-
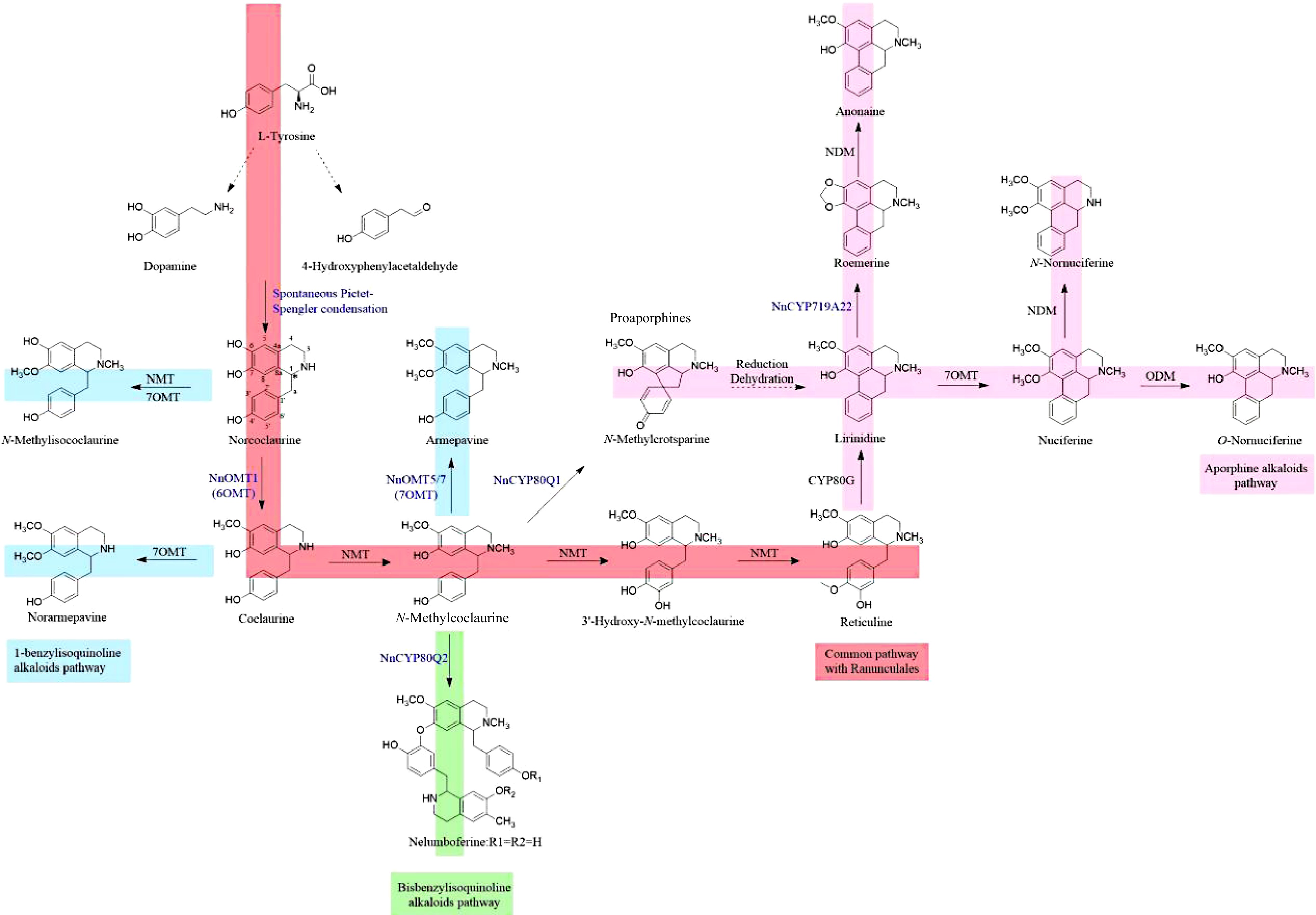
Figure 1.
Possible biosynthetic pathways of BIAs in sacred lotus.
-
No. Alkaloid Formula Enantiomer Organ Reference 1-Benzylisoquinoline 1 Norcoclaurine C16H17NO3 (+)-R and (−)-S L, E [27−30] 2 Coclaurine C17H19NO3 (+)-R L, E, F [27,29,31] 3 Norjuziphine C17H19NO3 NS F [32] 4 Isococlaurine C17H19NO3 NS F [33] 5 N-Methylcoclaurine C18H21NO3 (−)-R L, E, F [27,29,31] 6 6-Demethyl-4'-O-methyl-N-methylcoclaurine C18H21NO3 NS E [29] 7 Norarmepavine C18H21NO3 (+)-R F [31] 8 N-Methylisococlaurine C18H21NO3 NS L, E [29,34] 9 Norroefractine C18H21NO3 NS F [33] 10 Juziphine C17H19NO3 NS F [33] 11 Armepavine C19H23NO3 (−)-R and (+)-S L, E, S [29,31,35,36] 12 4'-O-Methyl-N-methylcoclaurine C19H23NO3 NS E [29] 13 Lotusine C19H24NO3+ NS E [29] 14 Isolotusine C19H24NO3+ NS E [29] 15 4'-O-Methylarmepavine C20H25NO3 NS L [37] Aporphine 16 Caaverine C17H17NO2 (−)-R L [35,38] 17 Asimilobine C17H17NO2 (−)-R L, F [31,38,39] 18 Glaziovine C18H19NO3 N/A F [33] 19 O-Nornuciferine C18H19NO2 (−)-R L, F [13,38,40] 20 N-Nornuciferine C18H19NO2 (−)-R L, E, F [13,29,38] 21 Lirinidine C18H19NO2 (−)-R L, F [13] 22 N-Methylasimilobine C18H19NO2 N/A F [32] 23 Roemerine C18H17NO2 (−)-R L, F [38,40−42] 24 Dehydronuciferine C19H19NO2 N/A L, R [13,34,41] 25 Dehydroanonaine C17H13NO2 N/A L [34] 26 Dehydroroemerine C18H15NO2 N/A L [34] 27 Pronuciferine C19H21NO3 (+)-R and (−)-S L, E, F [13,29,35,37] 28 Nuciferine C19H21NO2 (−)-R L, E, F [29,31,38,40] 29 7-Hydroxydehydronuciferine C19H19NO3 N/A L [38] 30 Lysicamine C18H13NO3 N/A L, F [13] 31 Cepharadione B C19H15NO4 N/A L [32] 32 Anonaine C17H15NO2 (−)-R L, F [38,41] 33 Liriodenine C17H9NO3 N/A L [38] Bisbenzylisoquinoline 34 Nelumboferine C36H40N2O6 NS E, S [41,43] 35 Liensinine C37H42N2O6 1R,1'R L, E, F, S [39−41,44] 36 Isoliensinine C37H42N2O6 1R,1'S E [40,44] 37 Dauriciline C36H40N2O6 NS S [5] 38 6-Hydroxynorisoliensinine C36H40N2O6 NS E [29] 39 N-Norisoliensinine C36H40N2O6 NS E [29] 40 Nelumborine C36H40N2O6 NS E [43] 41 Dauricinoline C37H42N2O6 NS S [5] 42 Neferine C38H44N2O6 1R,1'S E, S [40,41,44] 43 Dauricine C38H44N2O6 NS S, R [45] Tribenzylisoquinoline 1 Neoliensinine C63H70N3O10 1R,1'S,1''R E [44] Table 1.
Benzylisoquinoline alkaloids (BIAs) were identified in several organs of Nelumbo nucifera, together with their respective chemical formulas and stereochemical properties. L, lotus leaf; E, lotus embryo; F, lotus flower; S, lotus seed; R, lotus rhizome.
Figures
(1)
Tables
(1)