-

Figure 1.
Varieties of Bletilla at the traditional March Medicinal Market in Dali, Yunnan, China.
-

Figure 2.
(a)−(d) Bletilla striata (Thunb. ex Murray) Rchb. f. (e)−(h) Bletilla formosana (Hayata) Schltr. (i)−(l) Bletilla ochracea Schltr. (m), (n) Bletilla sinensis (Rolf) Schltr. (o), (p) Bletilla guizhouensis Jie Huang & G.Z. Chen (Photographed by Wang Meina, Zhu Xinxin, and He Songhua).
-

Figure 3.
Bletilla in Compendium of Materia Medica.
-

Figure 4.
Yurong powder made of Bletilla and other traditional Chinese medicines in Jingyue Quanshu.
-

-

-

-

-
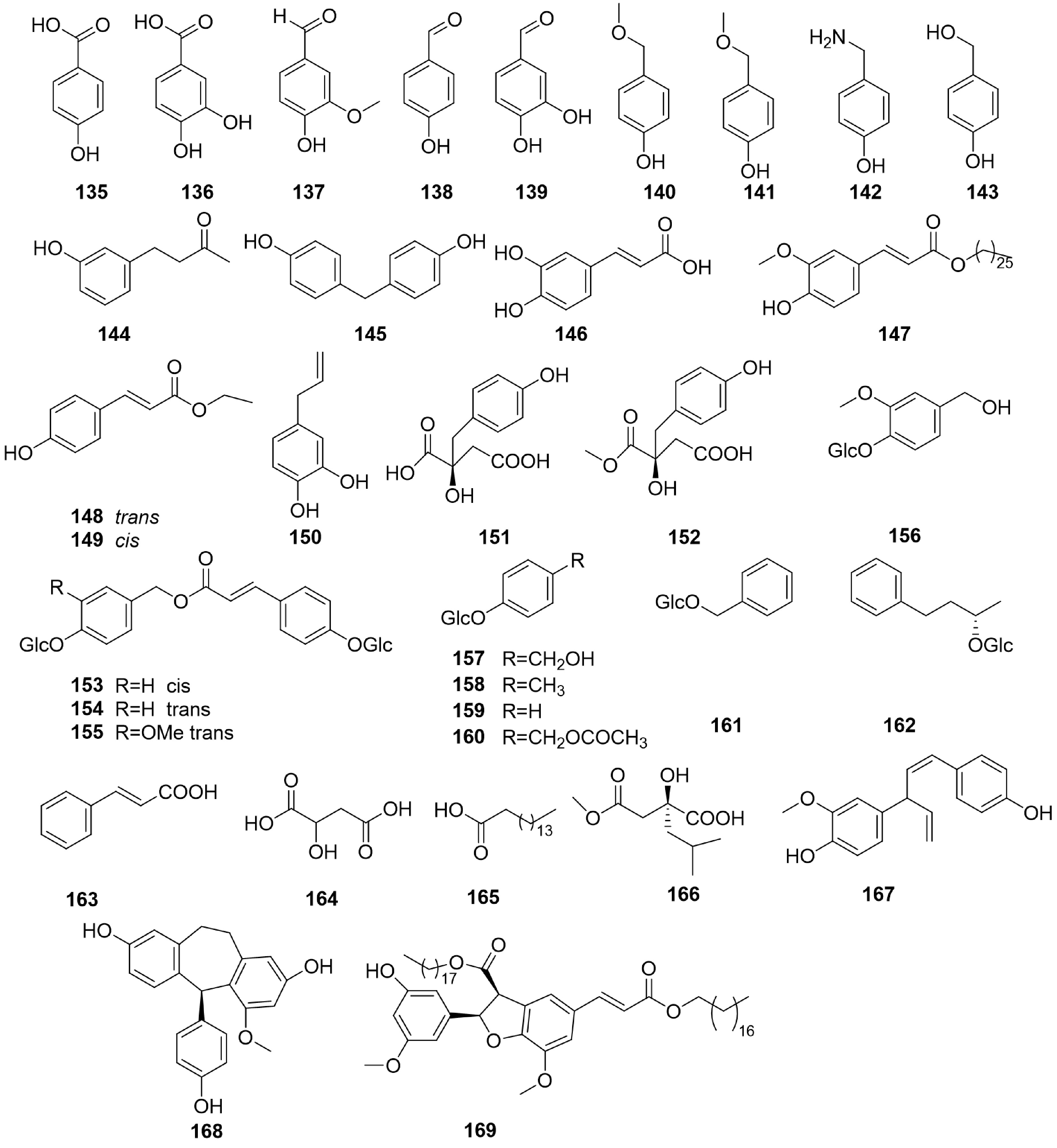
-
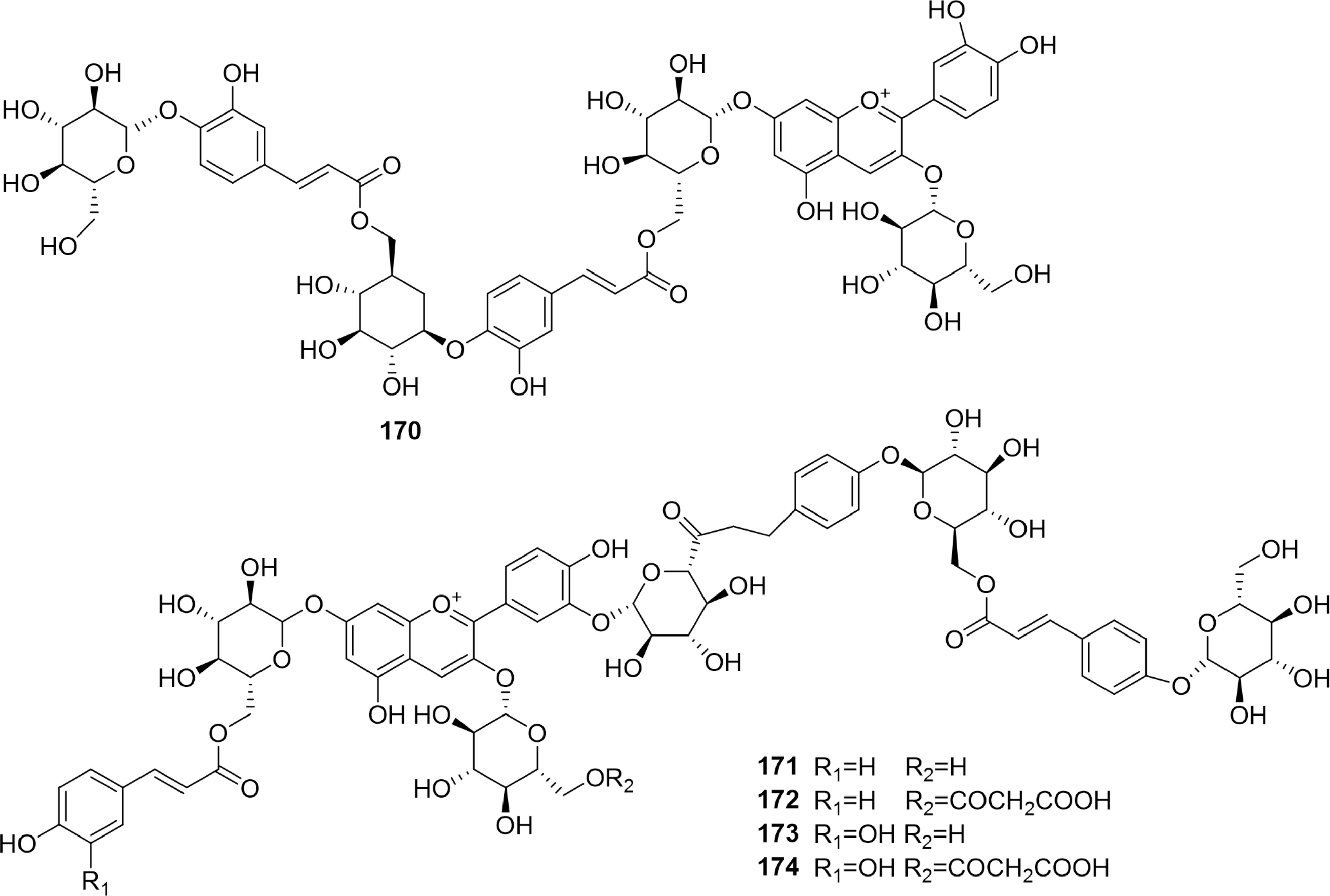
-
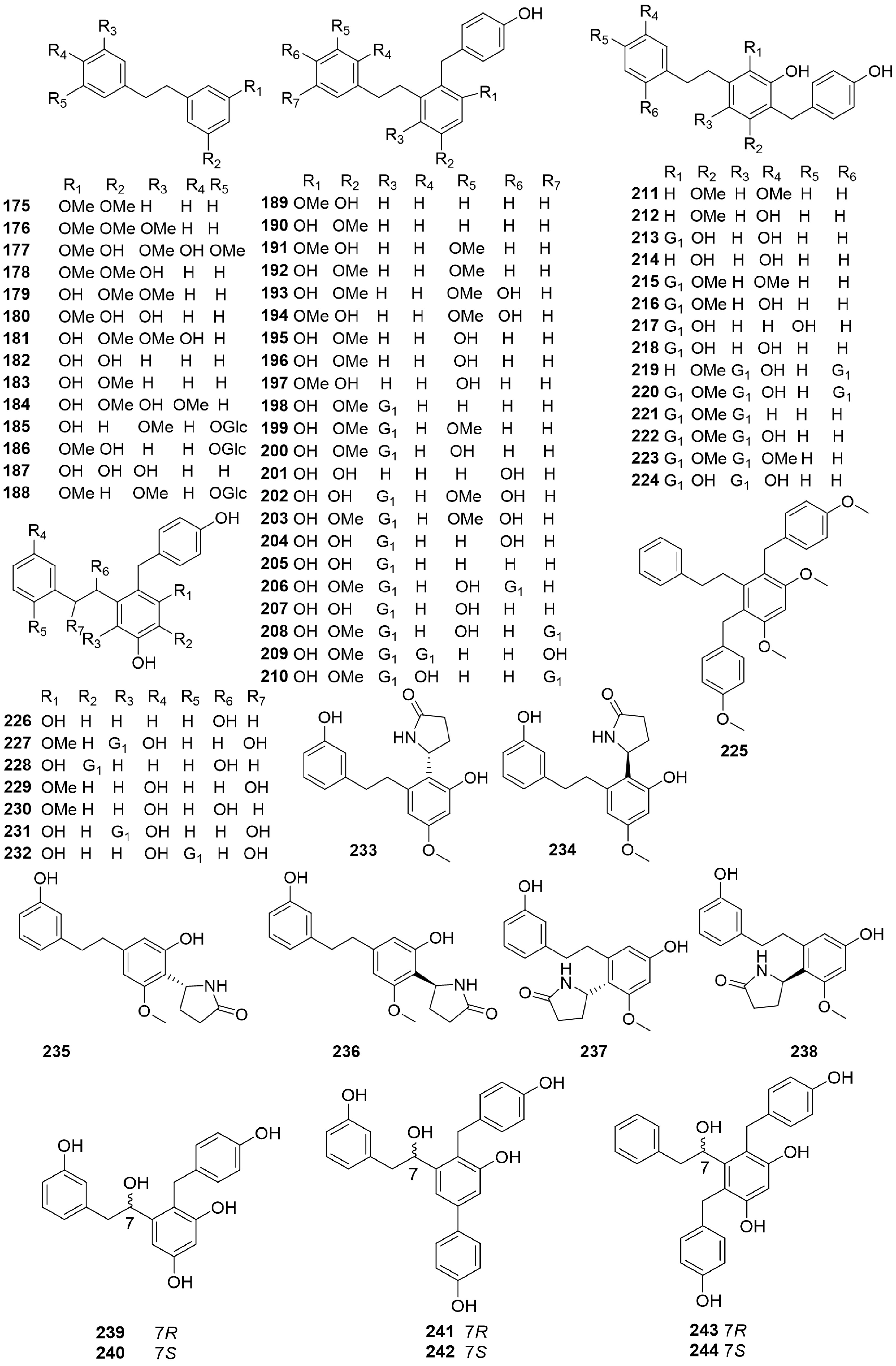
-
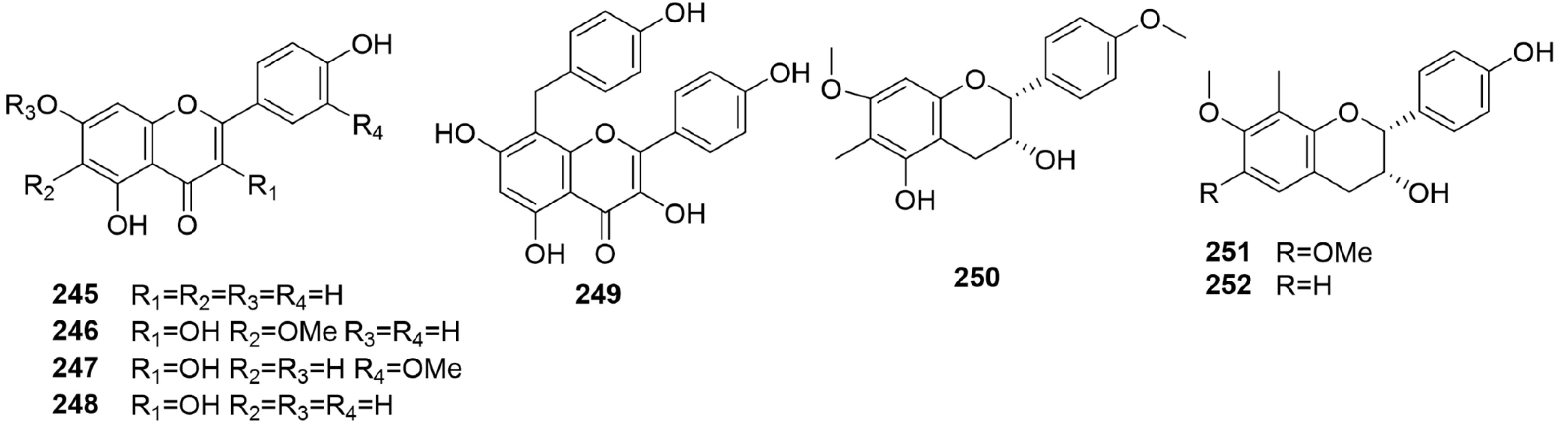
-
-
-
Morphological feature Bletilla striata Bletilla formosana Bletilla ochracea Bletilla foliosa Bletilla guizhouensis Plant height (cm) 18−60 15−80 25−55 15−20 45−60 Rhizome shape Compressed Compressed Somewhat compressed Subglobose Compressed Rhizome diameter (cm) 1−3 1−2 About 2 1−1.5 3−4 Stem characteristics Stout Enclosed by sheaths Stout Stout, short Thin Leaf shape Narrowly oblong Linear-lanceolate Oblong-lanceolate Elliptic-lanceolate Narrowly lanceolate Leaf size (cm) 8−29 × 1.5−4 6−40 × 0.5−4.5 8−35 × 1.5−2.8 5−12 × 0.8−3 25−45 × 1.2−4.5 Flower color Purplish red or pink Pale purple or pink Yellow Pale purple Deep purple Flower size Large Medium Medium Small to medium Large Inflorescence structure Branched or simple Branched or simple Simple Simple Branched Pedicel and ovary length (mm) 10−24 8−12 About 18 7−9 13−17 Sepal shape Narrowly oblong Lanceolate Lanceolate Linear-lanceolate Oblong-elliptic Petal shape Slightly larger than sepals Slightly narrower than sepals Oblique Lanceolate Oblong-elliptic Lip shape Obovate-elliptic Broadly elliptic Narrowly rhombic-obovate Narrowly oblong Narrowly oblong Lip color White with purplish veins Whitish to pale yellow with small dark purple spots Whitish to pale yellow with small dark purple spots White with purplish spots and purple edge White with deep purple edge Number of lip Lamellae 5 lamellae 5 undulate lamellae 5 longitudinal lamellae 3 fimbriate lamellae 7 longitudinal lamellae Column characteristics Subterete, dilated towards apex Subterete, dilated towards apex Slender, dilated towards apex Cylindric, dilated towards apex Suberect, with narrow wings Table 1.
The morphological differences among five species of Bletilla plants native to China.
-
Dynasty (Year) Title Author Original Chinese English translation Late Han
(184−220 AD)Mingyi Bielu / 白给生山谷, 叶如藜芦,
根白相连, 九月采Bletilla grows in the valley, with leaves like Veratrum nigrum L., root is white and connected. September is the time for harvesting. Wei-Jin period
(220−420 AD)WuPu Bencao Wu Pu 白根, 茎叶如生姜, 藜芦,
十月花, 直上, 紫赤色,
根白连, 二月, 八月, 九月采Bletilla, stems and leaves like Zingiber officinale Roscoe and V. nigrum. It blooms in October and is purple and red, the inflorescence is vertical and upward. The roots are white and connected. It can be dug in February, August, and September. the Northern and Southern
(420−589 AD)Bencao Jizhu Tao Hongjing 近道处处有之, 叶似杜若,
根形似菱米, 节间有毛It is everywhere near the road. The leaves are like Pollia japonica Thunb. The roots are like the fruit of Trapa natans L., and internode are many fibrous roots. Tang
(618−907 AD)Su Jing, Zhangsun Wuji, etc Tang materia medica 生山谷, 如藜芦, 根白连, 九月采 Born in the valley, with leaves like V. nigrum, root is white and connected. September is the time for harvesting. Song
(960−1279 AD)Su Song Commentaries on the Illustrations 白芨, 生石山上。春生苗,
长一尺许, 似栟榈及藜芦,
茎端生一台, 叶两指大, 青色,
夏开花紫, 七月结实, 至熟黄黑色。
至冬叶凋。根似菱米, 有三角白色, 角端生芽。二月, 七月采根Bletilla grow on the stone hill. It sprouts in spring and grows about a foot long. The seedlings are like Trachycarpus fortunei (Hook.) H. Wendl. and V. nigrum. The leaves are two finger-size. In summer, it blooms purple flowers and bears fruit in July. The ripe fruit is yellow-black. The leaves wither in winter. The root is like the fruit of T. natans, with three corners, white, and sprouting at the corners. The roots are dug in February and July. Ming
(1368−1644 AD)Li Shizhen Compendium of Materia Medica 一棵只抽一茎, 开花长寸许,
红紫色, 中心如舌, 其根如菱米,
有脐, 如凫茈之脐,
又如扁扁螺旋纹, 性难干Only one stem per herb. The flower is more than one inch long, red and purple, and the center resembles a tongue. Its root is similar to the fruit of T. natans, possessing an umbilicus akin to that of Eleocharis dulcis (N. L. Burman) Trinius ex Henschel. It has spiral veins and is challenging to dry. −, Anonymous. Table 2.
Morphological description of the plants belonging to Bletilla in the ancientChinese medicinal books.
-
Ethnic group Latin name Local name Used part Use method Medicinal effect Achang Bletilla striata (Thunb. ex Murray) Rchb. F. Baiji Tuber After the roots are dried, chew them orally or grind them into powder for external application Tuberculosis, hemoptysis, bleeding from gastric ulcer, burns and scalds Bai Baijier, Goubaiyou, Yangjiaoqi Tuber Treatment of tuberculosis hemoptysis, bronchiectasis hemoptysis, gastric ulcer hemoptysis, hematochezia, skin cracking Dai Yahejie Tuber Used for tuberculosis, tracheitis, traumatic injury, and detumescence De'ang Bagerao Tuber Tuberculosis, hemoptysis, bleeding from gastric ulcer, burns and scalds Dong Shaque, Sanjue Tuber Treat hematemesis and hemoptysis Jingpo Lahoiban, Pusehzuo tuber For tuberculosis, bronchiectasis, hemoptysis, gastric ulcer, hematemesis, hematuria, hematochezia, traumatic bleeding, burns, impotence Meng Moheeryichagan, Nixing Tuber For tuberculosis hemoptysis, ulcer bleeding, traumatic bleeding, chapped hands, and feet Miao Bigou, Wujiu, Sigou Tuber Used for hemoptysis of tuberculosis, bleeding of ulcer disease, traumatic bleeding, chapped hands, and feet Molao Dajieba Tuber Treat internal and external injuries caused by falls Tibetan Sanchabaiji Tuber Fresh chopped and soaked with honey; Powdered after sun-dried, then taken with honey and water Mainly used to treat cough, asthma, bronchitis, lung disease and a few gynecological diseases Tu Ruokeye Tuber After the roots are dried, chew them orally or grind them into powder for external application Treatment of tuberculosis, hemoptysis, bloody stool, chapped skin Wa Baiji Tuber After the roots are dried, chew them orally or grind them into powder for external application For tuberculosis, hemoptysis, gastrointestinal bleeding, scald and burn Yao Biegeidai Tuber Treat gastric ulcer, pulmonary tuberculosis, cough, hemoptysis, and hematemesis Yi Daibaij, Tanimobbaili, Niesunuoqi, Atuluobo Tuber Treatment of tuberculosis, hemoptysis, golden wound bleeding, burns, chapped hands and feet Zhuang Manggounu Tuber Treat stomachache and hemoptysis Bai Bletilla formosana (Hayata) Schltr. Baijier, Yangjiaoqi Tuber After the roots are dried, chew them orally or grind them into powder for external application It is used for emesis, hemoptysis due to tuberculosis, and hemoptysis due to gastric ulcer. External application for treatment of incised wound Miao Lianwu Tuber The effect is the same as that of B. striata Lisu Haibiqiu Tuber It can treat tuberculosis, hemoptysis, epistaxis, golden sore bleeding, carbuncle and swelling poison, scald by soup fire, chapped hands and feet Yi Niesunuoqi, Yeruomaoranruo, Atuluobo, Ribumama, Atuxixi, Abaheiji, Binyue, Ziyou Tuber It is used for tuberculosis, hemoptysis, traumatic injury, treatment of frostbite, burn, scald, bed-wetting of children and other diseases Bai Bletilla ochracea Schltr. Baijier, Yangjiaoqi Tuber After the roots are dried, chew them orally or grind them into powder for external application For hematemesis, epistaxis, hemoptysis due to tuberculosis, hemoptysis due to gastric ulcer; External application of golden sore and carbuncle Meng Moheeryichagan, Nixing tuber The effect is the same as that of B. striata Table 3.
The traditional medicinal knowledge of Bletilla in ethnic communities, China.
-
Pharmacological activity Tested substance/part Tested system/organ/cell Tested dose/dosing method Results Refs. Anti-inflammatory Ethanol extract of Bletilla striata RAW264.7 cells RAW264.7 cells were pre-treated with ethanol extract of B. striata for 1 h and then stimulated with LPS (200 ng/mL) for 12 h, 0.05% DMSO was applied as the parallel solvent control. The culture supernatant was collected for IL-6 and TNF-α detection. Ethanol extract of B striata significantly inhibited LPS-induced interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) expression at 2.5 µg/mL. [41] The ethyl acetate-soluble (EtOAc) extract of tubers of B. striata H2O2-induced PC12 cell injury model PC12 cells were seeded in 96-well multiplates at a density of 1.5 × 105 cells/mL. After overnight incubation at 37 °C with 5% CO2, 10 μM test samples and H2O2 (final concentration of 450 μM) were added into the wells and incubated for another 12 h. It protected the cells with the cell viabilities of 57.86 ± 2.08%, 64.82 ± 2.84%, and
64.11 ± 2.52%.[98] Ethanol extract of tubers of B. striata RAW264.7 cells Cells were treated with ethanol extracts (25 μM) dissolved in DMSO, in the presence of
1 μg/mL lipopolysacchride
(LPS) for 18 hThe anti-inflammatory activity with IC50 of 2.86 ± 0.17 μM. [54] PE extract of the tubers of B. striata LPS-stimulated BV2 cells Cells treated with extract
(0, 1, 10, 30, 100 μg/mL) and dihydropinosylvi (0, 1, 10, 30, 100 μM) in presence of LPS
(1 μg/mL)The anti-inflammatory activity with IC50 values of 96.0 μM. [96] Ethanol extract of the roots of B. striata Cox-1 and Cox-2 Treated with the ethanol extracts at various concentrations
(0, 1, 10, 100 μM)The compounds with sugar moieties displayed selective inhibition of Cox-2 (N90%). [38] B. striata polysaccharide (BSPb) Human mesangial cells (HMCs) HMCs were pre-treated with BSPb (5, 10, 20 μg/mL) BSPb efficiently mediated expression of NOX4 and TLR2, to attenuate generation of ROS and inflammatory cytokines. [12] Compounds extracted from the rhizomes of Bletilla ochracea RAW264.7 cells After 24 h preincubation,
cells were treated with serial dilutions in the presence of
1 μg/mL LPS for 18 h. Each compound was dissolved in DMSO and further diluted in medium to produce different concentrations. NO production in the supernatant was assessed by adding 100 μL of Griess regents.It showed the inhibitory effects with IC50 values in the range of 15.29–24.02 μM. [76] Compounds extracted from the rhizomes of B. ochracea Murine monocytic RAW264.7 cells After 24 h preinubation, RAW 264.7 cells were treated with compounds (25 μM) dissolved in DMSO, in the prenence of
1 μg/mL LPS for 18 h. NO production in each well was assessed by adding 100 μL of Giress regentIt showed the inhibitory effects with IC50 2.86 ± 0.17 μM. [86] Compounds extracted from the rhizomes of Bletilla formosana Elastase Release Assays Neutrophils (6 × 105 cells/mL) were equilibrated in MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide (100 μM) at 37 °C for 2 min and then incubated with a test compound or an equal volume of vehicle (0.1% DMSO, negative control) for 5 min. Most of the isolated compounds were evaluated for their anti-inflammatory activities. The results showed that IC50 values for the inhibition of superoxide anion generation and elastase release ranged from 0.2 to 6.5 μM and 0.3 to 5.7 μM, respectively. [49] Anti-tumor Two compounds from Bletilla striata A549 cells Compounds were tested for their ability to induce ROS generation in A549 cells at concentrations of 20 two compounds for 24 h, the cells were harvested to evaluate the ROS production. The two compounds exhibited antiproliferative effects using the MTT test; these effects may be due to cell cycle arrest and inducing ROS generation. [87] Stilbenoids from B. striata BCRP-transduced K562 (K562/BCRP) cells — It showed antimitotic activity and inhibited the polymerization of tubulin at IC50 10 μM. [78] Compounds extracted from the rhizomes of B. ochracea The human tumor cell lines HL-60 (acute leukemia), SMMC-7721 (hepatic cancer), A-549 (lung cancer), MCF-7 (breast cancer), and SW480 (colon cancer) 100 μL of adherent cells were seeded into each well of 96-well cell culture plates. After 12 h of incubation at 37 °C, the test compound was added. After incubated for 48 h, cells were subjected to the MTS assay. All isolated metabolites except 7 were evaluated for cytotoxic activity against five human cancer cell lines (HL-60, SMMC7721, A-549, MCF-7 and SW480). [76] Antiviral The tuber of B. striata Madin-Darby canine kidney model and embryonated eggs model As simultaneous treatment with 50% inhibition concentration (IC50) ranging from 14.6 ± 2.4 to 43.3 ± 5.3 μM. Phenanthrenes from B. striata had strong anti-influenza viral activity in both embryonated eggs and MDCK models. [107] The 95% ethanol
Extract of B. striataBALB/C mice — It has significant anti-influenza
virus effect in mice, which may be related to the increase of IL-2, INFα, INF-β and thus enhance the immune function of mice.[12] Antioxidant Compounds extracted from the rhizomes of B. formosana DPPH radical-scavenging assay Solutions containing 160 μL of various concentrations of sample extract, 160 μL of various concentrations of BHA, 160 μL of various concentrations of ascorbic acid, and the control (160 μL of 75% methanol) were mixed separately with 40 μL of 0.8 mM DPPH dissolved in 75% methanol. Each mixture was shaken vigorously and left to stand for 30 min at room temperature in the dark. Tthe seedlings grown by tissue culture of B. formosan collected in Yilan County had the best antioxidant capacity. In addition, B. formosana collected in Taitung County had the best scavenging capacity in the tubers, leaves and roots. [93] Fibrous roots of B. striata DPPH model and superoxide anion system The ABTS+ solution was prepared by reacting 7 Mm ABTS with 2.45 mM potassium persulfate (final concentrations both dissolved in phosphate buffer, 0.2 M, pH 7.4) at room temperature for 12–16 h in the dark. It removed free radicals and inhibit tyrosinase activity. [33] B. striata extracts (BM60) The murine macrophage cells NR8383, male SD mice (180~200 g) NR8383 were pretreated with extracts (1, 10 and 100 g/mL) for 4 h and then 65 stimulated with 1 g/mL of LPS for 24 h. Acute lung injury was induced in mice by nonhexposure intratracheal instillation of LPS (3.0 mg/kg). Administration of the BM60 extract of 35, 70, and 140 mg/kg (L, M, H) was performed by oral gavages. The BM60 treatment reduced the production of NO in NR8383 macrophages. Treatments with BM60 at the doses of 35, 70, 140 mg/kg significantly reduced macrophages and
neutrophils in the bronchoalveolar lavage fluid (BALF).[12] The crude
polysaccharides obtained from B. striataDPPH free radical scavenging activity Concentration
range of 2.5–5.0 mg/mLThe IC50 of BSPs-H was 6.532 mg/mL. [35] Hemostasis B. striata polysaccharide (BSP) Diabetes mellitus (DM) mouse models were induced by high fat-diet feeding combined with low-dose streptozocin injection DM mouse models were induced by high fat-diet feeding combined with low-dose streptozocin injection. The BSP solutions were applied on the surface of each wound at a volume of 50 μl. RD mice were assigned as normal controls and received saline treatment (n = 6). All mice were treated with vehicle or BSP once daily from the day of wounding (d0) until 12 days later (d12). BSP administration accelerated diabetic wound healing, suppressed macrophage infiltration, promoted angiogenesis, suppressed NLRP3 inflammasome activation, decreased IL-1β secretion, and improved insulin sensitivity in wound tissues in DM mice. [112] B. striata Micron Particles (BSMPs) Tail amputation model and healthy male Sprague-Dawley (SD) rats
(250 ± 20 g, 7 weeks of
age)Rats were divided into six groups of five treated with cotton gauze and BSMPs (350–250, 250–180, 180–125, 125–75, and < 75 μm), respectively. Compared to other BSMPs of different size ranges, BSMPs of 350–250 μm are most efficient in hemostasis. As powder sizes decrease, the degree of aggregation between particles and hemostatic BSMP effects declines. [109] Rhizoma Bletillae polysaccharide (RBp) Adult male SD rats weighing 220 ± 20 g After incubation for 1 min at 37 °C, 300 μL of PRP was dealt with different concentrations of RBp (50, 100, 150, and 200 mg/L) under continuous stirring, and the vehicle was used as the blank control. RBp significantly enhanced the platelet aggregations at concentrations of 50−200 mg/L in a concentration-dependent manner. [113] Antibacterial Bibenzyl derivatives from the tubers of Bletilla striata S. aureus ATCC 43300, Bacillus subtilis ATCC 6051, S. aureus ATCC 6538 and Escherichia coli ATCC 11775 Using a microbroth dilution method, bacteria were seeded at
1 × 106 cells per well (200 μL) in a
96-well plate containing Mueller-Hinton broth with different concentrations (from 1 to 420 μg/mL, 300 μg/mL and so on;
2-fold increments) of each test compound.It showed inhibitory activities with MIC of (3–28 μg/mL) against S. aureus ATCC6538 [116] The crude extract of B. striata S. album, A. capillaris, C. cassia They were seeded at 1 × 106 cells per well (200 μL) in a 96-well plate containing Mueller−Hinton broth (meat extracts 0.2%, acid digest of casein 1.75%, starch 0.15%) with different concentrations (from 1 to 128 μg/mL; 2-fold increments) of each test compound. It showed S. album (0.10%), A. capillaris (0.10%), and C. cassia (0.10%) to have the strongest antibacterial properties. [118] The ethyl acetate-soluble (EtOAc) extract of tubers of B. striata S. aureus ATCC 43300, S. aureus ATCC 6538, and Bacillus subtilis ATCC 6051) and Escherichia coli ATCC 11775) Bacteria were seeded at 1 × 106 cells per well (200 μL) in a 96-well plate containing Mueller Hinton broth with different concentrations (from 1 to 420 μg/ml; 2-fold increments) of each test compound. The extract was effective against three Gram-positive bacteria with minimum inhibitory concentrations (MICs) of 52–105 μg/ml. [98] The phenanthrene fraction (EF60) from the ethanol extract of fibrous roots of Bletilla striata pseudobulbs S. aureus ATCC 25923, S. aureus ATCC 29213, S. aureus ATCC 43300, E. coli ATCC 35218, and P. aeruginosa ATCC 27853, Bacillus subtilis 168 EF60 was active against all tested strains of Staphylococcus aureus, including clinical isolates and methicillin-resistant S. aureus (MRSA). The minimum inhibitory concentration (MIC) values of EF60 against these pathogens ranged from 8 to 64 μg/mL. EF60 could completely kill S. aureus ATCC 29213 at 2× the MIC within 3 h but could kill less than two logarithmic units of ATCC 43300, even at 4× the MIC within 24 h. The postantibiotic effects (PAE) of EF60 (4× MIC) against strains 29213 and 43300 were 2.0 and 0.38 h, respectively. [117] Anti-adhesive Bletilla striata extraction solution PPA was induced by cecal wall abrasion, and Bletilla striata was injected to observe its efect on adhesion in rats The rats in the sham operation group was not treated; the other rats of the three experimental groups were intraperitoneally injected with 8 ml of phosphate-buffered saline (Control group), 15% Bletilla striata extraction solution (BS group), and 0.2% hyaluronic acid solution (HA group), respectively. Bletilla striata decreased the development of abdominal adhesion in abrasion-induced model of rats and reduced the expression of the important substance which increased in PPAs. [120] Immunomodulatory B. striata polysaccharide (BSPF2) Mouse spleen cells To observe the immune activity of BSPF2, mouse spleen cells were stimulated with BSPF2 at 10–100 g/mL for 72 h. Immunological assay results demonstrated that BSPF2 significantly induced the spleen cell proliferation in a dose-dependent manner. [121] Anti-pulmonary fibrosis B. striata polysaccharide Clean grade male SD rats SD rats were randomly divided into 5 groups, sham operation group (equal volume of normal saline), model group (equal volume of normal saline), tetrandrine positive control (24 mg/kg) group and white and Polysaccharide low
(100 mg/kg) and high (400 mg/kg) dose groups.The Bletilla striata polysaccharide has remarkable regulation effect on anti-oxidation system and immune system, but cannot effectively prevent lung fibrosis. [127] Small molecule components of Bletilla striata Clean grade male SD rats SD rats were randomly divided into 5 groups, sham operation group (0.5 mL normal saline), model group (0.5 mL normal saline), and positive control group (tetrandrine 24 mg/kg) and low (20 mg/kg) and high (40 mg/kg) dosage groups of the small molecule pharmacological components of Bletilla, which were administered by gavage once a day for 2 consecutive months. The small molecule components of Bletilla striata can effectively prevent lung fibrosis though regulating the anti-oxidation system,immune system and cytokine level; SMCBS is one of the active components of Bletilla striata on silicosis therapy [124] —, not given. Table 4.
Summary of the pharmacological activities of Bletilla species.
Figures
(14)
Tables
(4)