-

Figure 1.
(a) Whole plant, (b) medicinal portion, and (c) commercial herbal pieces of Lindera aggregata.
-
Figure 2.
General geographical distribution of Lindera aggregata in China.
-
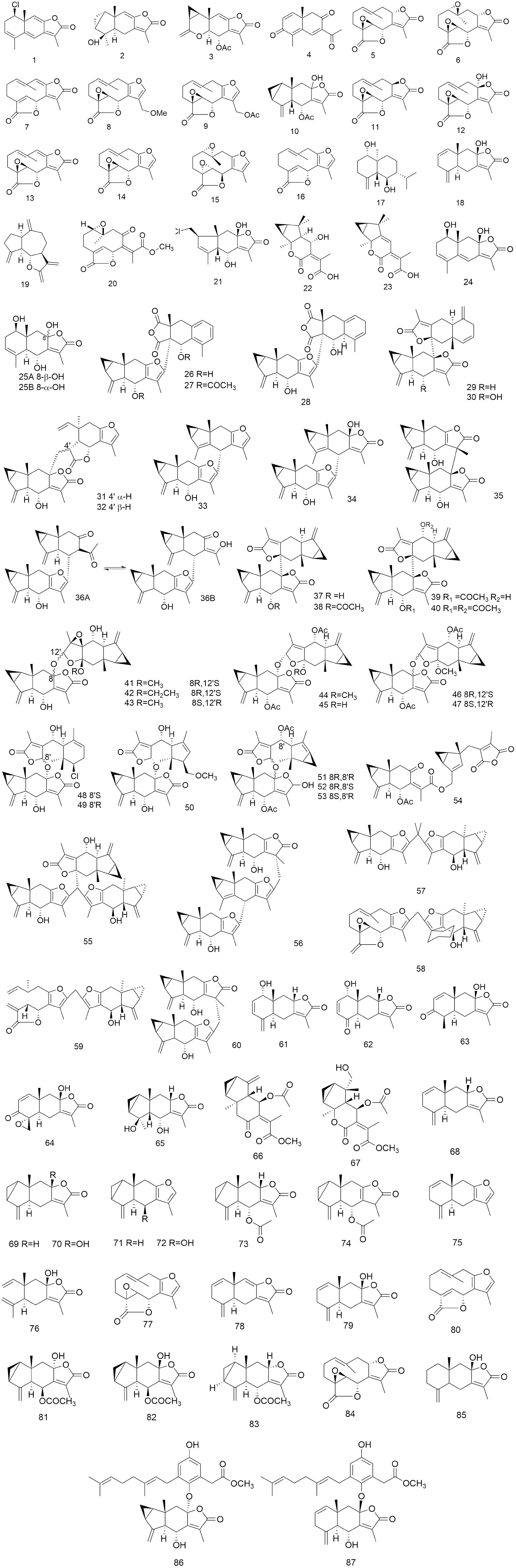
Figure 3.
Structures of sesquiterpenoids (1−87) isolated from Lindera aggregata.
-
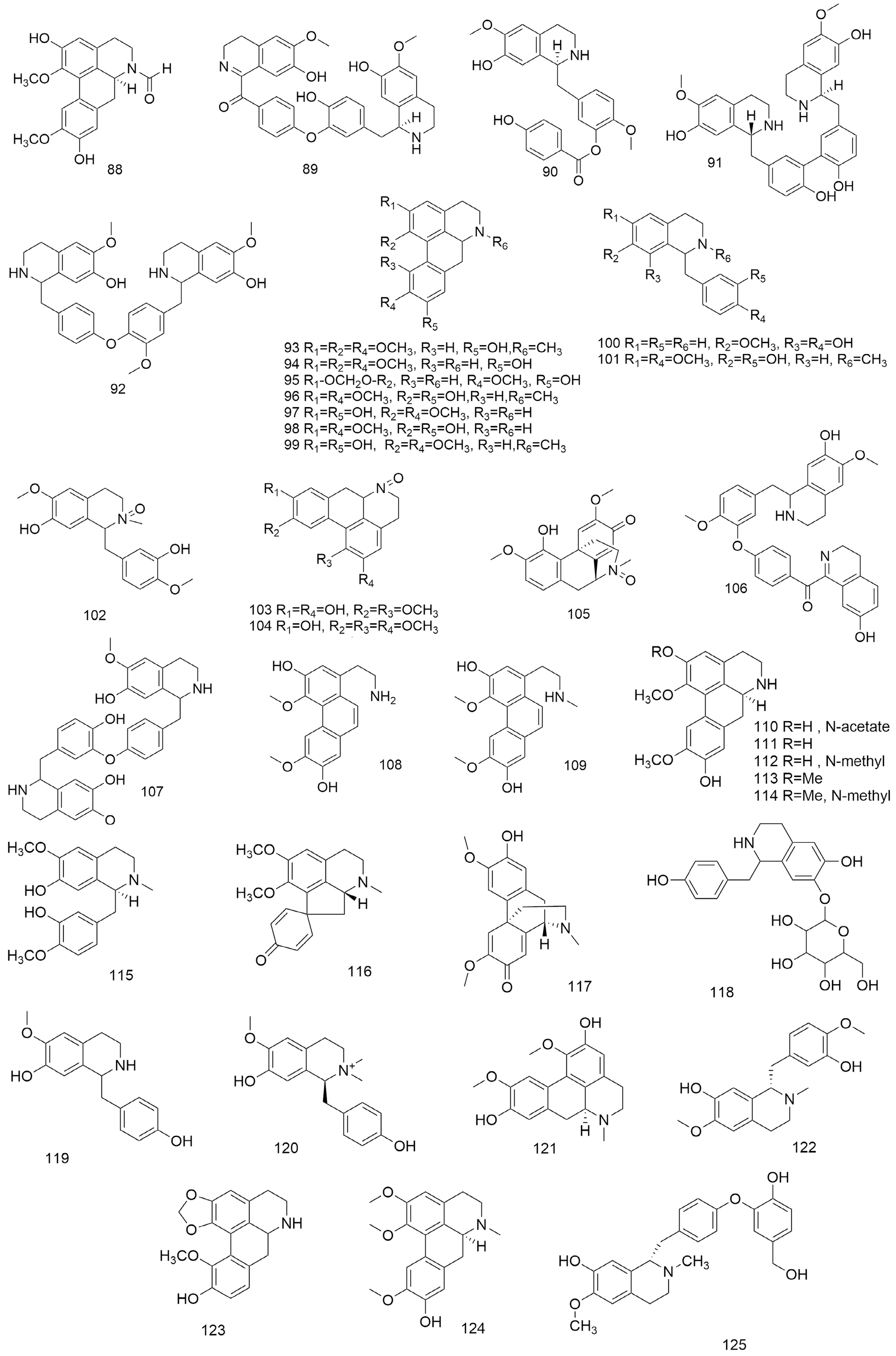
Figure 4.
Structures of alkaloids (88-125) isolated from Lindera aggregata.
-
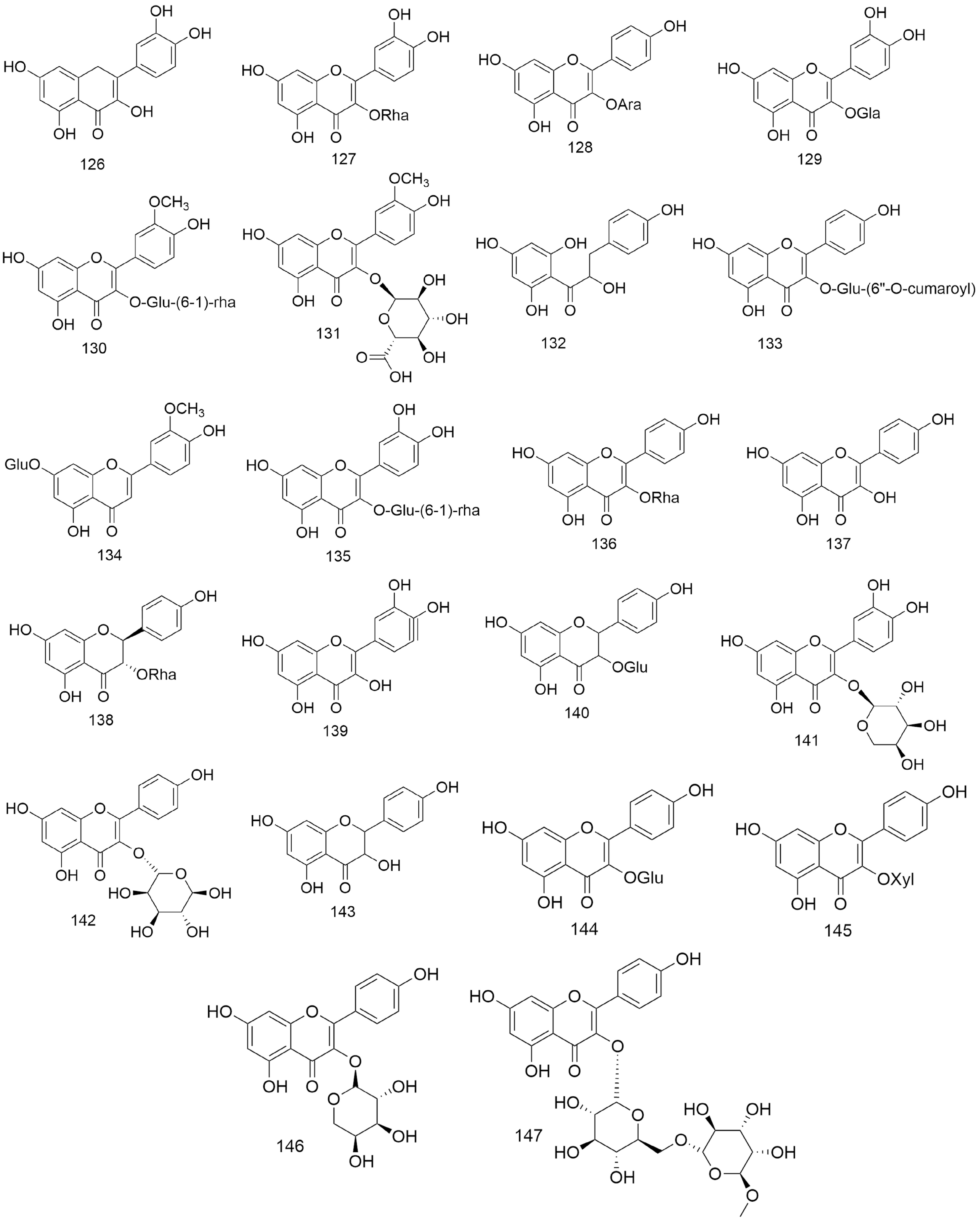
Figure 5.
Structures of flavonoids (126−147) isolated from Lindera aggregata.
-
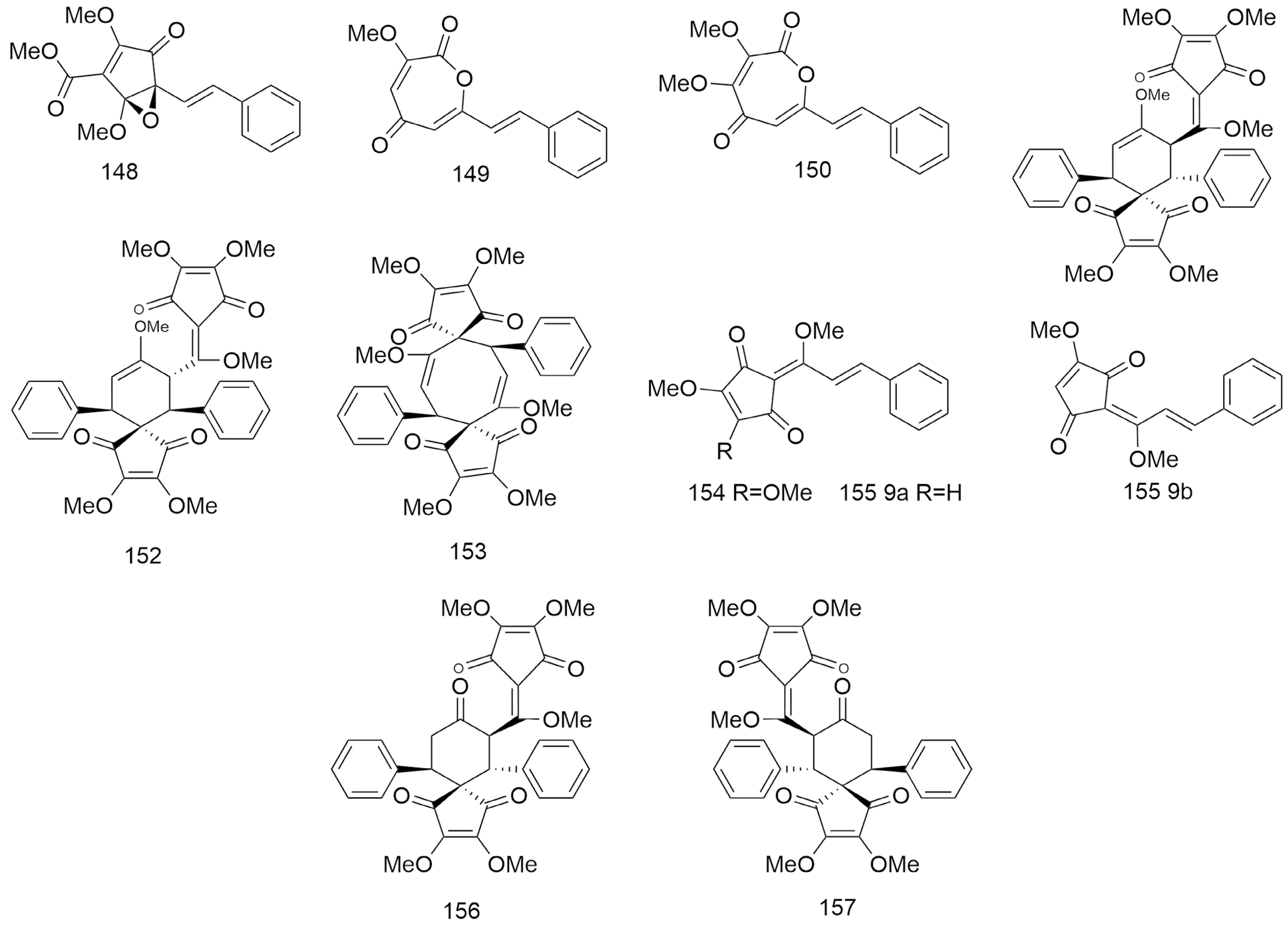
Figure 6.
Structures of cyclopentanedione derivatives and enantiomers of ketone derivatives (148−157) isolated from Lindera aggregata.
-
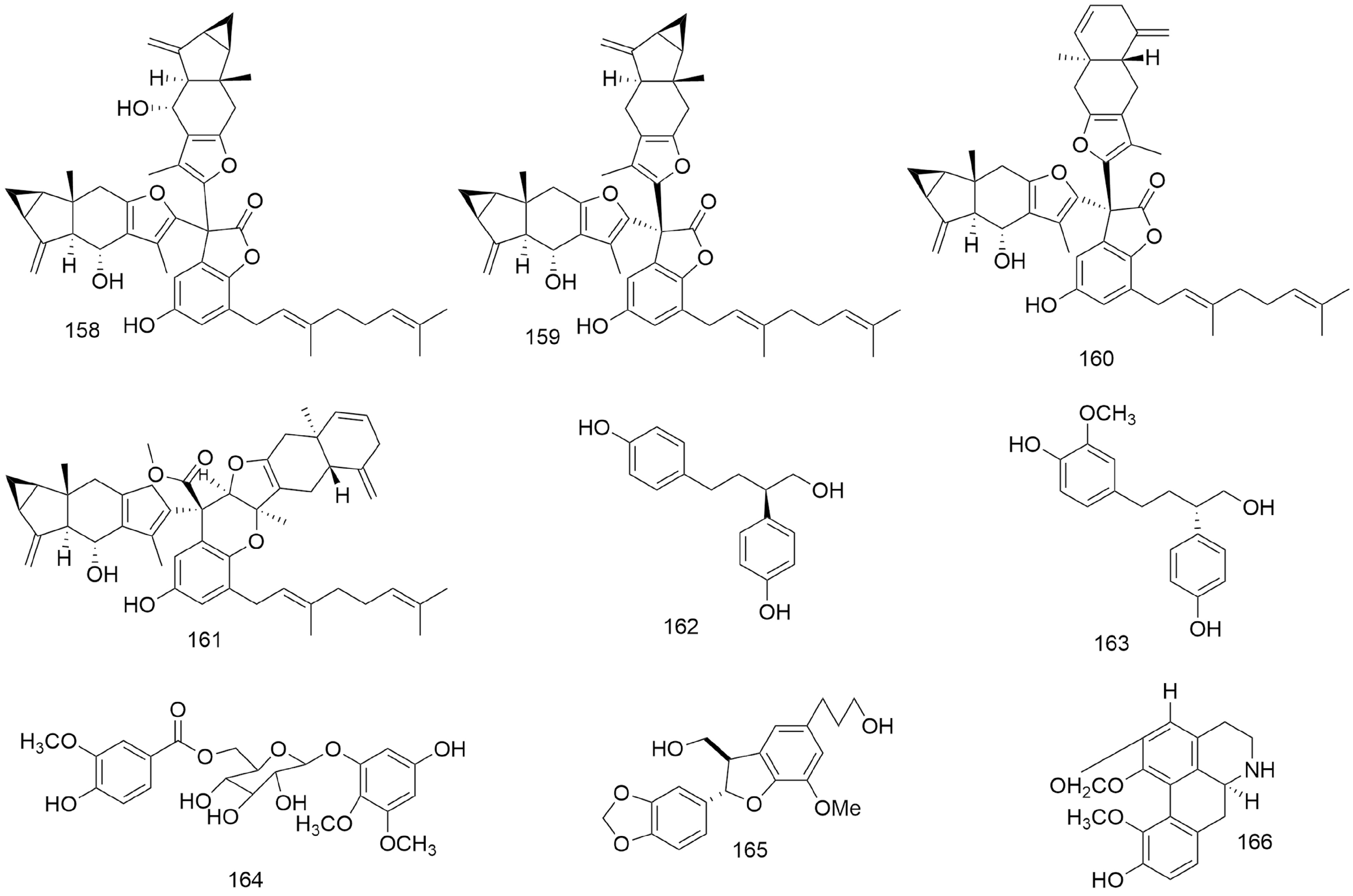
Figure 7.
Structures of disesquiterpenoid−geranylbenzofuranone conjugates, benzenoids and benzenoid glycosides and other compounds isolated from Lindera aggregata.
-
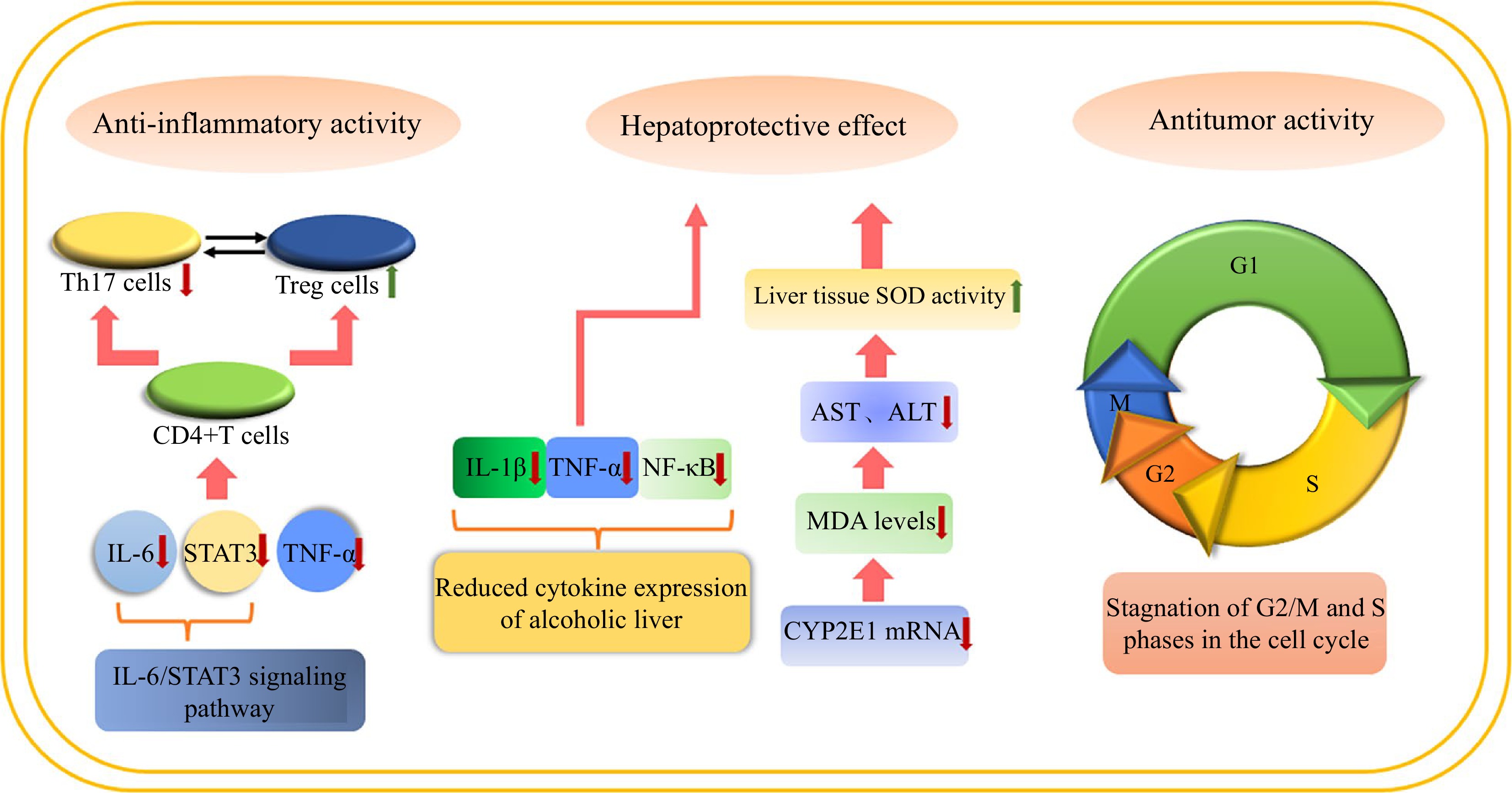
Figure 8.
The main biological activities and corresponding mechanisms of Lindera aggregata.
-
Preparation name Composition Role of LA in prescription Traditional and clinical uses References Suo Quan Wan Lindera aggregata, Alpinia oxyphylla, Dioscorea polystachya Leading role Treatment of nocturnal enuresis and frequent urination caused by kidney deficiency Weishi Jiacangfang (《魏氏家藏方》) Tiantai Wuyao San Lindera aggregata, Aucklandia costus, Foeniculum vulgare, Citrus reticulata, Alpinia officinarum, Areca catechu, Melia azedarach, Croton tiglium Leading role Treat small intestinal hernia, reduce abdominal pain and induce testicles Sheng Ji Zong Lu (《圣济总录》) Wu Yao Tang Lindera aggregata, Cyperus rotundus, Aucklandia costus, Angelica sinensis, Glycyrrhiza uralensis Leading role Treatment of irregular menstruation, dysmenorrhea, premenstrual syndrome, chronic pelvic inflammatory disease, chronic hepatitis, hyperplasia of mammary glands, and chronic gastritis Ji Yin Gangmu
(《济阴纲目》)Wu Mo Yin Zi Lindera aggregata, Aquilaria sinensis, Areca catechu, fruit of Citrus aurantium, Aucklandia costus Leading role Relieve depression, treat anger and convulsion Yifang Jijie
(《医方集解》)Zheng Qi Tian Xiang San Lindera aggregata, Cyperus rotundus, Citrus reticulata, Perilla frutescens, Zingiber officinale Leading role Treat menstrual irregularities, chest and side pain Yixue Gangmu
(《医学纲目》)Wu Yao San Lindera aggregata, Cyperus rotundus, Alpinia officinarum, Paeonia lactiflora Leading role Reconcile milk to treat children's night crying Therapeutics of Children’s Disease (《小儿药证直诀》) Jia Wei Wu Yao Tang Lindera aggregata, Cyperus rotundus, Amomum villosum, Aucklandia costus Leading role Promote blood circulation, regulate menstruation, and relieve pain Ji Yin Gangmu
(《济阴纲目》)Bai He Tang Lilium brownii var. viridulum, Lindera aggregata Supporting role Treatment of heartache and epigastric pain Shifang Kuoge
(《时方歌括》)Bu Xin Tang Angelica sinensis, Rehmannia glutinosa, Paeonia lactiflora, Corydalis yanhusuo, Lindera aggregata, Paeonia × suffruticosa, Polygala tenuifolia, (Poria cocos Schw.), Dimocarpus longan Supporting role Treatment of heartache and limb chills Yu An (《玉案》) Si Mo Tang Panax ginseng, Areca catechu, Aquilaria sinensis, Lindera aggregata Supporting role Treatment of chest tightness and anorexia Ji Sheng Fang
(《济生方》)Tong Yu Jian Angelica sinensis, Carthamus tinctorius, Crataegus pinnatifida, Lindera aggregata, Citrus reticulata, Cyperus rotundus, Alisma plantago-aquatica Supporting role Activating blood circulation and removing blood stasis, promoting “Qi” and relieving pain Complete Collection of Jingyue's Treatise (《景岳全书》) Nuan Gan Jian Angelica sinensis, Lycium chinense, (Poria cocos Schw.), Foeniculum vulgare, Cinnamomum cassia, Lindera aggregata, Aquilaria sinensis Supporting role Treat liver and kidney colds, abdominal pain, and hernia Complete Collection of Jingyue's Treatise (《景岳全书》) Ai Fu Nuan Gong Wan Ambrosia artemisiifolia, Cyperus rotundus, Cinnamomum cassia, Angelica sinensis, Paeonia lactiflora Pall. Phlomoides umbrosa (Turcz.) Kamelin & Makhm., Lindera aggregata, Morinda officinalis, Kadsura heteroclita Supporting role Treatment of menstrual irregularity and dysmenorrhea Shenshi Zhen Sheng Shu
(《沈氏尊生书》)Liu Mo Tang Aquilaria sinensis, Aucklandia costus, Areca catechu, Lindera aggregata, fruit of Citrus aurantium, Rheum palmatum Supporting role Treatment of bloating and constipation Zhengzhi Zhunshen
(《证治准绳》)Table 1.
Examples of traditional Chinese medicine prescriptions containing Lindera aggregata.
-
Class Compounds Part of the plant Chromatographic methods Type of extract Reference Sesquiterpenes Linderaggredin A 1 Whole plants Chromatography, 1H NMR spectrum, HSQC NMR spectral, HMBC spectral, NOESY spectra Methanol extract Kuo et al.[25] Linderaggredin B 2 Whole plants Chromatography, 1H NMR spectrum, HSQC NMR spectral, HMBC spectral, NOESY spectra Methanol extract Kuo et al.[25] Linderaggredin C 3 Whole plants Chromatography, 1H NMR spectrum, HSQC NMR spectral, HMBC spectral, NOESY spectra Methanol extract Kuo et al.[25] Linderaggredin D 4 Whole plants Chromatography, 1H NMR spectrum, HSQC NMR spectral, HMBC spectral, NOESY spectra Methanol extract Kuo et al.[25] Linderanlide A 5 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral, CC Ethanol extract Qiang et al.[26] Linderanlide B 6 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral, CC Ethanol extract Qiang et al.[26] Linderanlide C 7 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral, CC Ethanol extract Qiang et al.[26] Linderanlide D 8 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral, CC Ethanol extract Qiang et al.[26] Linderanlide E 9 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral, CC Ethanol extract Qiang et al.[26] Linderanlide F 10 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral, CC Ethanol extract Qiang et al.[26] Sesquiterpenoids (6) 11 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral Ethanol extract Qiang et al.[26] Sesquiterpenoids (7) 12 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral Ethanol extract Qiang et al.[26] Sesquiterpenoids (8) 13 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral Ethanol extract Qiang et al.[26] Sesquiterpenoids (9) 14 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral Ethanol extract Qiang et al.[26] Sesquiterpenoids (10) 15 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral Ethanol extract Qiang et al.[26] Sesquiterpenoids (11) 16 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral Ethanol extract Qiang et al.[26] Sesquiterpenoids (13) 17 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral Ethanol extract Qiang et al.[26] Sesquiterpenoids (14) 18 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral Ethanol extract Qiang et al.[26] Sesquiterpenoids (15) 19 Roots UV, IR, NMR, HR-ESI-MS, and CD spectra, CD spectral Ethanol extract Qiang et al.[26] Neolindenenonelactone 20 Roots Fast atom bom-bardment mass spectroscopy, one-dimensional nuclear magnetic resonance spectroscopy, two-dimensional-nuclear magnetic resonance spectroscopy, ilica gel column chromatography,TLC EtOH extract Cheng et al.[27] Linderagalactones A 21 Roots HRESIMS, 2D NMR, ECD spectra, HMBC spectrum, NOESY spectrum, silica gel GF254
plates, C18 reversed-phase silica gel, TLCEtOH extract Gan et al.[28] Linderagalactones B 22 Roots HRESIMS, 2D NMR, ECD spectra, HMBC spectrum, NOESY spectrum, silica gel GF254
plates, C18 reversed-phase silica gel, TLCEtOH extract Gan et al.[28] Linderagalactones C 23 Roots HRESIMS, 2D NMR, ECD spectra, HMBC spectrum, NOESY spectrum, silica gel GF254
plates, C18 reversed-phase silica gel, TLCEtOH extract Gan et al.[28] Linderagalactones D 24 Roots HRESIMS, 2D NMR, ECD spectra, HMBC spectrum, NOESY spectrum, silica gel GF254
plates, C18 reversed-phase silica gel, TLCEtOH extract Gan et al.[28] Linderagalactones E 25 Roots HRESIMS, 2D NMR, ECD spectra, HMBC spectrum, NOESY spectrum, silica gel GF254
plates, C18 reversed-phase silica gel, TLCEtOH extract Gan et al.[28] Linderanoid A 26 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid B 27 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid C 28 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid D 29 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid E 30 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid F 31 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid G 32 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid H 33 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid I 34 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid J 35 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid K 36 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid L 37 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid M 38 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid N 39 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderanoid O 40 Roots UV spectra, ECD spectra, NMR Spectrum Ethanol extract Liu et al.[29] Linderaggrenolide A 41 Roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] LinderaggrenolideB 42 Roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] Linderaggrenolide C 43 Roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] Linderaggrenolide D 44 Roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] Linderaggrenolide E 45 Roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] Linderaggrenolide F 46 Roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] Linderaggrenolide G 47 Roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] Linderaggrenolide H 48 Roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] Linderaggrenolide I 49 Roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] Linderaggrenolide J 50 Roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] Linderaggrenolide K 51 roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] Linderaggrenolide L 52 roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] Linderaggrenolide N 53 roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] Linderaggrenolide N 54 roots NMR spectra, UV spectra, ECD spectra, HRESIMS, Silica gel column chromatography,
thin layer chromatographyEtOH extract Liu et al.[30] Aggreganoid A 55 \ IR spectrum, NMR spectra, ECD spectra EtOH extract Liu et al.[31] Aggreganoid B 56 \ NMR spectra EtOH extract Liu et al.[31] Aggreganoid C 57 \ positive HR-ESIMS spectrum, NMR spectra, ECD spectra EtOH extract Liu et al.[31] Aggreganoid D 58 \ NMR spectra EtOH extract Liu et al.[31] Aggreganoid E 59 \ NMR spectra EtOH extract Liu et al.[31] Aggreganoid F 60 \ NMR spectra EtOH extract Liu et al.[31] Linderolide G 61 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Linderolide H 62 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Linderolide I 63 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Linderolide J 64 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Linderolide K 65 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Linderolide L 66 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Linderolide M 67 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Lindestrenolide 68 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Shizukanolide 69 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Chloranthalactone D 70 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Lindenene 71 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Lindenenol 72 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Lindenonolide H 73 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Lindenanolide A 74 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Lindestrene 75 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] 8-hydroxyisogermafurenolide 76 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Linderane 77 Roots UV spectra, IR spectra, CD spectra, NMR spectra, EI-mass spectra, CC, TLC Methanol extract Liu et al.[32] Dehydrolindestrenolide II 78 Leaves NMR spectra, TLC, Silica gel column chromatography Ethanol extract Zhang et al.[33] Hydroxylinderstrenolide III 79 Leaves NMR spectra, TLC, Silica gel column chromatography Ethanol extract Zhang et al.[33] Linderalactone IV 80 Leaves NMR spectra, TLC, Silica gel column chromatography Ethanol extract Zhang et al.[33] 6-acetyl lindenanolide B-1 I 81 Leaves NMR spectra, TLC, Silica gel column chromatography Ethanol extract Zhang et al.[33] 6-acetyl lindenanolide B-2 I 82 Leaves NMR spectra, TLC, Silica gel column chromatography Ethanol extract Zhang et al.[33] Lindenanolide H (2) 83 Leaves TLC, HPLC, NMR spectra Ethanol extract Sun et al.[34] Lindenanolide A (3) 84 Leaves TLC, HPLC, NMR spectra Ethanol extract Sun et al.[34] Atractylenolide III(4) 85 Leaves TLC, HPLC, NMR spectra Ethanol extract Sun et al.[34] Linderin A 86 Roots IR spectra, NMR spectra, HR-ESI-MS, Thin-layer chromatography, silica gel G
precoated platesEthanol extract Wen et al.[35] Linderin B 87 Roots IR spectra, NMR spectra, HR-ESI-MS, Thin-layer chromatography, silica gel G
precoated platesEthanol extract Wen et al.[35] Alkaloid Linderaggrine B 88 Whole plants Chromatography, 1H NMR spectrum, NOESY spectra Methanol extract Kuo et al.[25] (1′S)-12′-hydroxyl-linderegatine 89 Roots UV spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] (1S)-5′-O-p-hydroxybenzoyl norreticuline 90 Roots UV spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] (1R, 1′R)-11,11′-biscoclaurine 91 Roots UV spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Costaricine 92 Roots UV spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] N-methyllauro-tetanine 93 Roots UV, spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Laurotetanine 94 Roots UV, spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Actinodaphnine 95 Roots UV, spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Isoboldine 96 Roots UV, spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Laurolitsine 97 Roots UV, spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Norisoboldine 98 Roots UV, spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Boldine 99 Roots UV, spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Norjuziphine 100 Roots UV, spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Reticuline 101 Roots UV spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Reticuline n-oxide 102 Roots UV spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Boldine n-oxide 103 Roots UV spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] N-methyllaurotetanine n-oxide 104 Roots UV spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Salutaridinen-oxide 105 Roots UV spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Linderegatine 106 Roots UV spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Lindoldhamine 107 Roots UV spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Secolaurolitsine 108 Roots UV spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] Secoboldine 109 Roots UV spectra, HRESIMS spectra, Waters HPLC column, NMR spectroscopy, ECD spectra,
Thin-layer chromatography, Silica gel column chromatographyEtOH extract Yang et al.[36] (+)-norboldine acetate 110 Roots 2D NMR spectra, 1H NMR spectra, IR spectra, NMR spectra, RP-18 column EtOH extract Gan et al.[37] (+)-norboldine 111 Roots 2D NMR spectra, 1H NMR spectra, IR spectra, NMR spectra, Silica gel column chromatography EtOH extract Gan et al.[37] (+)-boldine 112 Roots 2D NMR spectra, IR spectra, NMR spectra, RP-18 column EtOH extract Gan et al.[37] (+)-laurotetanine 113 Roots 2D NMR spectra, IR spectra, NMR spectra, Silica gel column chromatography EtOH extract Gan et al.[37] (+)-N-methyllaurotetanine114 Roots 2D NMR spectra, IR spectra, NMR spectra, Silica gel column chromatography EtOH extract Gan et al.[37] (+)-reticuline 115 Roots 2D NMR spectra, IR spectra, NMR spectra, Silica gel column chromatography EtOH extract Gan et al.[37] (−)-pronuciferine 116 Roots 2D NMR spectra, IR spectra, NMR spectra, Silica gel column chromatography EtOH extract Gan et al.[37] Pallidine 117 Roots 2D NMR spectra, IR spectra, NMR spectra, RP-18 column EtOH extract Gan et al.[37] Demethylcoclaurine-7-o- glucoside 118 Roots \ Methanol extract Peng et al.[38] Coclaurine 119 Roots \ Methanol extract Peng et al.[38] Magnocurarine 120 Roots \ Methanol extract Peng et al.[38] Boldine 121 Roots \ Methanol extract Peng et al.[38] Reticuline 122 Roots \ Methanol extract Peng et al.[38] Hernangerine 123 Roots \ Methanol extract Peng et al.[38] N-methyllaurotetanine124 Roots \ Methanol extract Peng et al.[38] Karakoramine 125 Roots \ Methanol extract Peng et al.[38] Flavonoids Quercetin 126 Leaves Infrared spectrum, NMR spectra, TLC Ethanol extract Zhang et al.[39] Quercetin-3-O-rhamnoside 127 Leaves Infrared spectrum, NMR spectra, TLC Ethanol extract Zhang et al.[39] Kampferol-3-O-L-arabinopyranoside 128 Leaves Infrared spectrum, NMR spectra, TLC Ethanol extract Zhang et al.[39] Quercetin-3-O-β-D-galactopyranoside 129 Leaves Infrared spectrum, NMR spectra, TLC Ethanol extract Zhang et al.[39] Isorhamnetin-3-O-[β-D-glucopyranosy-l (6→1)- rhamno-side]130 Leaves Infrared spectrum, NMR spectra, TLC Ethanol extract Zhang et al.[39] Kampferol-3-O-α-glicurinoside 131 Leaves Infrared spectrum, NMR spectra, TLC Ethanol extract Zhang et al.[39] Nubigenol 132 Leaves Infrared spectrum, NMR spectra, TLC Ethanol extract Zhang et al.[40] Kaempferol-3-O-(6″-trans-p-coumaroyl)-β-D-glucopyranoside 133 Leaves Infrared spectrum, NMR spectra, TLC Ethanol extract Zhang et al.[40] Chrysoeriol-7-β-D-glucopyranoside 134 Leaves Infrared spectrum, NMR spectra, TLC Ethanol extract Zhang et al.[40] Rutin 135 Leaves Infrared spectrum, NMR spectra, TLC Ethanol extract Zhang et al.[40] Kaempferol-3-O-L-rhamnoside 136 Leaves NMR spectra, TLC Ethanol extract Xiao et al.[41] Kaempferol 137 Leaves NMR spectra, TLC Ethanol extract Xiao et al.[41] Dihydrokaempferol-3-O-L-rhamnoside 138 Leaves NMR spectra, TLC Ethanol extract Xiao et al.[41] Quercetin 139 Leaves NMR spectra, TLC Ethanol extract Xiao et al.[41] Kaempferol-3-O-D-glucopyranoside 140 Leaves NMR spectra, TLC Ethanol extract Xiao et al.[41] Avicularin 141 Leaves NMR spectra, TLC Ethanol extract Luo et al.[42] Afzelin 142 Leaves NMR spectra, TLC Ethanol extract Luo et al.[42] Dihydrokaempferol 143 Leaves NMR spectra, TLC Ethanol extract Luo et al.[42] Astragaline 144 Leaves NMR spectra, TLC Ethanol extract Luo et al.[42] Kaempfero-l3-O-β-D-xylopyranoside 145 Leaves NMR spectra, TLC Ethanol extract Luo et al.[42] Juglalin 146 leaves NMR spectra, TLC Ethanol extract Luo et al.[42] Kaempfero-l3-O-(2″-O-β-D-glucopyranosyl)-α-L-rhamnopyranoside 147 leaves NMR spectra, TLC Ethanol extract Luo et al.[42] Cyclopentenedione derivatives (±)-lindepentone A 148 Roots NMR spectra, ESIMS, HRESIMS, Silica gel column chromatography, High performance liquid chromatography EtOAc extract Chen et al.[45] Lindoxepine A 149 Roots NMR spectra, ESIMS, HRESIMS, Silica gel column chromatography, High performance liquid chromatography EtOAc extract Chen et al.[45] Lindoxepine B 150 Roots NMR spectra, ESIMS, HRESIMS, Silica gel column chromatography, High performance liquid chromatography EtOAc extract Chen et al.[45] Epi-bi-linderone 151 Roots NMR spectra, ESIMS, HRESIMS, Silica gel column chromatography, High performance liquid chromatography EtOAc extract Chen et al.[45] Bi-linderone 152 Roots NMR spectra, ESIMS, HRESIMS, Silica gel column chromatography, High performance liquid chromatography EtOAc extract Chen et al.[45] Linderaspirone A 153 Roots NMR spectra, ESIMS, HRESIMS, Silica gel column chromatography, High performance liquid chromatography EtOAc extract Chen et al.[45] Methyllinderone 154 Roots NMR spectra, ESIMS, HRESIMS, Silica gel column chromatography, High performance liquid chromatography EtOAc extract Chen et al.[45] Methyllucidone (a pair of cis–trans isomers, 9a and 9b) 155 Roots NMR spectra, ESIMS, HRESIMS, Silica gel column chromatography, High performance liquid chromatography EtOAc extract Chen et al.[45] Enantiomers of ketone derivatives (+)-demethoxy-epi-bi-linderone (4a) 156 Roots NMR spectra, ESIMS, HRESIMS,Silica gel column chromatography, High performance liquid chromatography EtOAc extract Chen et al.[45] (-)-demethoxy-epi-bi-linderone (4b) 157 Roots NMR spectra, ESIMS, HRESIMS,Silica gel column chromatography, High performance liquid chromatography EtOAc extract Chen et al.[45] Disesquiterpenoid−geranylbenzofuranone conjugates Linderalide A 158 Roots UV spectra, CD spectra, NMR spectra, Waters XBridge C18 (5 μm, 250 × 10 mm2, i.d.) columns, Siliabond C18 ODS column, Silica gel column chromatography Ehanol extract Liu et al.[46] Linderalide B 159 Roots UV spectra, CD spectra, NMR spectra, Waters XBridge C18 (5 μm, 250 ×10 mm2, i.d.) columns, Siliabond C18 ODS column, Silica gel column chromatography Ehanol extract Liu et al.[46] Linderalide C 160 Roots UV spectra, CD spectra, NMR spectra, Waters XBridge C18 (5 μm, 250 ×10 mm2, i.d.) columns, Siliabond C18 ODS column, Silica gel column chromatography Ehanol extract Liu et al.[46] Linderalide D 161 Roots UV spectra, CD spectra, NMR spectra, Waters XBridge C18 (5 μm, 250 ×10 mm2, i.d.) columns, Siliabond C18 ODS column, Silica gel column chromatography Ehanol extract Liu et al.[46] Benzenoids and linderagatin A 162 Roots 1D (1H, 13C) spectra, 2D NMR (COSY, NOESY, HSQC and HMBC) spectra, ECD spectra, CC, TLC MeOH extracts Ma et al.[47] linderagatin B (1-2) 163 Roots 1D (1H, 13C) spectra, 2D NMR (COSY, NOESY, HSQC and HMBC) spectra, ECD spectra, CC, TLC MeOH extracts Ma et al.[47] benzenoid glycoside 6′-O-vanilloyl-5-hydroxy-2,3-dimethoxyphenol 1-O-β-D-gluco-pyranoside 164 Whole plants Chromatography, 1H NMR spectrum, HMBC spectral, HSQC NMR spectral Methanol extract Kuo et al.[25] Others 9,9′-dihydroxy-3,4-methylenedioxy-3′-methoxy [7-O-4′,8-5′]Lignans 165 Leaves TLC, HPLC, NMR spectra Ethanol extract Sun et al.[34] Hernangerine 166 Roots TLC, NMR spectra Ethanol extract Zhu et al.[48] ‘\’ Denotes no useful information found in the study. Table 2.
The main compounds isolated from Lindera aggregata.
-
Pharmacological activity Tested substance Model Tested living system/ organ/cell Result Dose range Time period of application References Anti-inflammatory activity Ethanol extract C57BL/6 mice Colonic tissue Regulated the IL-6 signaling pathway to modulate the balance of Th17 and Treg cells, thus attenuated DSS-induced colitis in mice 1–1.7 g/kg 21 d Lai et al.[49] Ethanol extract SD rats Feces and serum Exerted anti-UC effects on the rat model induced by TNBS and that the mechanism might be associated with the inhibition of inflammatory cytokines, such as IL-6 and TNF-α 0.5, 1, 2 g /kg 9 d Lai et al.[50] \ C57BL/6 mice Colonic tissue Promoted Treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD+/SIRT1/SUV39H1/H3K9me3 signaling pathway 40 mg/kg 10 d Qi et al.[51] Ethanol extract ICR mice Paw Exhibited a potential therapeutic effect on CIA in mice as the main active constituent of LA responsible for the benefits for RA remedy 10, 20, 40 mg/kg 20 d Luo et al.[52] \ SD rats Cell Been able to prevent IL-1b-induced release of IL-6 from rat FLS, key producers of IL-6 in synovial membranes of joints 3, 10, 30, 60 mM 14 d Wei et al.[53] \ ICR mice Cell Suppressed osteoclast differentiation through Preventing the accumulation of TRAF6-TAK1 complexes and activation of MAPKs/NF-kB/c-Fos/NFATc1 Pathways 3, 10, 30 mM 5 d Wei et al.[54] \ Wistar rats Paw Attenuated osteoclast differentiation and Inflammatory bone Erosion in an aryl hydrocarbon receptor-Dependent Manner 15 mg/kg 14 d Wei et al.[55] \ Wistar rats Paw Ameliorated collagen-induced arthritis through regulating the balance between Th17 and regulatory T cells in gut-associated lymphoid tissues 15, 30 mg/kg 14 d Tong et al.[56] Ethanol extract SD rats Paw Had obvious therapeutic effect on adjuvant arthritis in rats 200, 100, 50 mg/kg 11 d Liu et al.[57] \ Sprague – Dawley rats Synovial tissue Reduced the number of blood vessels and the expression of growth factors in the synovium of AA rats, inhibited VEGF-induced in vitro angiogenesis in HUVECs \ 10 d Lu et al.[58] Hepatoprotective effect WYSTW (water extract) SD rats Serum and liver tissue Suppressed NF-κB, TNF- α expression, reducing IL-1β 2 g/kg 10 d Wang et al.[59] WYCTW (ethanol extract) SD rats Serum and liver tissue Significantly reduced serum ALT content and serum AST content, suppressed NF-κB, TNF-α expression, reducing IL-1β 2 g/kg 10 d Wang et al.[59] WYCTC1 (alcohol extract petroleum ether extract) SD rats Serum and liver tissue Significantly reduced serum ALT content and serum AST content, suppressed NF-κB, TNF-α expression, reducing IL-1β 2 g/kg 10 d Wang et al.[59] WYCTC2 (ethyl acetate extract of LA alcohol extract) SD rats Serum and liver tissue Significantly reduced serum ALT content and serum AST content, suppressed NF-κB, TNF-α expression, reducing IL-1β 2 g/kg 10 d Wang et al.[59] WYCTC3 (alcohol extract n-butanol extract) SD rats Serum and liver tissue Significantly reduced serum ALT content and serum AST content, suppressed NF-κB, TNF-α expression, reducing IL-1β 2 g/kg 10 d Wang et al.[59] WYCTC4 (alcohol extract water extract) SD rats Serum and liver tissue Significantly reduced serum ALT content and serum AST content, suppressed NF-κB, TNF-α expression, reducing IL-1β 2 g/kg 10 d Wang et al.[59] Water extract SD rats Liver tissue and blood Had preventive effect on alcoholic liver injury by inhibiting serum ACT and AST levels, and this beneficial effect might be associated with anti-inflammation and anti-oxidation 1 ml/100 g 10 d Wang et al.[62] Ethanol extract SD rats Liver tissue and blood Had preventive effect on alcoholic liver injury by inhibiting serum ACT and AST levels, and this beneficial effect may be associated with anti-inflammation and anti-oxidation 1 ml/100 g 10/d Wang et al.[62] WYSTW SD rats Serum and small intestine tissue Had the effect of protecting liver 4 g/kg 33 d Ji et al.[63] WYCTW SD rats Serum and small intestine tissue Had the effect of protecting liver 4 g/kg 33 d Ji et al.[63] WYCTC1 (alcohol extract petroleum ether extract) SD rats Serum and small intestine tissue Had the effect of protecting liver 4 g/kg 33 d Ji et al.[63] WYCTC2 (ethyl acetate extract of LA alcohol extract) SD rats Serum and small intestine tissue Had the effect of protecting liver 4 g/kg 33 d Ji et al.[63] WYCTC3 (alcohol extract n-butanol extract) SD rats Serum and small intestine tissue Had the effect of protecting liver 4 g/kg 33 d Ji et al.[63] Ethanol extract RAW 264.7 cells Cell Inhibitory activities on nitric oxide production induced by lipopolysaccharide in mouse macrophage RAW 264.7 cells,
with IC50 values of 37.8 and 38.7 μM, respectively5, 10, 20, 40, 50 μM/ml 26 h Yang et al.[36] WYSTW SD rats Serum and liver tissue Increased serum SOD activity, decreased the expression of CYP2E1 mRNA 2 g/kg 10 d Tang et al.[60] WYCTW SD rats Serum and liver tissue Increased serum SOD activity, decreased the expression of CYP2E1 mRNA 2 g/kg 10 d Tang et al.[60] WYCTC1 SD rats Serum and liver tissue Increased serum SOD activity, decreased the expression of CYP2E1 mRNA 2 g/kg 10 d Tang et al.[60] WYCTC2 SD rats Serum and liver tissue Increased serum SOD activity, decreased the expression of CYP2E1 mRNA 2 g/kg 10 d Tang et al.[60] WYCTC3 SD rats Serum and liver tissue Increased serum SOD activity, decreased the expression of CYP2E1 mRNA 2 g/kg 10 d Tang et al.[60] WYCTC4 SD rats Serum and liver tissue Increased serum SOD activity, decreased the expression of CYP2E1 mRNA 2 g/kg 10 d Tang et al.[60] Ethanol extract SD rats Serum and liver tissue Better reduced the content of serum MDA, increased the activity of SOD in serum and liver tissue, and reduced the expression of CYP2E1 mRNA in rats with acute alcoholic liver injury 1 ml/100 g 10 d Chen et al.[61] Ethanol extract ICR mice Serum and liver tissue Reduced the serum transaminase activity and the production of lipid peroxidation intermediate MDA in CCl4 liver injured mice, and significantly enhanced the TAOC and SOD activities 50, 100, 200 mg/kg 7 d Gu et al.[64] Ethanol extract Human umbilical vein endothelial cells (HUVEcs) Cell Improved the ability of endogenous antioxidation 62.5, 125, 250, 500 µM 5 h Han et al.[44] \ SD rats Serum and liver tissue Increased the activity of SOD and GSH Px in serum of model rats and reduced the content of MDA, improved the antioxidant capacity against the liver injury induced by CCl4 in rats 5, 15, 45 mg/kg 6 weeks Chen et al.[65] Anti-tumor activity Ethanol extract Human colon carcinoma cell line HCT-116 Cell Cytotoxic activities against human colon carcinoma cell line (HCT-116), with IC50 values of 51.4 and 27.1 μM, respectively 2.5, 5, 10, 20, 40, 50, 80, 100 μM/ml 24~25 h Yang et al.[66] Ethanol extract Human colon carcinoma cell line HCT-116 Cell The inhibition of cell proliferation in HCT116 occurred via induction of apoptosis and arrested of the G2/M and S cell
cycle phases\ \ Yang et al.[66] Volatile oil extract A549, Eca-109and so on Cell Leaf essential oil exhibited significant cytotoxicity against all the cells tested with a potential selectivity for cancerous cells 12.5~400 μg/mL 28 h Yan et al.[68] Volatile oil extract HepG2 Cell Inhibited HepG2 cell proliferation and induced HepG2 cell apoptosis 50, 100, 150, 200 μg/mL 8 h Yan et al.[67] Volatile oil extract A549 Cell Inhibited cell proliferation 6.25, 12.5, 25, 50, 100, 200, 400 μg/ml 4 h Yan et al.[70] Volatile oil extract Bel7402 Cell Inhibited cell proliferation 6.25, 12.5, 25, 50, 100, 200, 400 μg/ml 4 h Yan et al.[70] Volatile oil extract Eca-109 Cell Inhibited cell proliferation 6.25, 12.5, 25, 50, 100, 200, 400 μg/ml 4 h Yan et al.[70] Volatile oil extract HeLa Cell Inhibited cell proliferation 6.25, 12.5, 25, 50, 100, 200, 400 μg/ml 4 h Yan et al.[70] Volatile oil extract HT29 Cell Inhibited cell proliferation 6.25, 12.5, 25, 50, 100, 200, 400 μg/ml 4 h Yan et al.[70] Volatile oil extract MDA-MB-231 Cell Inhibited cell proliferation 6.25, 12.5, 25, 50, 100, 200, 400 μg/ml 4 h Yan et al.[70] Volatile oil extract PC-3 Cell Inhibited cell proliferation 6.25, 12.5, 25, 50, 100, 200, 400 μg/ml 4 h Yan et al.[70] Volatile oil extract SGC-7901 Cell Inhibited cell proliferation 6.25, 12.5, 25, 50, 100, 200, 400 μg/ml 4 h Yan et al.[70] Volatile oil extract SW1990 Cell Inhibited cell proliferation 6.25, 12.5, 25, 50, 100, 200, 400 μg/ml 4 h Yan et al.[70] Volatile oil extract U-2 OS Cell Inhibited cell proliferation 6.25, 12.5, 25, 50, 100, 200, 400 μg/ml 4 h Yan et al.[70] \ SGC-7901 Cell Induced the apoptosis of SGC-7901 by regulating the expression of p53, Bax, Bcl-2 and other key proteins 0, 160, 200, 240 μmol/L 24 h Liang et al.[69] \ BALB/c nude mice Tumor tissues Regulated the BCL-2/caspase-3/PARP pathway and suppressed tumor growth in a human glioblastoma multiforme xenograft mouse model 1, 2.5, 5 mg/kg, 14 d Hwang et al.[71] \ Human A549 lung cancer cells Cell Inhibited the invasion and migration of the A549 cancer cells and exhibited a dose-response association. 1, 5, 10, 20 µM 24 h Chuang et al.[72] \ The human OC cell lines SKOV-3 and OVCAR-3 Cell Decreased phophorylation of serine 727 and tyrosine 705 of STAT3 and expression of survivin, a STAT3-regulated gene 0, 5, 10, 20, 50 µM 48 h Rajina et al.[73] Anti-hyperlipidemic effect Water extract SD rats Serum and liver tissue Had lipid-lowering effect on hyperlipidemia model of rats 1, 3, 9 g/kg 6 weeks Chen et al.[75] Water extract SD rats Serum and renal tissue Significantly promoted the reduction of TG, TC and LDL-C in rats fed with high-fat diet 0.33, 0.66, 2.00 g/kg·bw 45 d Chen et al.[76] Water extract ICR mice Serum and liver tissue Had the effect of lowering blood lipid, improved the steatosis
of liver cells, and had a good therapeutic effect on fatty liver50, 100, 200 mg/kg 4 weeks Cao et al.[77] Water extract ICR mice Serum AqLA-L treatment regulated the disorders of the serum lipid and liver function, reduced hepatic GLU contents both in normal and HCL mice 0.3, 0.6, 1.2 g/kg 10 d Wang et al.[78] \ SD rats Serum and liver tissue Reduced serum lipid level, improved liver cell lipid accumulation, and increased AMPK α Protein phosphorylation level, activating AMPK α to promote lipid metabolism 1.6, 0.8 g/kg 8 weeks Sun et al.[79] Water extract rats of SPF Serum and liver tissue Had significant improving effect on changes in pathology of the liver tissues in rat models with hyperlipidemia. Its mechanism is probably realized by blocking TLR- 4/NF- κB signaling pathway, and reducing protein expression of TNF-α and IL-2 1.6, 0.8 g/kg 8 weeks Han et al.[80] Antibacterial
activityPolyphenol water extract Staphylococcus aureus Diameter of bacteriostatic ring Significantly inhibited the growth of Staphylococcus aureus 2.5 mg/mL 24 h Shen et al.[81] \ H.pylori Bcterial growth Ihibited he growth of H.pylori 2, 4, 8, 16, 32, 64, 128 μg/mL \ Tan et al.[74] Analgesic effect Water extract SD rats Serum Inhibited the serum MTL level of IBS-D rats and increased serum Sec level 0.94, 1.88, 3.76 g/kg 14 d Xiao et al.[83] Ethanol extract Zebrafish model Total motion distance Had analgesic effect 100 μg/mL 1 h Peng et al.[38] Renal protection Ethanol extract SD rats Renal tissue Mitigated adenine-induced CKD by modulating the metabolic profile and TGF-β/Smad signaling pathway 150 mg/kg 14 d Cai et al.[85] Water extract SD rats Renal tissue Mitigated adenine-induced CKD by modulating the metabolic profile and TGF-β/Smad signaling pathway 150 mg/kg 14 d Cai et al.[85] Inhibition is emptying effect Aqueous extract SD rats Plasma Suppressed gastric emptying rate, increased the content of cAMP, reduced the content of cGMP, increased the ratio of cAMP / cGMP 10 mL/kg 20 min Nie et al.[86] Volatile oil extract SD rats Plasma Suppressed gastric emptying rate, increased the content of cAMP, reduced the content of cGMP, increased the ratio of cAMP / cGMP 10 mL/kg 20 min Nie et al.[86] Ethanol extract SD rats Plasma Reduce the content of cGMP 10 mL/kg 20 min Nie et al.[86] Ether extract SD rats Plasma \ 10 mL/kg 20 min Nie et al.[86] Ethanol-extraction ether extract SD rats Plasma Suppressed gastric emptying rate, increased the ratio of
cAMP/cGMP10 mL/kg 20 min Nie et al.[86] Intestinal microbial regulation \ SD rats Blood and faeces Improved the species diversity of intestinal flora in rats, increased the stability of bacterial community structure, and regulated intestinal microorganisms in rats with alcoholic liver injury 3, 2, 1 g/kg 20 d Xu et al.[87] Antidepressant effect Water extract C57/BL6 mice Blood Reduced the serum level of corticosterone and expression of caspase-3, while increased expression of BDNF in vivo and increased cell viability in corticosterone treated PC12 cells, which was accompanied by decreased caspase-3 expression and the ratio of Bax/Bcl-2 mRNA expression as well as increased BDNF expression in vitro. 30, 100, 300 mg/kg 2 weeks Choi et al.[88] Table 3.
Pharmacological activities of Lindera aggregata.
Figures
(8)
Tables
(3)