-
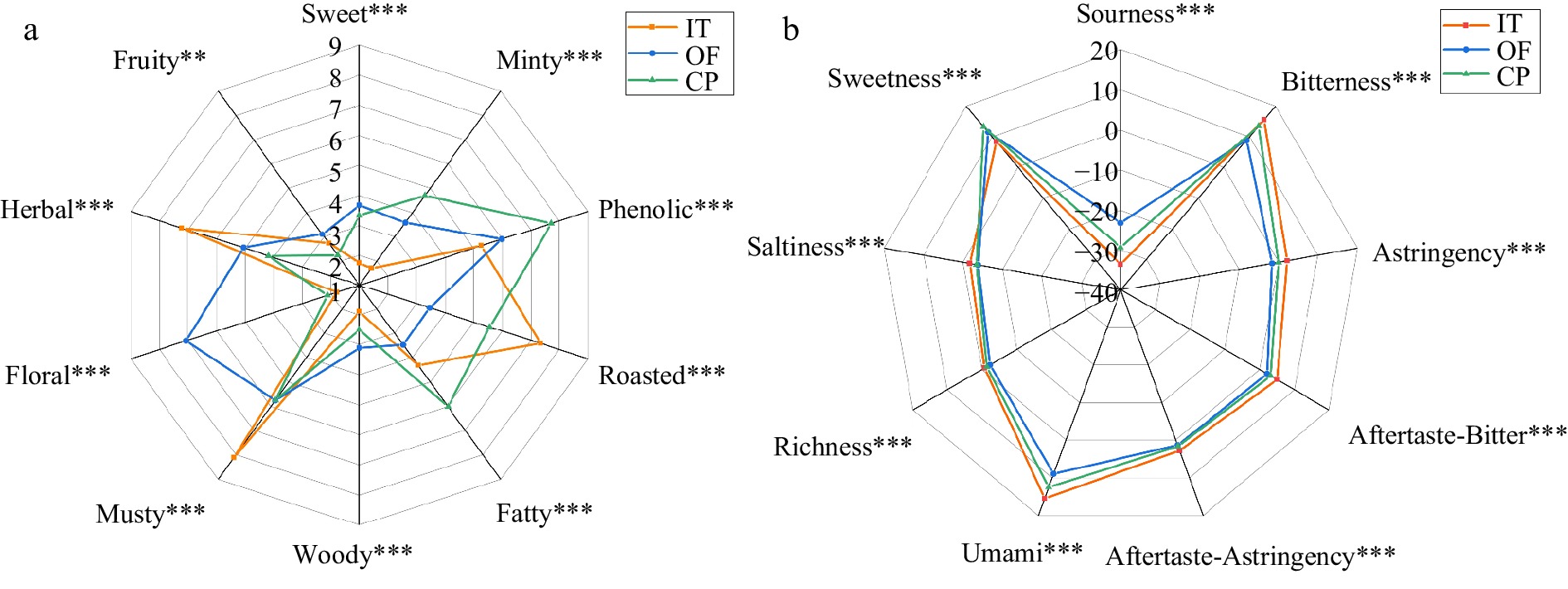
Figure 1.
Sensory spider plot of three CPT infusion samples, (a) sensory evaluation of three CPT samples based on ten aroma attributes, (b) taste profiles of three CPT samples by E-tongue. Note: *, ** and *** significant at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001.
-
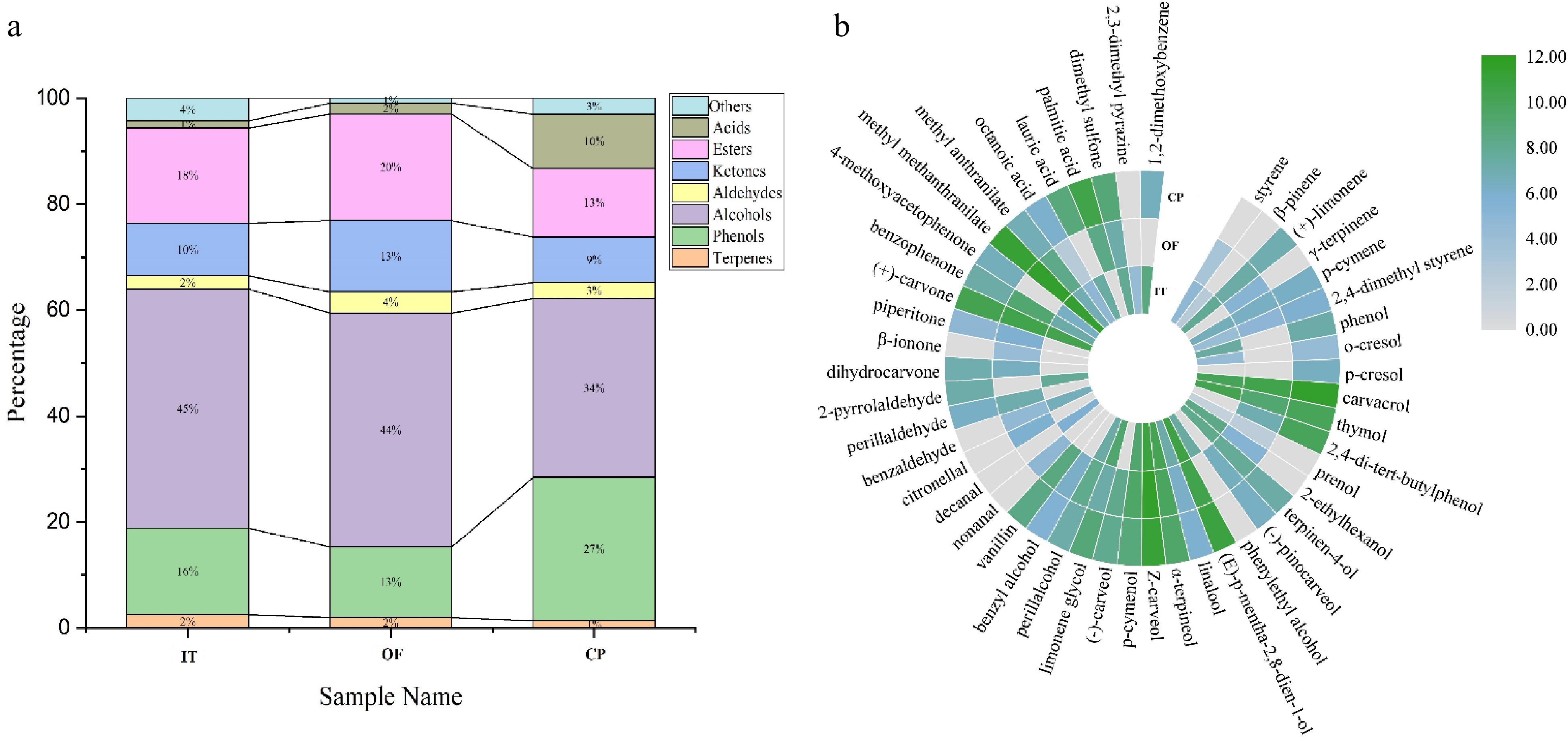
Figure 2.
Distribution map of volatile aroma substances, (a) species distribution profile of volatile compounds, (b) concentration distribution of each volatile compounds in three CPT infusion samples.
-
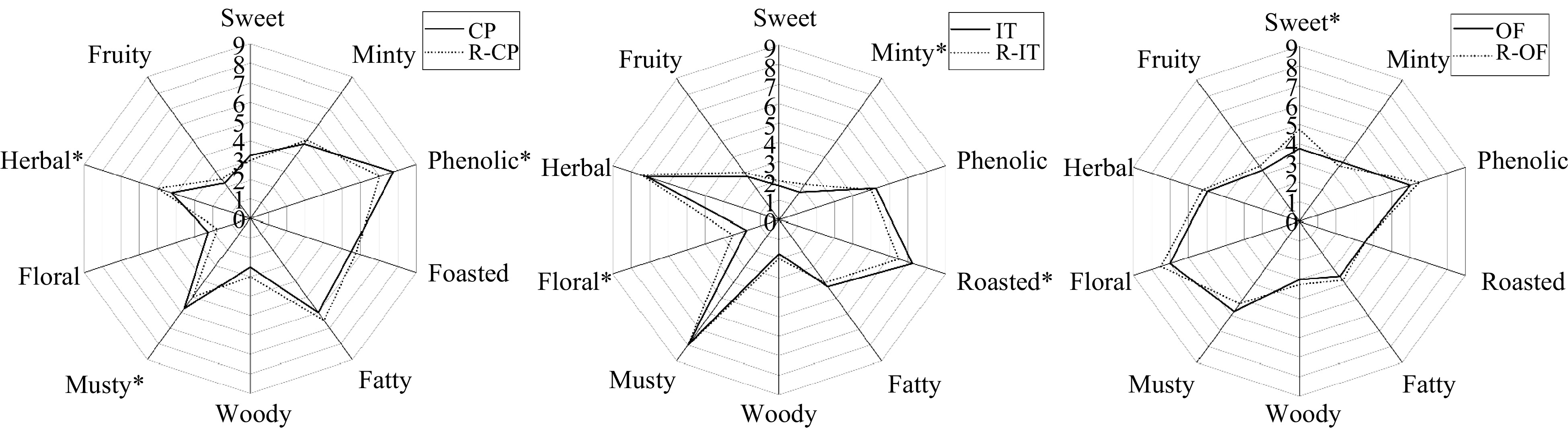
Figure 3.
Descriptive sensory analysis radar diagram of recombination model and corresponding CPT samples. Note: The sensorial parameters indicated with * are significantly different between samples (p ≤ 0.05).
-
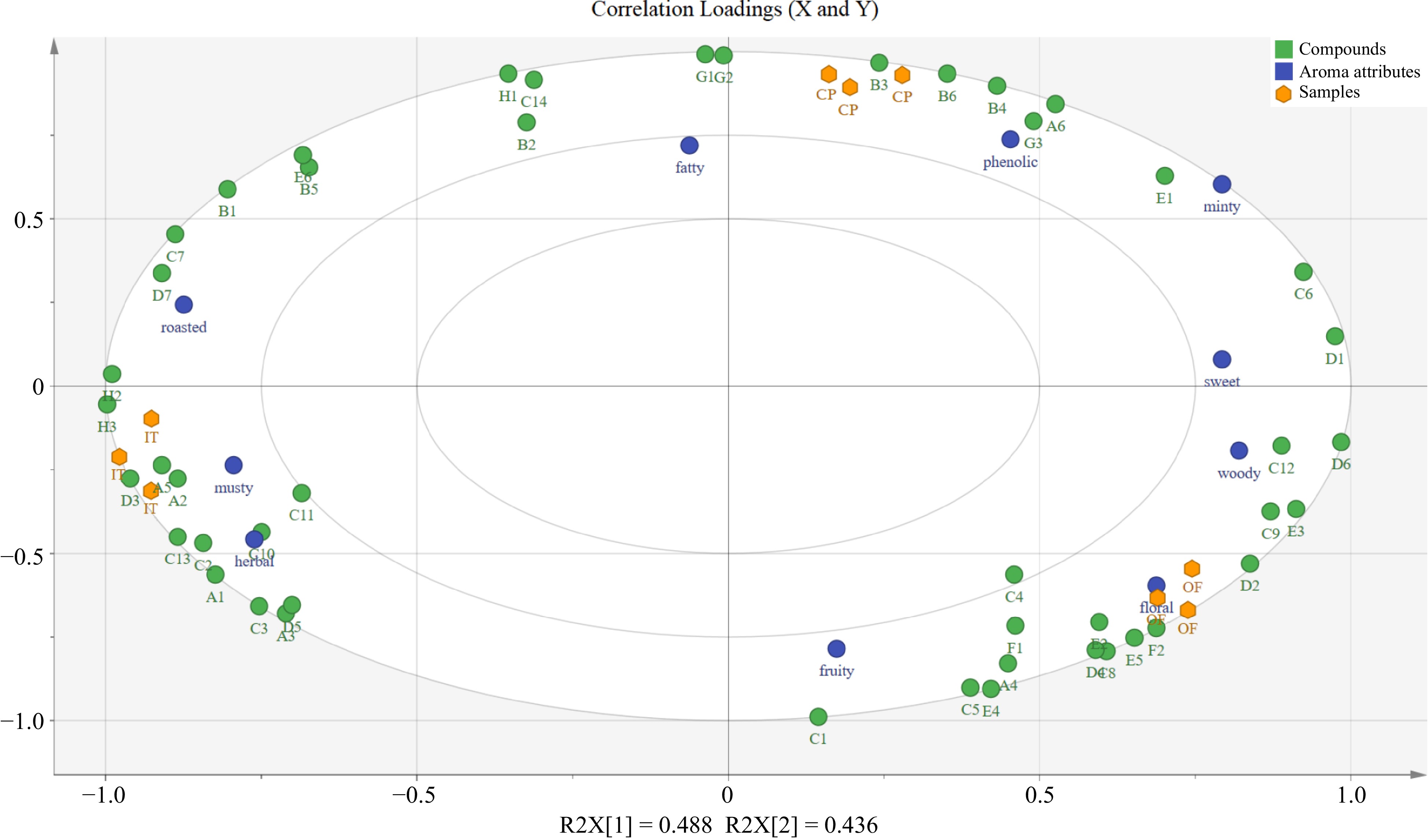
Figure 4.
Correlation loadings plot for aroma-active compounds (X-matrix) and sensory attributes (Y-matrix) of three CPT samples.
-
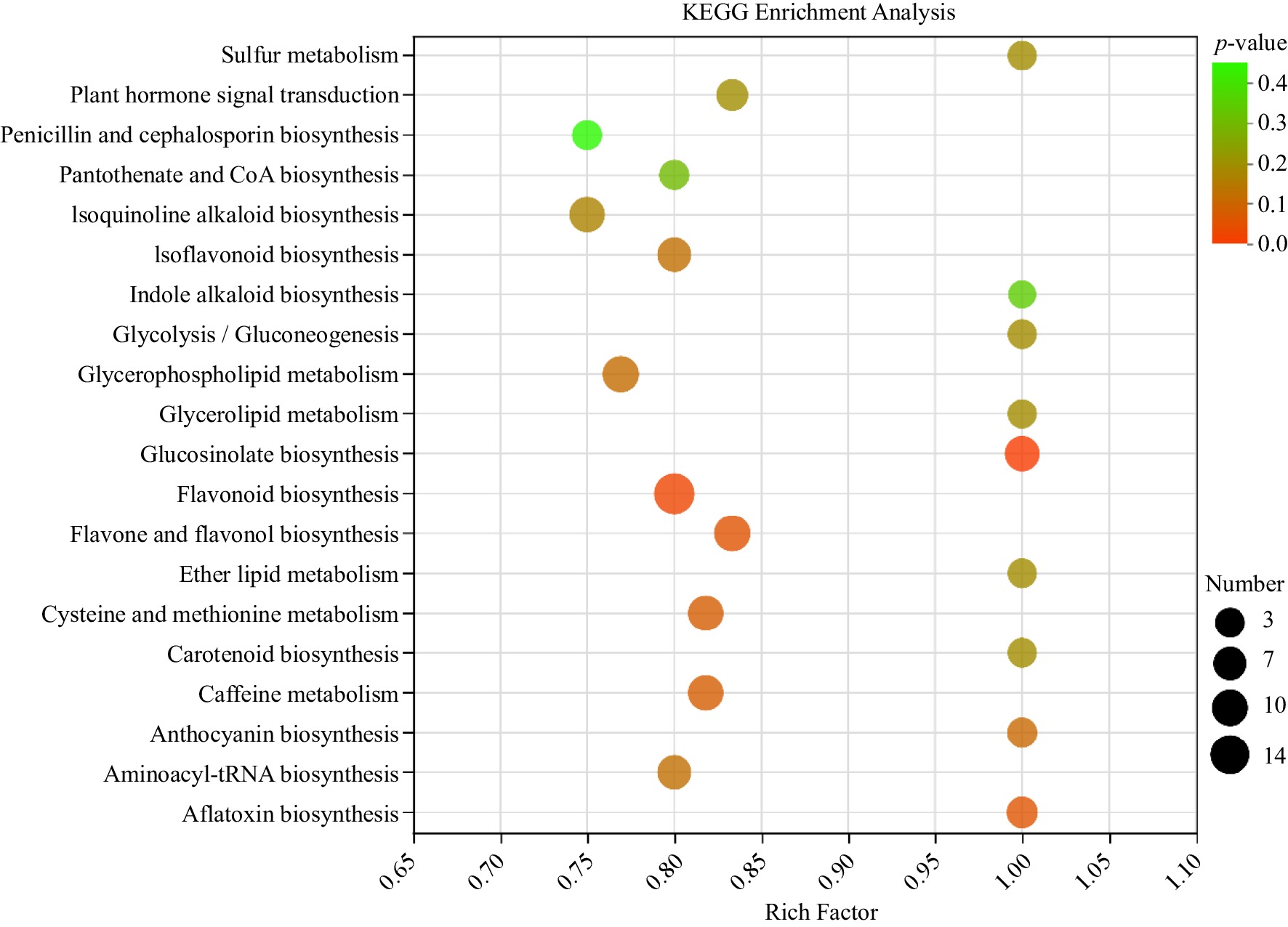
Figure 5.
KEGG enrichment analysis of TOP20 metabolic pathways in untargeted metabolomics.
-
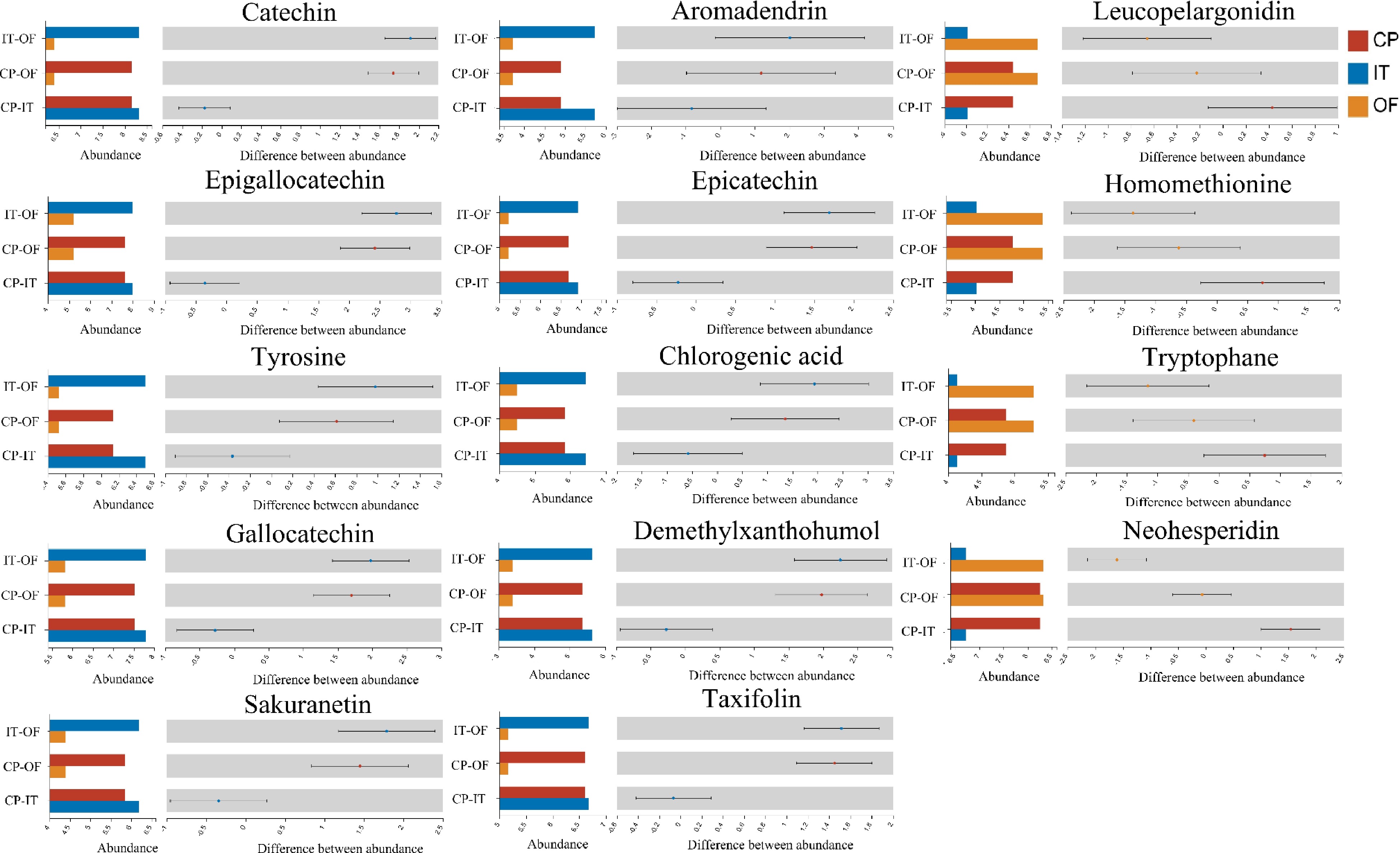
Figure 6.
Non-volatile compounds that significantly contributed to taste of three CPT samples.
-
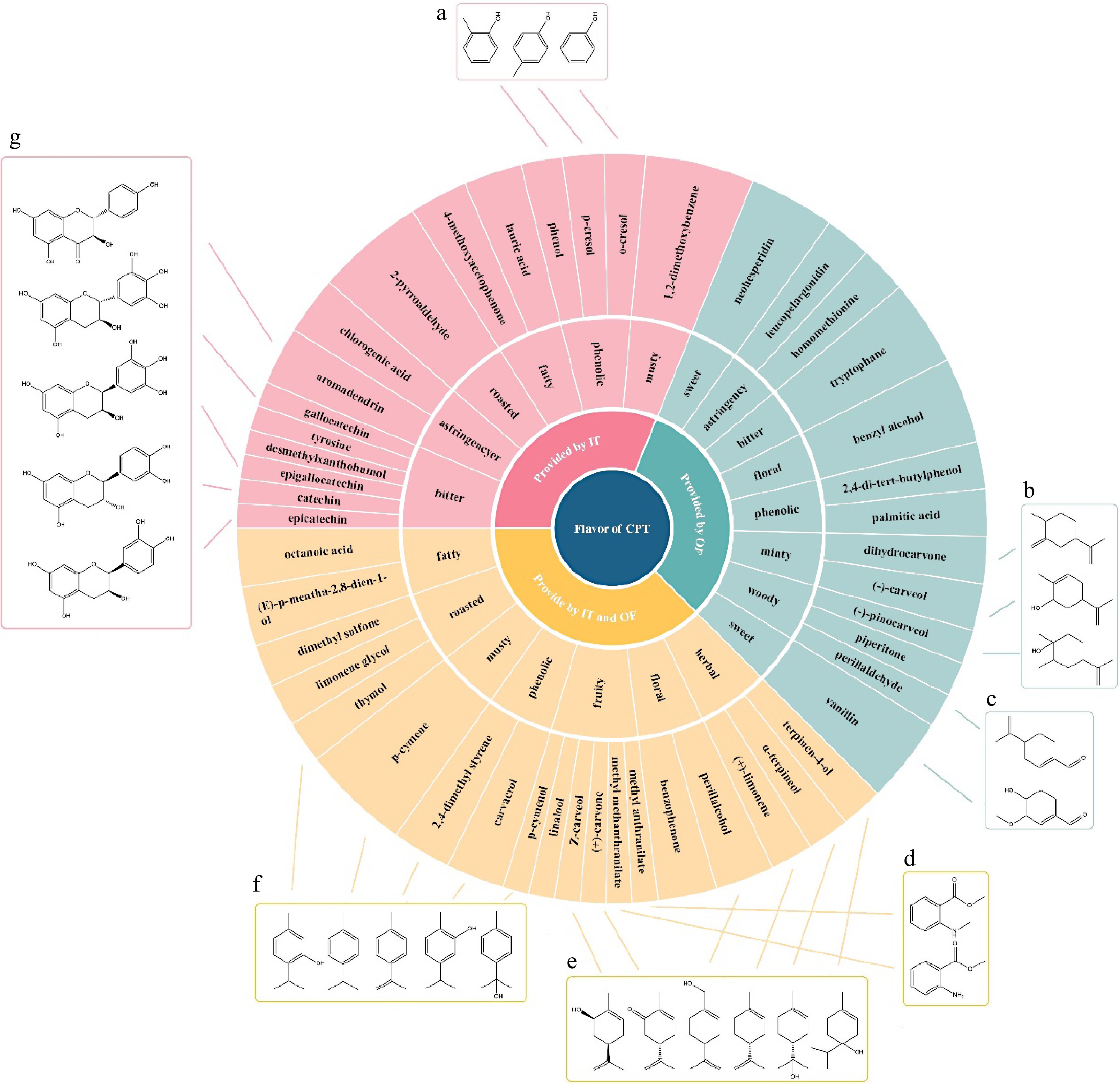
Figure 7.
Flavor wheel of key flavor compounds of CPT infusion samples. Compounds marked with molecular structure in frames (a)−(g) were specific to the IT, OF or both IT and OF.
-
No. Compounds RIa Aroma descriptionb FDc IMd HP-INNOWAX HP-5MS cal. ref. cal. ref. IT OF CP Alkenes A1 styrene 1295 1272 − 890 sweet, balsam, floral(acacia) 2 1 − MS, RI, S, O A2 β-pinene 1135 1115 − 978 woody, pine, hay, green 2 − − MS, RI, S, O A3 (+)-limonene 1232 − 1001 − citrus, herbal, sweet 4 4 4 MS, RI, S, O A4 γ-terpinene 1277 1255 − 1064 oily, woody, lime, herbal − 1 − MS, RI, S, O A5 p-cymene 1305 1280 1103 1026 musty, woody, spice 1024 1024 1024 MS, RI, S, O A6 2,4-dimethyl styrene 1459 1433 1076 1078 phenolic, spicy, soil, plastic 2048 64 2 MS, RI, S, O Phenols B1 phenol 2037 2028 − 981 phenolic, plastic, rubber 2 − 4 MS, RI, S, O B2 o-cresol 1998 2010 1060 musty, phenolic, herbal, leathery 1 − 1 MS, RI, S, O B3 p-cresol 2950 2079 1097 1098 phenolic, floral(narcissus) − − 8 MS, RI, S, O B4 carvacrol 2226 2225 1296 1307 spice, woody, phenolic 2048 1024 1024 MS, RI, S, O B5 thymol 2239 2172 1292 1297 herbal, phenolic, roasted 1024 1024 1024 MS, RI, S, O B6 2,4-di-tert-butylphenol 2323 2330 1512 1513 phenolic − 2048 2048 MS, RI, S, O Alcohols C1 prenol 1340 1323 − 778 fruity, green, floral(lavender) 2 4 − MS, RI, S, O C2 linalool 1553 1549 1101 1104 herbal, green, floral(rose), 16 8 8 MS, RI, S, O C3 α-terpineol 1714 1170 1190 1191 pine, citrus, woody, floral(lilac) 1024 512 32 MS, RI, S, O C4 Z-carveol 1842 1869 1220 1220 vegetable,green, caraway 16 8 4 MS, RI, S, O C5 p-cymenol 1868 1851 1185 1188 sweet, fruity(cherry), camphor 128 8 4 MS, RI, S, O C6 (-)-carveol 1884 1846 1231 1225 minty, green, herbal, spicy − 4 4 MS, RI, S, O C7 limonene glycol 2298 2325 1345 1342 minty, roasted, 2 1 1 MS, RI, S, O C8 perillalcohol 2018 2021 − 1300 spicy(cardamom), floral(violet) 1 2 1 MS, RI, S, O C9 benzyl alcohol 1903 1885 − 1034 floral(rose), phenolic − 2048 2 MS, RI, S, O C10 2-ethylhexanol 1496 1490 − 1026 citrus, fresh, floral, oil, sweet 2048 4 − MS, RI, S, O C11 terpinen-4-ol 1602 1636 1177 herbal, woody, earthy, musty 4 32 64 MS, RI, S, O C12 (-)-pinocarveol 1679 1666 1137 1140 warm, woody, fennel, cereal − 4 2 MS, RI, S, O C13 phenylethyl alcohol 1940 1923 − 1121 floral(rose) 16 − − MS, RI, S, O C14 (E)-p-mentha-2,8-dien-1-ol 1645 1641 1120 1121 fatty, popcorn, minty 4 4 1024 MS, RI, S, O Aldehydes D1 vanillin 2615 2550 1397 1394 sweet(chocolate), creamy − 1 4 MS, RI, S, O D2 nonanal 1410 1396 − 1102 waxy, fatty, orange − 64 − MS, RI, S, O D3 decanal 1513 1504 − 1195 sweet, waxy, citrus(orange), floral 4 − − MS, RI, S, O D4 citronellal 2023 1488 − 1158 sweet, floral, herbal, waxy, citrus − 4 − MS, RI, S, O D5 benzaldehyde 1556 1529 − 961 almond, fruity(cherry) 16 8 − MS, RI, S, O D6 perillaldehyde 1814 1807 1276 1279 woody, pine, sweet(balsam), minty − 32 16 MS, RI, S, O D7 2-pyrrolaldehyde 2065 2048 1013 1015 musty, beefy, burnt, roasted, smoky 1024 − 1024 MS, RI, S, O Ketones E1 dihydrocarvone 1636 1645 1198 1200 herbal, minty, rubber, rice − 16 8 MS, RI, S, O E2 β-ionone 1964 1953 1485 1490 powdery, floral(orris), woody − 8 − MS, RI, S, O E3 piperitone 1758 1743 1266 1268 woody, minty, camphor − 8 4 MS, RI, S, O E4 (+)-carvone 1764 1744 1244 1245 minty, fruity, spice 1024 2048 1024 MS, RI, S, O E5 benzophenone 2533 2505 − 1625 floral (rose, geranium) 4 4 2 MS, RI, S, O E6 4-methoxyacetophenone 2106 2120 1341 1345 fatty, sweet, anisic 2 − 2 MS, RI, S, O Esters F1 methyl methanthranilate 2100 2068 1408 1402 fruity, musty, sweet 2048 2048 2048 MS, RI, S, O F2 methyl anthranilate 2283 2257 − 1338 floral (orange flower), fruity(grape) 1024 2048 1024 MS, RI, S, O Acids G1 octanoic acid 2033 2070 1191 fatty, waxy, rancid, oily, green, cheesy 2 1 4 MS, RI, S, O G2 lauric acid 2489 2502 1570 fatty, fruity(coconut), oily 2 − 2 MS, RI, S, O G3 palmitic acid 2512 2890 1968 1964 phenolic, waxy, fatty − 2 4 MS, RI, S, O Others H1 dimethyl sulfone 1944 1912 − 915 roasted, sulfurous, burnt 2 2 2 MS, RI, S, O H2 2,3-dimethyl pyrazine 1373 1352 − 911 nutty, butter, coffee, caramel, roasted 4 − − MS, RI, S, O H3 1,2-dimethoxybenzene 1743 1740 1145 1143 musty, creamy, phenolic, sweet 2 − 1 MS, RI, S, O a Retention index of compounds on an HP-INNOWAX column and HP-5MS column. Cal means the RI value calculated by the formula. Ref means the RI value confirmed by comparison retention index to reference standards in the same condition (https://webbook.nist.gov/). b Aroma description. The aroma description vocabulary was generated by the GC-O evaluation team by comparing the aroma characteristics at actual concentrations with the literature and spectral library descriptions. c FD factor, flavor dilution factor determined on a HP-INNOWAX column. '−' means not being detected. d Identification method: MS means identified by comparison with the NIST mass spectral library 11 Vision database; RI means confirmed by comparison retention index; S means confirmed by authentic standard chemicals; O means confirmed by aroma descriptor. Table 1.
Identification analysis of volatile compounds in citrus Pu-erh tea samples.
-
No. Compounds OT (mg/kg)A Concentration (mg/kg)B OAVC ACI%D IT OF CP IT OF CP IT OF CP A1 styrene 0.065 26.54a 8.29b − 408.26 127.49 − 0.0526 0.0041 − A2 β-pinene 0.14 4.09a − − 29.24 − − 0.0038 − − A3 (+)-limonene 0.034 233.62a 159.49b 120.07c 6871.15 4691.00 3531.55 0.8852 0.1513 0.9724 A4 γ-terpinene 1 − 37.35a − − 37.35 − − 0.0012 − A5 p-cymene 7.2 94.59a 80.77b 76.74c 13.14 11.22 10.66 0.0017 0.0004 0.0029 A6 2,4-dimethyl styrene 0.085 16.71c 30.69b 56.52a 196.62 361.01 664.97 0.0253 0.0116 0.1831 B1 phenol 5 179.14a − 150.89b 35.83 − 30.18 0.0046 − 0.0083 B2 o-cresol 1.4 19.59b − 20.07a 14.00 − 14.34 0.0018 − 0.0039 B3 p-cresol 0.0039 − − 83.12a − − 21311.63 − − 5.7137 B4 carvacrol 2.29 1070.68c 1413.74b 2900.15a 467.54 617.35 1266.44 0.0602 0.0199 0.3487 B5 thymol 1.7 1215.72a 590.69c 1011.75b 715.13 347.46 595.14 0.0921 0.0112 0.1542 B6 2,4-di-tert-butylphenol 0.5 − 120.60b 955.01a − 241.20 1910.02 − 0.0078 0.5259 C1 prenol 0.25 1.78b 2.88a − 7.12 11.54 − 0.0009 0.0004 − C2 linalool 0.00022 150.37a 69.68b 58.20c 683488.63 316718.51 264532.69 88.0482 10.2151 72.8359 C3 α-terpineol 1.2 1139.07a 883.02b 694.31c 949.23 735.85 578.59 0.1223 0.0237 0.1593 C4 Z-carveol 0.25 1732.26c 2991.85a 2243.32b 6929.04 11967.40 8973.28 0.8926 0.3860 2.4707 C5 p-cymenol ND 607.73b 713.37a 445.52c − − − − − − C6 (-)-carveol 0.25 − 285.04a 274.93b − 1140.16 1099.72 − 0.0368 0.3028 C7 limonene glycol ND 610.42a 148.96c 474.19b − − − − − − C8 perillalcohol 1.1 169.07b 279.38a 147.65c 153.70 253.98 134.23 0.0198 0.0082 0.0370 C9 benzyl alcohol 2.54 − 69.75a 48.40b − 27.46 19.05 − 0.0009 0.0052 C10 2-ethylhexanol 0.3 333.98a 42.32b − 1113.25 141.07 − 0.1434 0.0045 − C11 terpinen-4-ol 1.2 282.27a 220.39b 155.74c 235.22 183.66 129.78 0.0303 0.0059 0.0357 C12 (-)-pinocarveol ND − 93.72a 78.28b − − − − − − C13 phenylethyl alcohol 0.086 177.55a − − 2064.56 − − 0.2660 − − C14 (E)-p-mentha-2,8-dien-1-ol ND 1658.63b 1349.01c 1829.85a − − − − − − D1 vanillin 0.053 − 402.51a 356.40b − 7594.58 6724.47 − 0.2449 1.8515 D2 nonanal 0.0011 − 28.40a − − 25817.10 − − 0.8327 − D3 decanal 0.003 54.60a − − 18200.57 − − 2.3446 − − D4 citronellal 0.006 − 58.38a − − 9729.28 − − 0.3138 − D5 benzaldehyde 0.75 98.19a 23.93b − 130.92 31.91 − 0.0169 0.0010 − D6 perillaldehyde 0.03 − 134.70a 75.84b − 4490.11 2528.15 − 0.1448 0.6961 D7 2-pyrrolaldehyde 65 225.12a − 147.44b 3.46 − 2.27 0.0004 − 0.0006 E1 dihydrocarvone 3.25 − 82.27b 128.87a − 25.31 39.65 − 0.0008 0.0109 E2 β-ionone 0.000007 − 18.17a − − 2595440.13 − − 83.7102 − E3 piperitone 0.68 − 55.93a 28.08b − 82.25 41.29 − 0.0027 0.0114 E4 (+)-carvone 0.16 1285.06b 1406.29a 1215.37c 8031.61 8789.32 7596.06 1.0346 0.2835 2.0915 E5 benzophenone ND 149.77c 591.43a 157.09b − − − − − − E6 4-methoxyacetophenone ND 76.68b − 94.61a − − − − − − F1 methyl methanthranilate 0.349 2611.54b 2902.11a 2352.80c 7482.93 8315.50 6741.56 0.9640 0.2682 1.8562 F2 methyl anthranilate 0.003 116.02b 307.63a 105.30c 38672.96 102541.93 35101.20 4.9819 3.3073 9.6647 G1 octanoic acid 3 27.78b 4.16c 64.19a 9.26 1.39 21.40 0.0012 0.0000 0.0059 G2 lauric acid 10 175.08b − 431.46a 17.51 − 43.15 0.0023 − 0.0119 G3 palmitic acid 10 − 337.30b 1437.30a − 33.73 143.73 − 0.0011 0.0396 H1 dimethyl sulfone ND 243.11b 145.86c 492.16a − − − − − − H2 2,3-dimethyl pyrazine 0.8 20.18a − − 25.23 − − 0.0033 − − H3 1,2-dimethoxybenzene ND 389.68a − 92.04b − − − − − − A The odor detection thresholds in water were obtained from previous studies[12,45] and online database (www.vcf-online.nl/VcfHome.cfm). B Concentration (mg/kg), The concentration of each volatile compound was calculated based on the calibration equation in Supplemental Table S3. C OAV (Odor activity value). D ACI (Aroma character impact value). All results were expressed as mean value (n = 3). Values bearing different lowercase letters (a, b, c) were significantly different (p ≤ 0.05). Table 2.
Quantitative analysis of volatile compounds in citrus Pu-erh tea samples.
-
No. Odorants omitted from the complete recombinant Numbera Significanceb IT OF CP IT OF CP 1 octanoic acid, lauric acid, palmitic acid 6 9 11 * ** ** 1−1 octanoic acid 5 6 5 * * * 1−2 lauric acid 5 2 5 * − * 1−3 palmitic acid 2 6 7 − * * 2 thymol, carvacrol, phenol, o-cresol, p-cresol, 2,4-di-tert-butylphenol 8 7 9 ** * ** 2−1 thymol 9 8 8 ** ** ** 2−2 carvacrol 6 7 7 * * * 2−3 phenol 4 1 5 * − * 2−4 o-cresol 5 3 4 * − * 2−5 p-cresol 2 4 8 − * * 2−6 2,4-di-tert-butylphenol 8 7 8 ** * ** 3 linalool, perillalcohol, p-cymenol, limonene glycol, terpinen-4-ol, prenol, 2-ethylhexanol 15 15 15 *** *** *** 3−1 linalool 14 15 14 *** *** *** 3-2 perillalcohol 5 6 5 * * * 3−3 p-cymenol 2 3 2 − − − 3−4 limonene glycol 1 2 3 − − − 3−5 terpinen-4-ol 8 9 8 ** ** ** 3−6 prenol 5 4 1 * * − 3−7 2-ethylhexanol 6 5 3 * * − 4 phenylethyl alcohol, α-terpineol, (E)-p-mentha-2,8-dien-1-ol, (−)-pinocarveol, benzyl alcohol, (−)-carveol, Z-carveol 15 14 15 *** *** *** 4−1 phenylethyl alcohol 6 1 2 * − − 4−2 α-terpineol 8 7 7 ** * * 4−3 (E)-p-mentha-2,8-dien-1-ol 1 2 2 − − − 4−4 (−)-pinocarveol 2 2 3 − − − 4−5 benzyl alcohol 3 7 6 − * * 4−6 (−)-carveol 3 7 8 − * ** 4−7 Z-carveol 14 13 13 *** *** *** 5 p-cymene, β-pinene, styrene, 2,4-dimethyl styrene, (+)-limonene, γ-terpinene, 1,2-dimethoxybenzene 13 11 11 *** ** ** 5−1 p-cymene 6 6 5 * * * 5−2 β-pinene 6 3 3 * − − 5−3 styrene 11 3 3 ** − − 5−4 2,4-dimethyl styrene 10 9 11 ** ** ** 5−5 (+)-limonene 15 10 9 *** ** ** 5−6 γ-terpinene 3 7 2 − * − 5−7 1,2-dimethoxybenzene 3 2 1 − − − 6 benzaldehyde, 2-pyrrolaldehyde, perillaldehyde, decanal, nonanal, citronellal, vanillin 9 12 13 ** *** *** 6−1 benzaldehyde 8 4 1 ** * − 6−2 2-pyrrolaldehyde 6 3 7 * − * 6−3 perillaldehyde 2 7 6 − * * 6−4 decanal 9 2 2 ** − − 6−5 nonanal 3 8 4 − ** * 6−6 citronellal 4 12 5 * *** * 6−7 vanillin 5 12 13 * *** *** 7 2,3-dimethyl pyrazine, methyl methanthranilate, methyl anthranilate, dimethyl sulfone 14 15 15 *** *** *** 7−1 2,3-dimethyl pyrazine 6 3 4 * − * 7−2 methyl methanthranilate 14 15 15 *** *** *** 7−3 methyl anthranilate 13 14 15 *** *** *** 7−4 dimethyl sulfone 5 3 3 * − − 8 (+)-carvone, dihydrocarvone, piperitone, benzophenone, 4-methoxyacetophenone, β-ionone 13 14 15 *** *** *** 8−1 (+)-carvone 12 13 13 *** *** *** 8−2 dihydrocarvone 3 6 7 − * * 8−3 piperitone 4 7 8 * * ** 8−4 benzophenone 4 5 4 * * * 8−5 4-methoxyacetophenone 2 3 4 − − * 8−6 β-ionone 4 13 5 * *** * a The number of panelists who perceived the aroma difference by means of a triangle test. Fifteen panelists were invited for aroma omission experiment. b Levels of significance, defined based on the number of panelists who were able to determine the difference in aroma omission. −, not significant (0−3, p > 0.05); *, significant (4−7, p ≤ 0.05); **, highly significant (8-11, p ≤ 0.01); ***, very highly significant (12−15, p ≤ 0.001). Table 3.
Omission tests of three citrus Pu-erh tea based on aroma recombination model.
Figures
(7)
Tables
(3)