-
Figure 1.
Five different species and varieties of water lilies selected in this study.
-
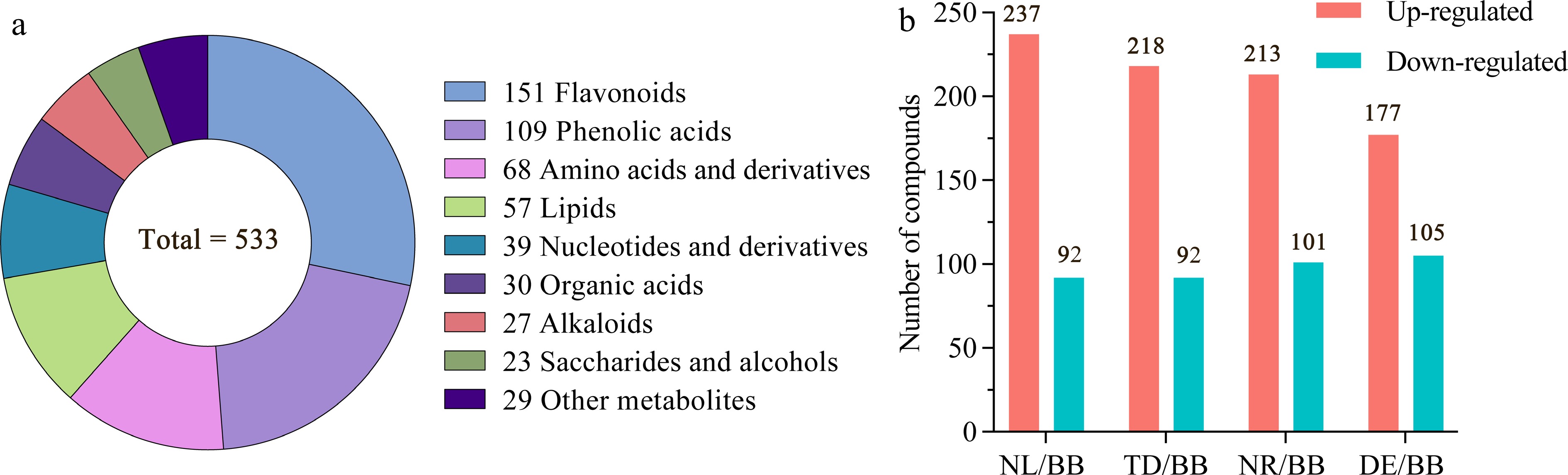
Figure 2.
Overview of the non-volatile components. (a) Quantitative distribution of chemical classes of volatile compounds. (b) Number of differentiated compounds with fold change ≥ 2 or ≤ 0.5. Note: NL, N. lotus; NR, N. rubra; TD, Nymphaea 'Texas Dawn'; BB, Nymphaea 'Blue Bird'; DE, Nymphaea 'Detective Erika'.
-
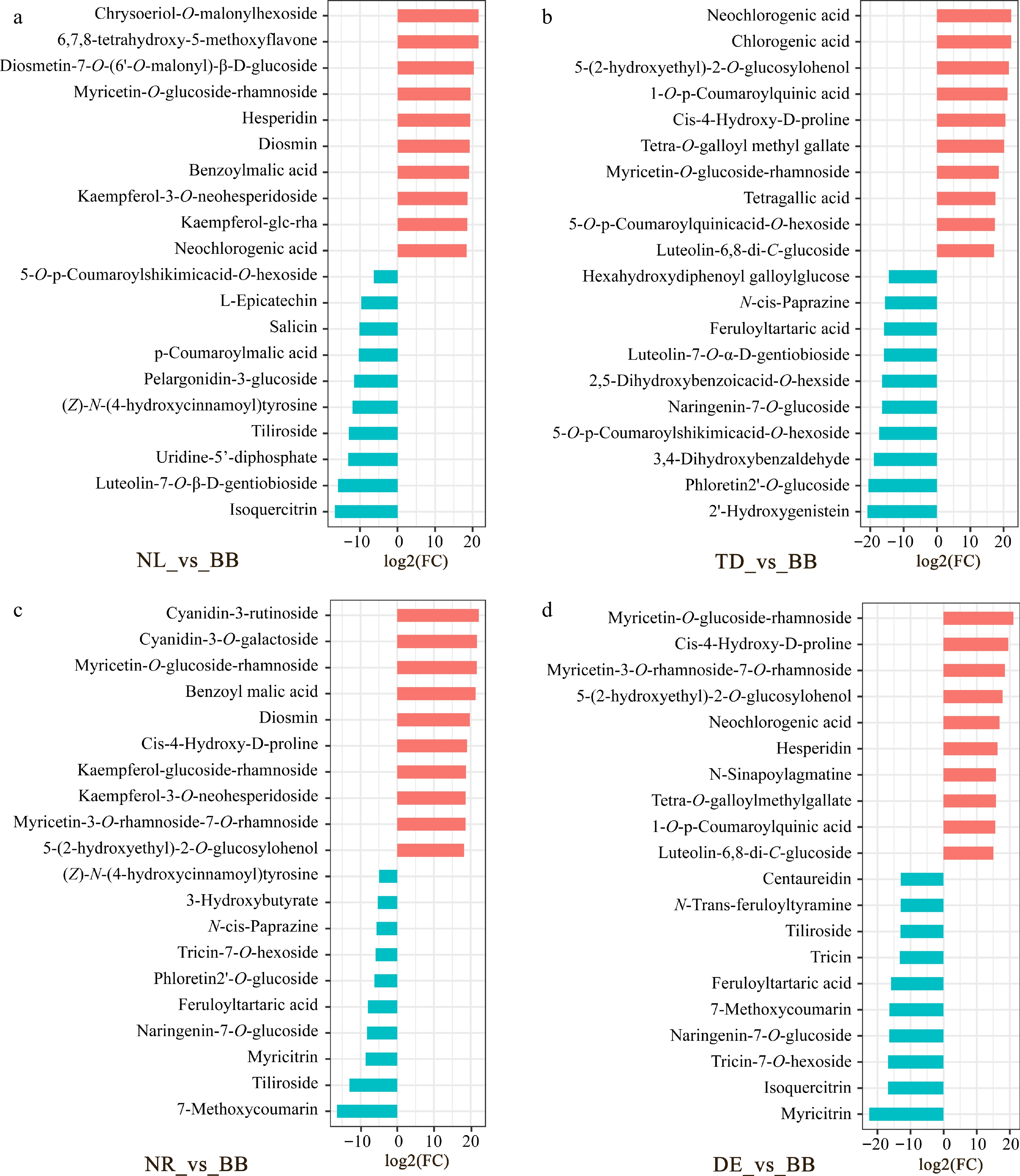
Figure 3.
The highest fold change values of non-volatile metabolites. (a) Fold change plot of NL vs BB. (b) Fold change plot of TD vs BB. (c) Fold change plot of NR vs BB. (d) Fold change plot of DE vs BB. Note: The horizontal coordinate is the log2FC of the differentially metabolized metabolite, and the vertical coordinate is the differentially metabolized metabolite. Red represents up-regulated differentially expressed metabolites, cyan represents down-regulated differentially expressed metabolites. NL, N. lotus; NR, N. rubra; TD, Nymphaea 'Texas Dawn'; BB, Nymphaea 'Blue Bird'; DE, Nymphaea 'Detective Erika'.
-
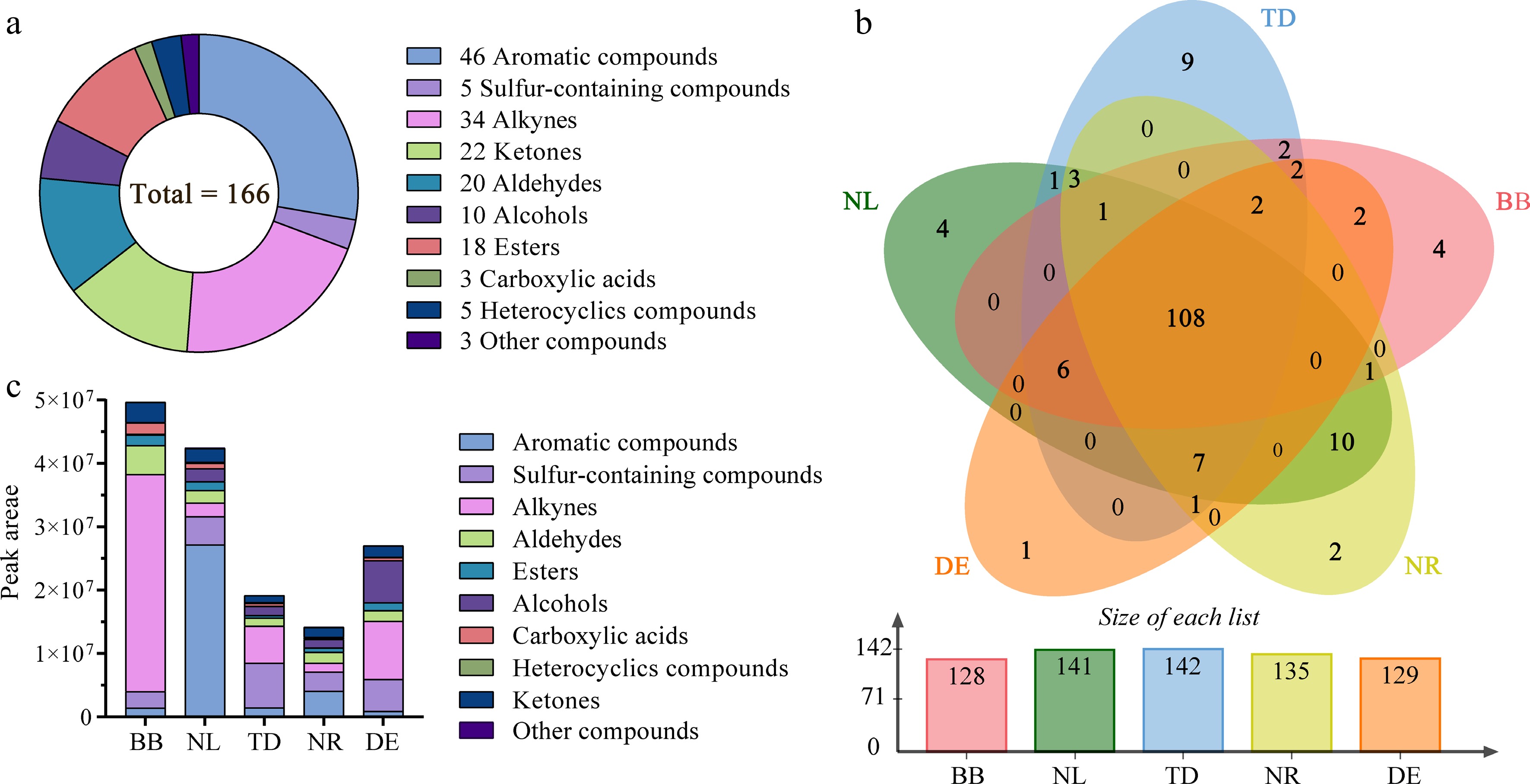
Figure 4.
Overview of the volatile components. (a) Quantitative distribution of chemical classes of volatile compounds. (b) Venn plot; numbers represent the identified metabolites. (c) Relative abundance of different types of volatile compounds. Note: NL, N. lotus; NR, N. rubra; TD, Nymphaea 'Texas Dawn'; BB, Nymphaea 'Blue Bird'; DE, Nymphaea 'Detective Erika'.
-
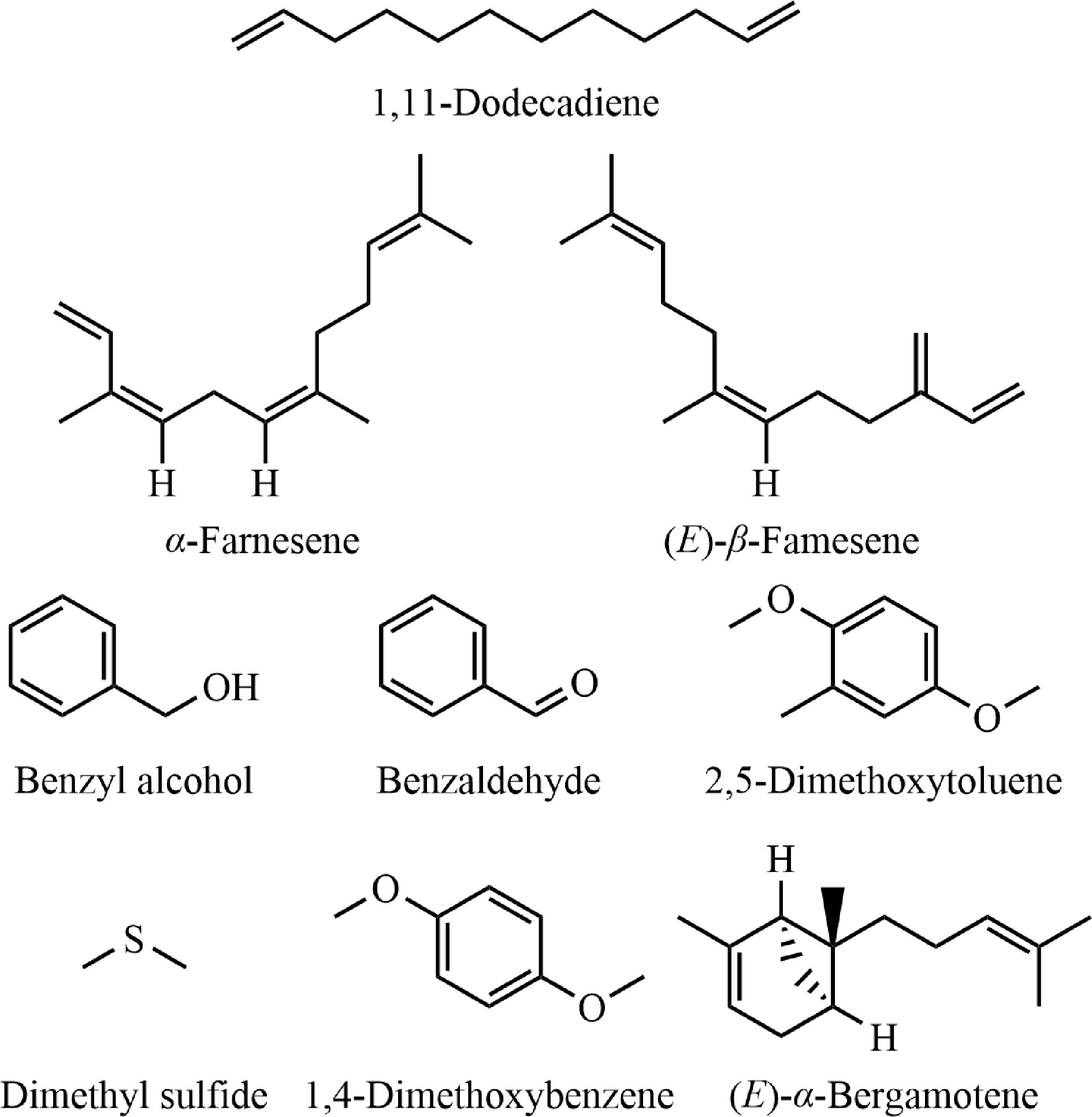
Figure 5.
Molecular formulas of major volatile compounds.
-
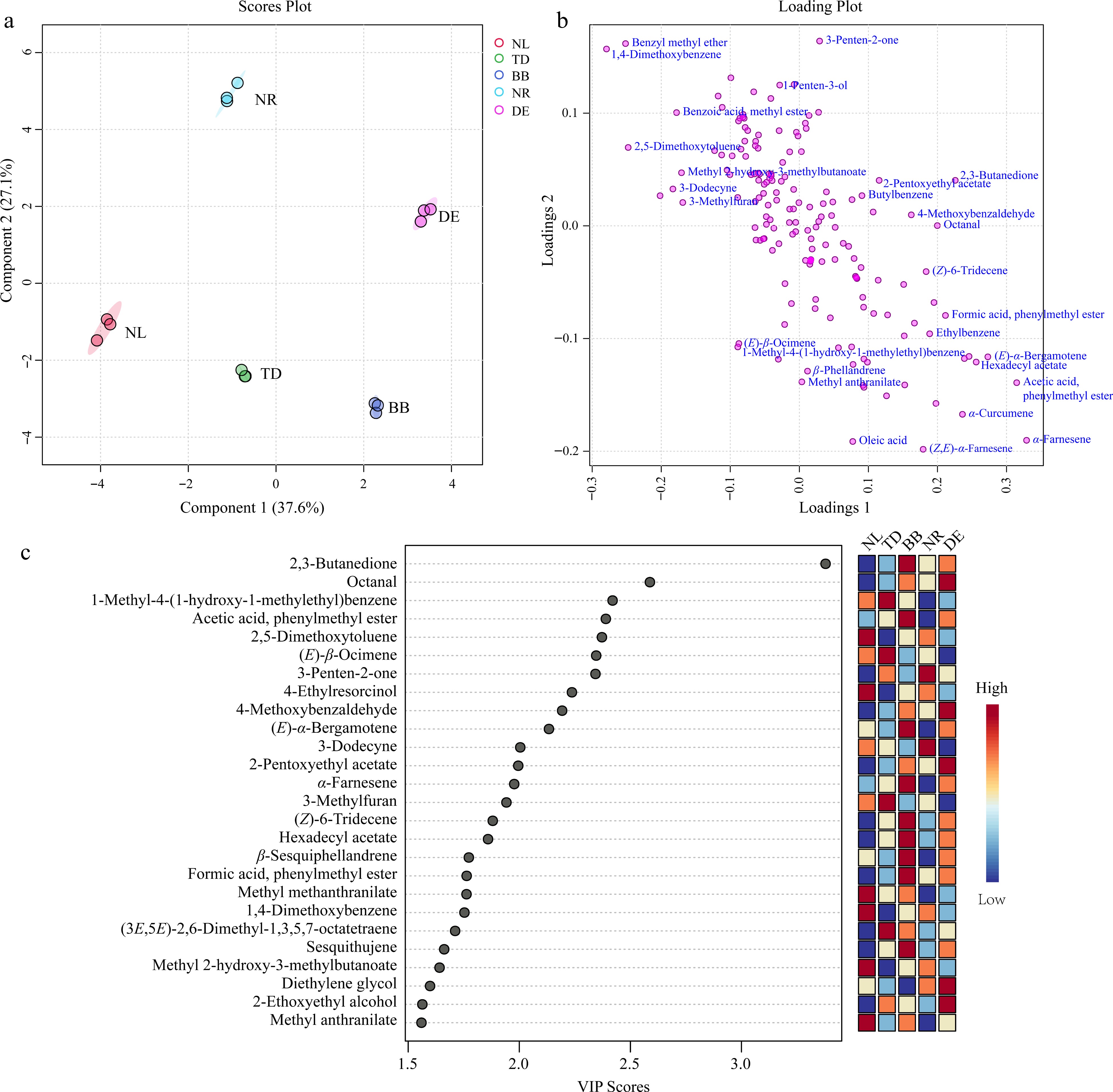
Figure 6.
The partial least squares discriminant analysis (PLS-DA) of the volatile compounds. (a) Score plot of PLS-DA. (b) The loading plot of PLS-DA. (c) Variable importance in the project (VIP) plot of PLS-DA. Note: NL, N. lotus; NR, N. rubra; TD, Nymphaea 'Texas Dawn'; BB, Nymphaea 'Blue Bird'; DE, Nymphaea 'Detective Erika'.
-
No. Compounds Class RI (ref)[a] RI (cal)[b] CAS Ion Odor type[c] Flavor[c] Relative content (%) BB NL TD NR DE 1 Benzyl alcohol Alcohols 1036 ± 4 1037 100-51-6 79 Floral Floral, rose, balsamic 0.11 4.48 6.32 8.13 23.83 2 Benzaldehyde Aldehydes 962 ± 3 969 100-52-7 77 Fruity Almond, burntsugar, sweet 8.10 2.13 3.17 5.22 3.91 3 Hexanal Aldehydes 800 ± 2 801 66-25-1 41 Green Green, fatty, leafy 0.15 0.69 0.61 3.84 0.34 4 Benzeneacetaldehyde Aldehydes 1045 ± 4 1049 122-78-1 91 Green Green, floral, honey 0.16 0.14 1.03 0.09 0.85 5 1,11-Dodecadiene Alkenes 1179 ± 2 1763 5876-87-9 67 / / 27.30 3.67 12.62 7.04 11.45 6 (E)-β-Famesene Alkenes 1457 ± 2 1453 18794-84-8 41 Woody Woody, citrus, herbal 18.28 0.39 0.84 0.38 1.27 7 α-Farnesene Alkenes 1508 ± 2 1506 502-61-4 41 Woody Citrus, herbal, neroli 14.44 0.17 10.38 0.18 16.41 8 (E)-α-Bergamotene Alkenes 1433 ± 3 1437 13474-59-4 93 Woody Woody 2.02 0.11 0.03 0.03 1.65 9 β-Sesquiphellandrene Alkenes 1524 ± 2 1528 20307-83-9 69 Herbal Herbal, fruity, woody 3.43 0.12 0.15 0.10 1.37 10 (E)-β-Ocimene Alkenes 1049 ± 2 1048 3779-61-1 93 / Herbal, sweet 0.01 0.03 3.89 0.04 0.01 11 2,5-Dimethoxytoluene Aromatic compounds 1251 ± 5 1249 24599-58-4 137 / / 0.38 56.18 0.49 12.18 0.52 12 1,4-Dimethoxybenzene Aromatic compounds 1168 ± 9 1166 150-78-7 123 Green Green, hay, sweet 0.05 3.62 0.02 6.63 0.02 13 Phenol Aromatic compounds 980 ± 4 981 108-95-2 94 Phenolic Phenolic, plastic, rubbery 0.17 0.87 0.56 1.46 0.62 14 Acetic acid Carboxylic acids 610 ± 10 581 64-19-7 45 Acidic Pungent, sour 3.42 2.01 2.18 0.77 1.58 15 Benzoic acid, methyl ester Esters 1094 ± 3 1096 93-58-3 105 Phenolic Phenolic, wintergreen, almond 0.03 0.57 0.07 1.86 0.03 16 Ethyl acetate Esters 612 ± 5 613 141-78-6 43 Ethereal Ethereal fruity sweet 0.38 0.39 0.14 0.00 2.06 17 Acetic acid, phenylmethyl ester Esters 1164 ± 2 1166 140-11-4 108 Floral Floral, fruity, jasmine 1.88 0.01 0.06 0.02 0.45 18 6-Methyl-5-heptene-2-one Ketones 986 ± 2 984 110-93-0 43 Citrus Citrus, green, musty 1.29 2.16 2.15 3.67 3.05 19 (E)-3-Penten-2-one Ketones 735 ± N/A 744 3102-33-8 69 / / 0.21 1.01 0.31 1.58 0.40 20 (E)-Geranylacetone Ketones 1453 ± 2 1448 3796-70-1 43 Floral Fruity, fresh, rose 2.15 0.76 0.65 0.72 1.18 21 2-Heptadecanone Ketones 1902 ± 7 1900 2922-51-2 58 / / 1.68 0.41 1.18 0.70 0.80 22 Dimethyl sulfide Sulfur-containing compounds 520 ± 7 553 75-18-3 47 Sulfurous Sulfurous, sweet corn 4.37 8.76 33.61 18.75 16.67 23 Carbon disulfide Sulfur-containing compounds 549 ± 13 565 75-15-0 76 / Sweet 0.55 1.17 1.96 1.29 1.39 24 Benzothiazole Sulfur-containing compounds 1229 ± 8 1240 95-16-9 135 Sulfurous Sulfurous, rubbery, vegetable, cooked 0.29 0.48 0.56 1.16 0.53 Total relative content (%) 90.92 91.45 83.19 77.29 90.83 RI, Retention index, Ion, Qualitative ion; [a] RI (ref): The RI values (median ± deviation) were the reference values for semi-standard non-polar (DB-5) column obtained from NIST 2014; [b] RI (cal): The RI values were calculated from C8-C40 n-alkanes; [c] odor type and flavor were obtained from website (www.thegoodscentscompany.com/search2.html). Note: NL, N. lotus; NR, N. rubra; TD, N. 'Texas Dawn'; BB, N. 'Blue Bird'; DE, N. 'Detective Erika'. Table 1.
Comparison of the main volatile compounds in five water lily samples.
Figures
(6)
Tables
(1)