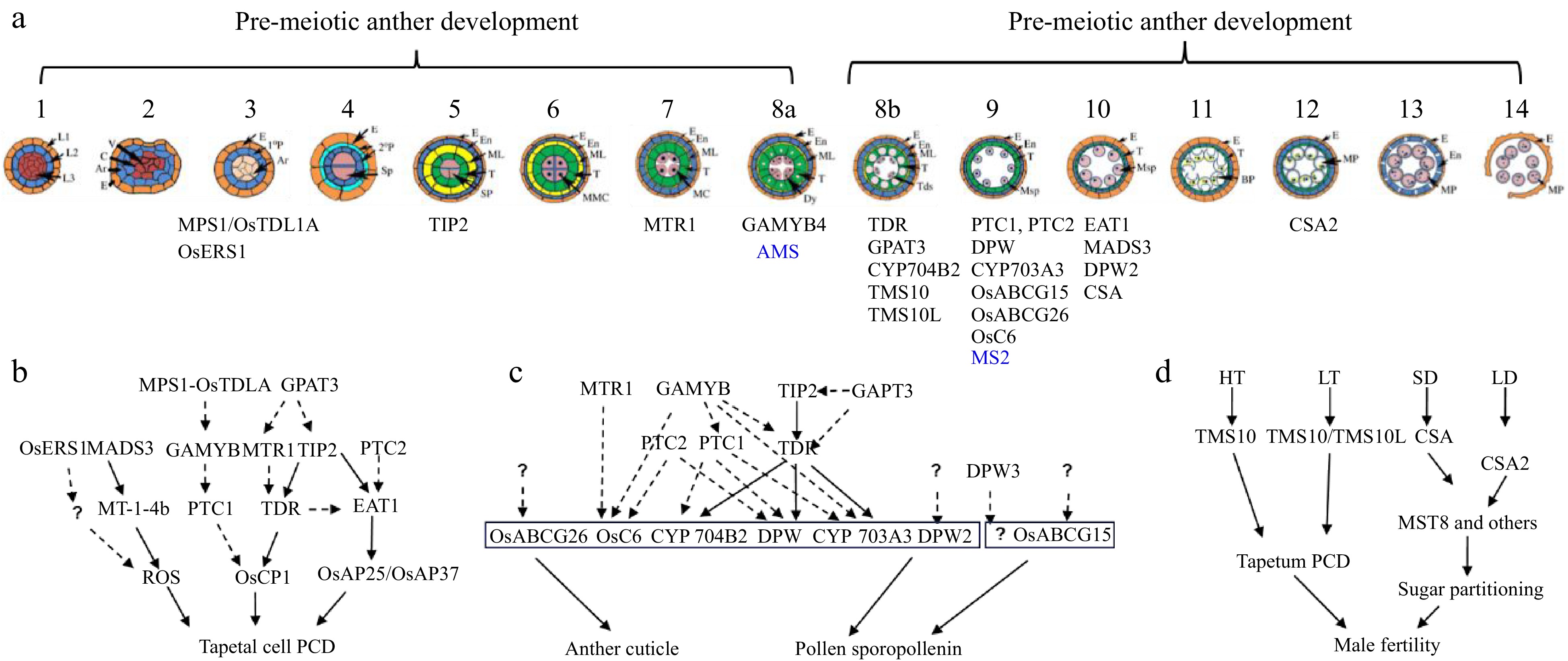
Figure 2.
Anther and pollen development and its adaptation to the environment. (a) Schematic overview of anther development in dicot and monocot plants. Genes identified by Prof. Zhang with a role in anther development are shown (blue for Arabidopsis and black for rice). (b) Regulatory aspects of anther development. (c) Pathways and networks related to rice tapetal programmed cell death (PCD), and anther cuticle and pollen sporopollenin development, all identified by Prof. Zhang. Solid arrows represent information obtained from biochemical binding assays, while dashed arrows indicate data from transcriptional analysis. Question marks indicate ongoing gaps in knowledge. (d) Prof. Zhang has identified two molecular pairs, TMS10/TMS10L and CSA/CSA2, that mediate rice responses to high/low temperature (HT, LT) and long-short day length (LD, SD), respectively.
-
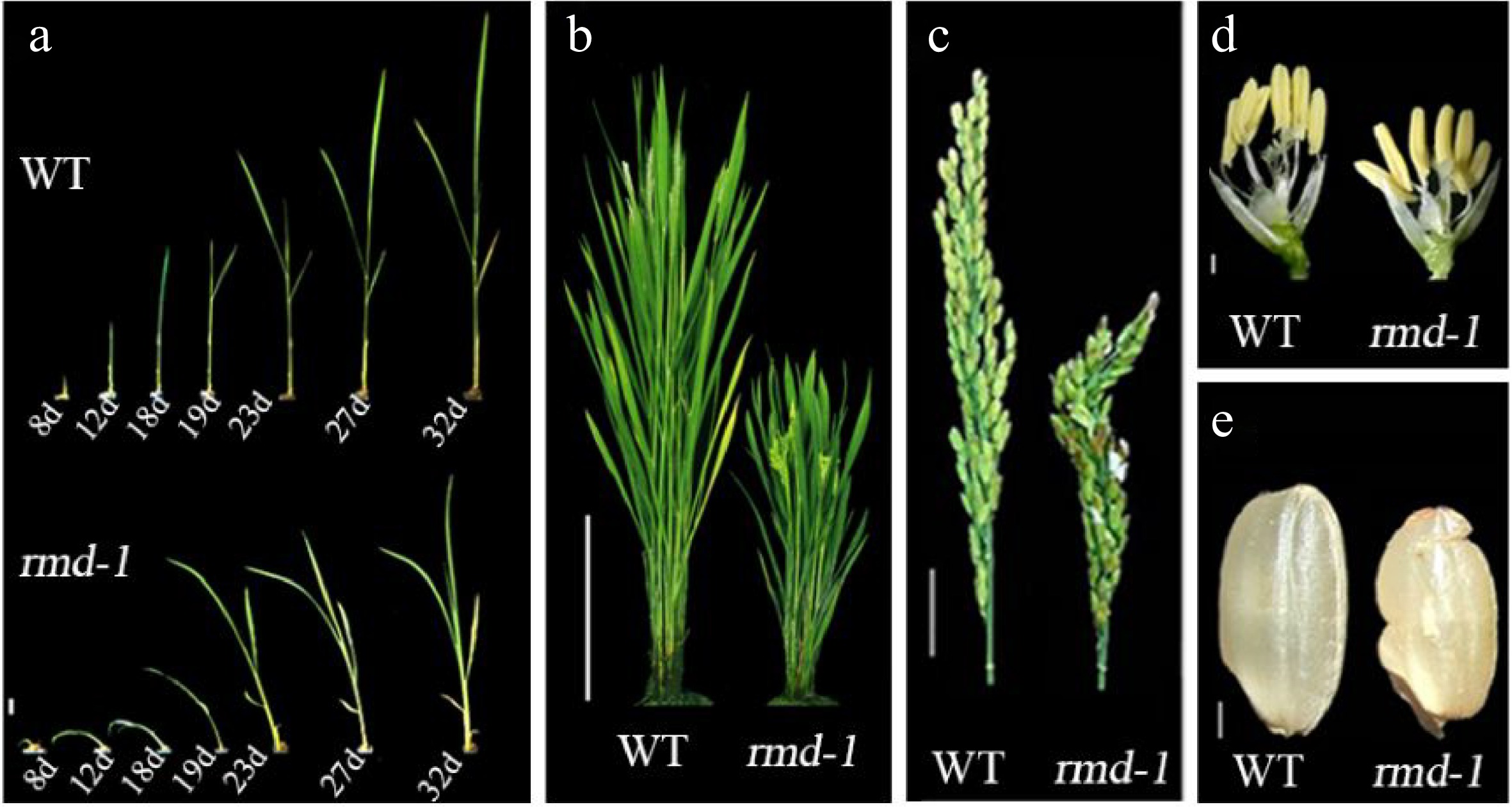
Figure 3.
rmd mutants exhibit pleiotropic defects compared with wild type (WT) plants[75]. (a) Seedlings; d, days after germination. Bar = 8 mm. (b) Rice plants at heading stage. Bar = 20 cm. (c) Panicle and rachis of plants at heading stage. Bar = 2 cm. (d) Individual flowers from plants at heading stage. Bar = 2 mm. (e) Seeds. Bar = 2 mm.
-
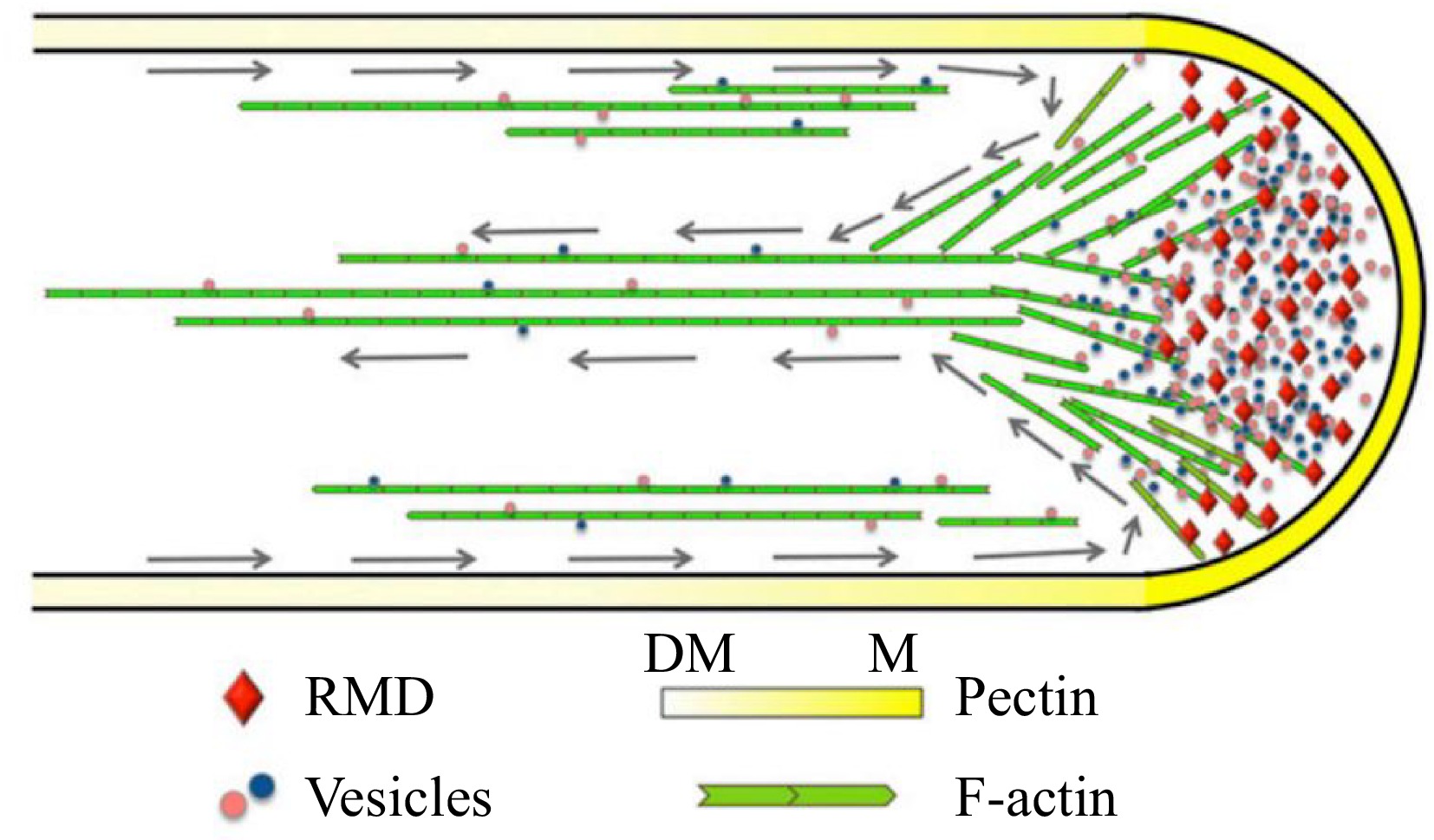
Figure 4.
Model depicting the function of RMD in rice pollen tube growth[76]. RMD serves as a crucial regulatory factor that plays a significant role in the maintenance of the spatial arrangement of cytoplasmic streaming, which is vital for the determination of pectin distribution and subsequent tip growth of the rice pollen tube. Gray arrows trace the reverse-fountain cytoplasmic streaming pattern. DM, demethyl-esterified; M, methyl-esterified.
-
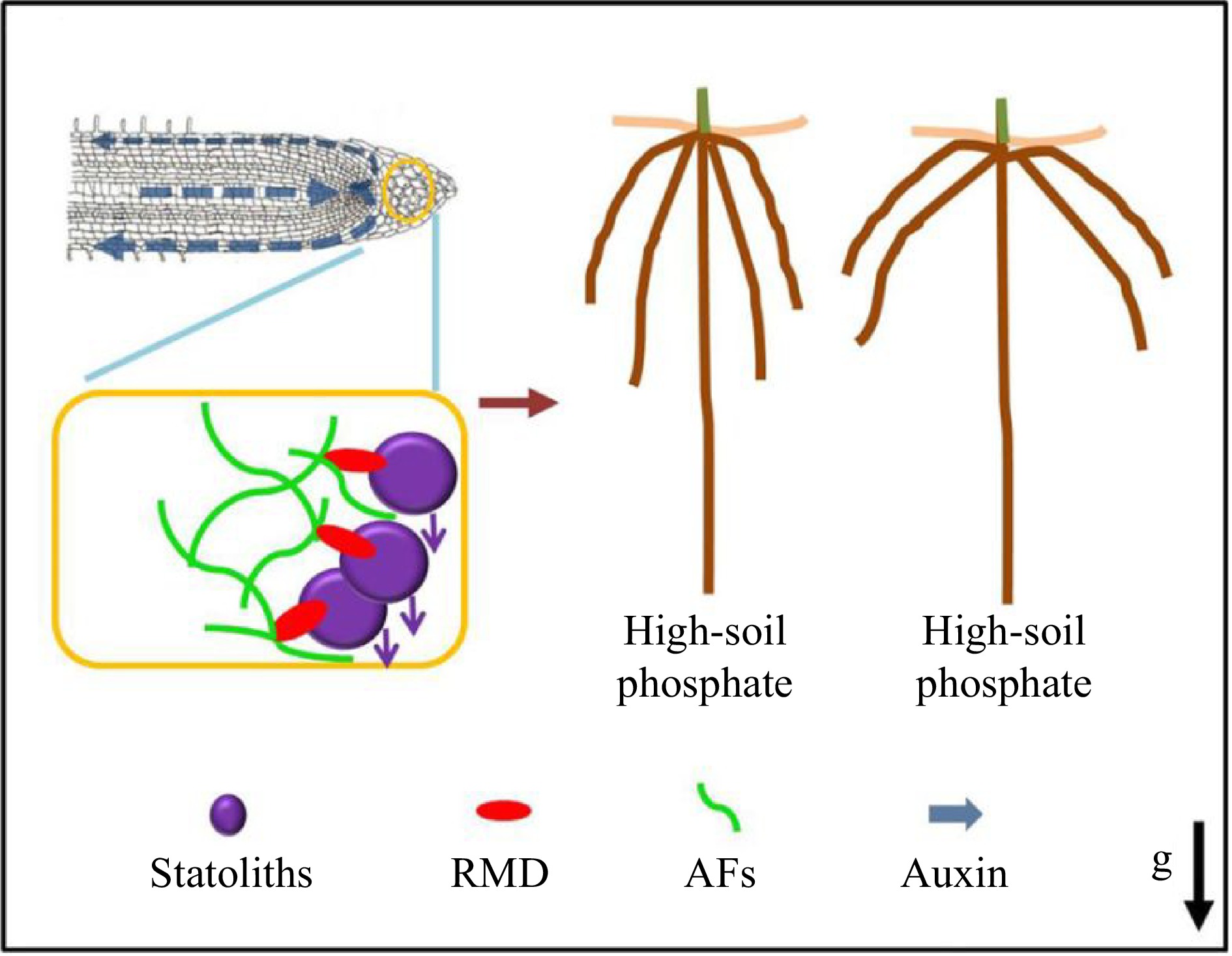
Figure 5.
RMD-dependent regulation of root crown angle by phosphate[77]. Prof. Zhang and collaborators proposed a model whereby low soil phosphate conditions and higher RMD levels in columella cells promote stronger interactions between AFs and statoliths that delay statolith sedimentation, resulting in less robust auxin-driven root gravitropic response and shallower crown root angle. In contrast, lower RMD levels in response to high soil phosphate ultimately result in a steeper crown root angle.
Figures
(5)
Tables
(0)