-
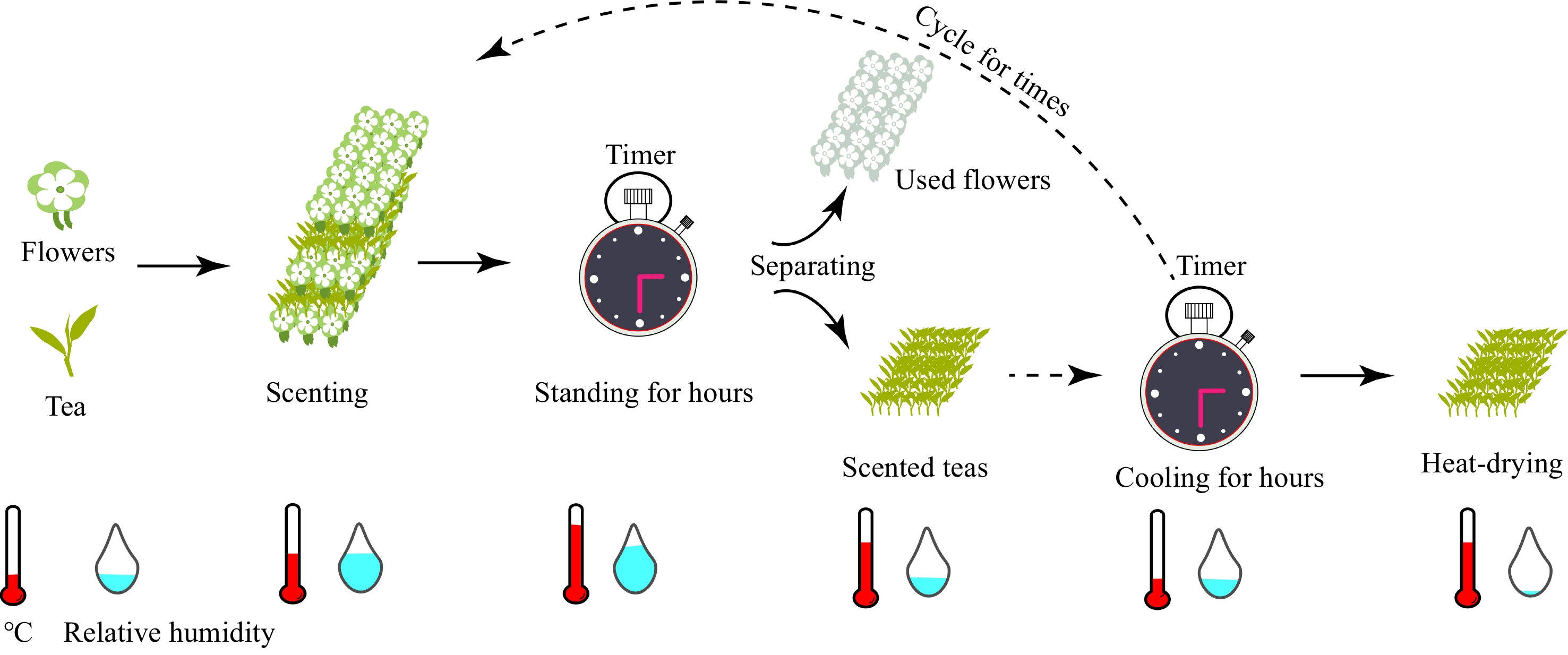
Figure 1.
Reduced form model of the scenting processes. The flowers are firstly mixed with tea and scented for hours at high temperature and relative humidity, then, the scented teas are separated and the flowers are used. The scented teas will then be mixed with flowers again. Finally, the scented teas will be heat-dried for hours to fix the aroma with tea leaves.
-
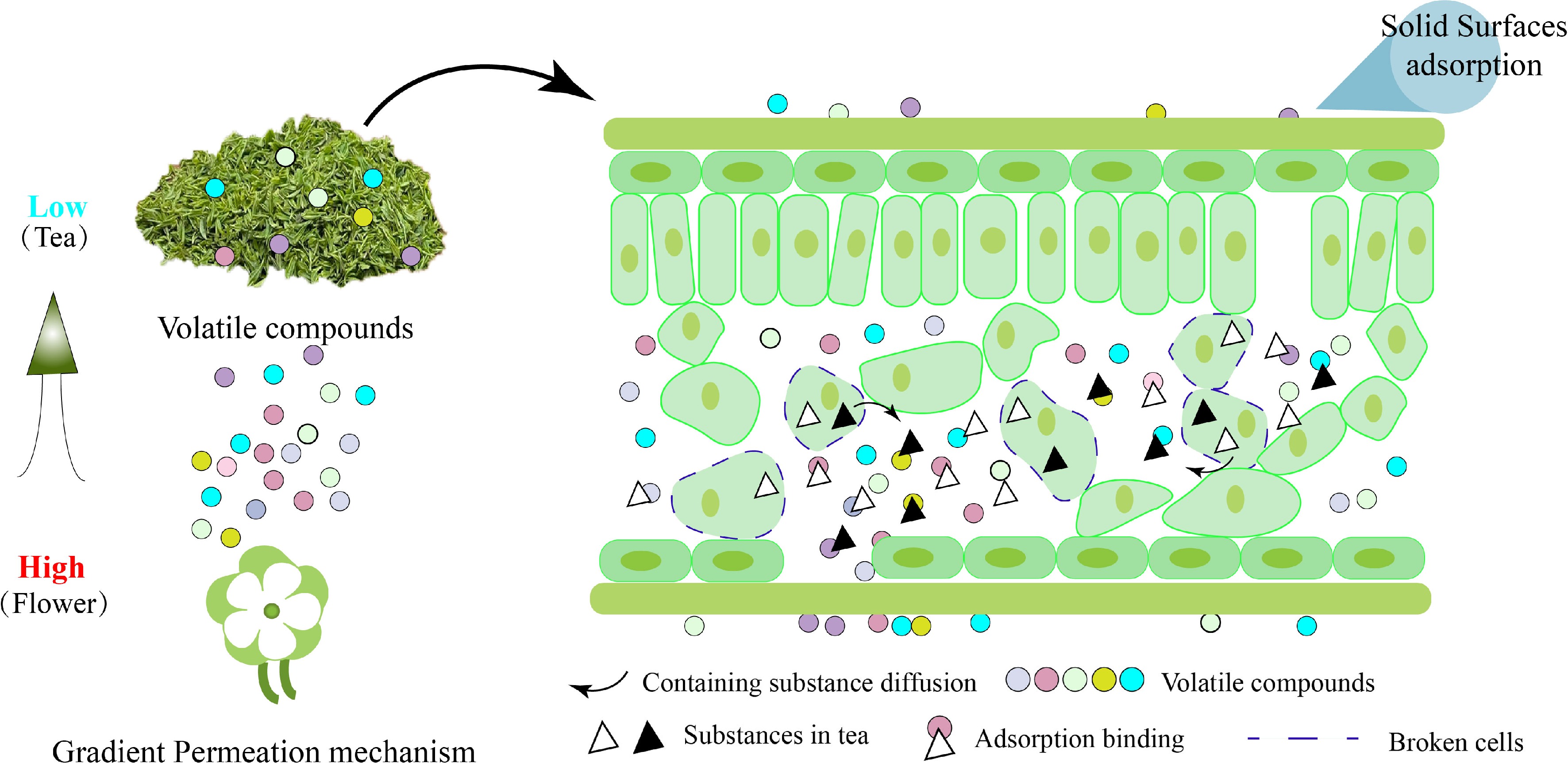
Figure 2.
Schematic diagram of waste tea residue adsorbing aroma substances. The aroma compounds will volatilize from higher concentrations to lower. The physical adsorption mainly happened on the solid surfaces of tea leaves. The chemical adsorption mainly depended on the bonding effect of volatile compounds with containing substances diffused from broken cells.
-
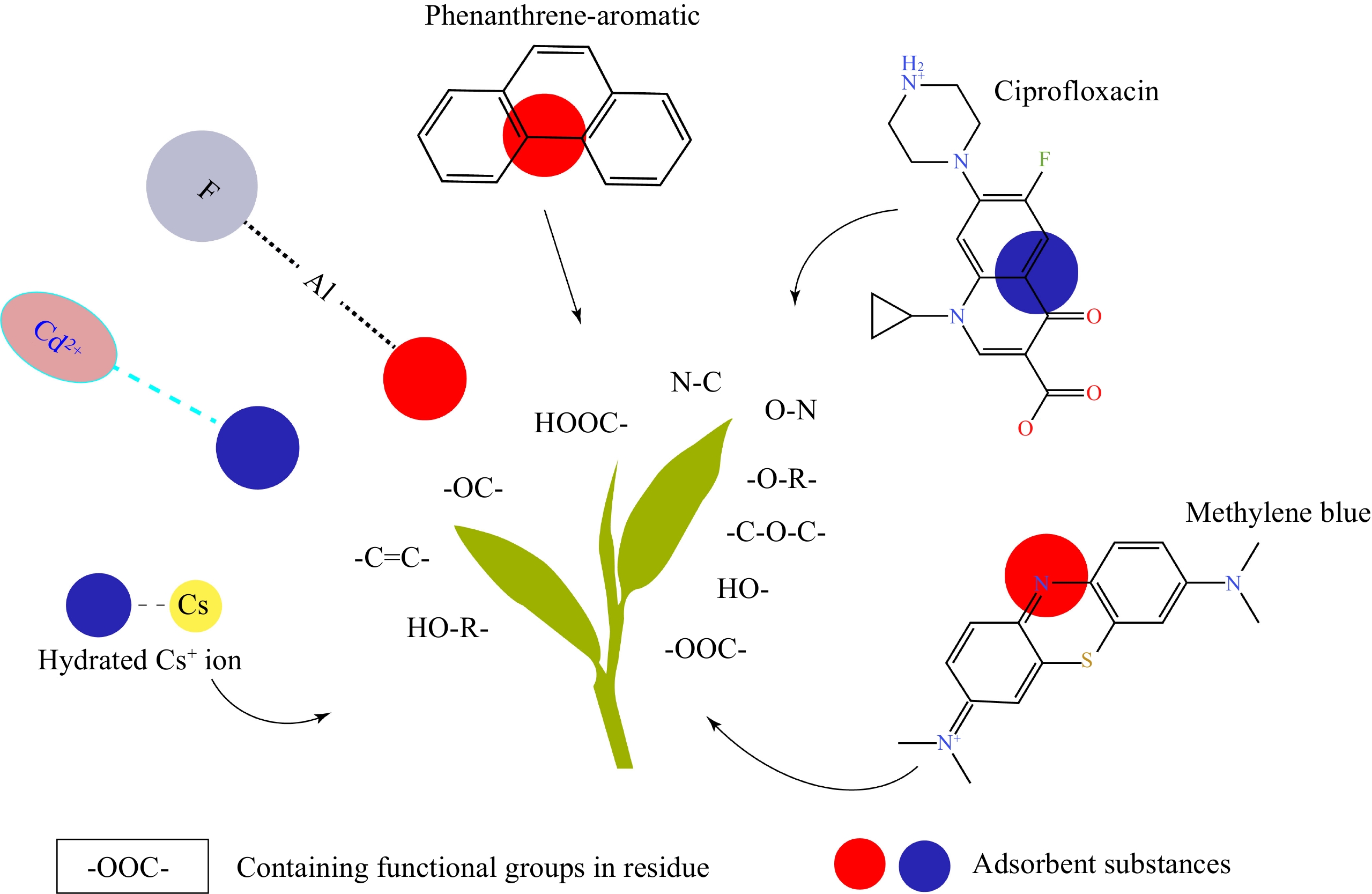
Figure 3.
Possible binding mechanisms between tea residue and adsorped compounds, which rely on the intermolecular force between containing functional groups in residue and adsorped compounds.
-
Adsorption models Equation The main parameters Field of application Ref. Freundlich model $ s={K}_{F}{C}^{N} $ s is the adsorption concentration (μg/g), C is the liquid phase equilibrium solution concentration (μg/mL), KF is the adsorption capacity coefficient [(μg/g)/(μg/mL)],
N (dimensionless) is the description Freundlich exponent of isothermal nonlinearity.Monolayer adsorption and
non-uniform surface adsorption[40] Langmuir model $ \dfrac{{c}_{e}}{Q\mathrm{e}}=\dfrac{{c}_{e}}{{Q}_{max}}+\dfrac{1}{{Q}_{max}{K}_{L}} $ Where Ce is the equilibrium concentration, Qe is the adsorption amount of the dye at equilibrium, Qmax is the maximum adsorption amount of the dye, and
KL is the Langmuir constant.Uniform surface adsorption [14] Freundlich model $ \mathit{ln}{Q}_{e}=\mathrm{ln}{K}_{F}+\dfrac{1}{n}\mathrm{ln}{C}_{e} $ Among them, KF is the Freundlich constant, and
n is the heterogeneity factor.Monolayer adsorption and
non-uniform surface adsorption[41] Dubinin–Radushkevich model $ {l}_{n}{Q}_{e}=\mathrm{ln}{Q}_{m}-K{\varepsilon }^{2} $ In the formula, K is the coefficient related to the free energy of adsorption (mol/kJ2), Qm is the maximum
adsorption capacity (mg/g), ε is the Polanyi adsorption potential.Adsorption behavior of exogenous substances on surfaces [42] Temkin model $ {Q}_{\mathrm{e}}=\dfrac{RT}{{B}_{T}}\mathrm{ln}\left({A}_{T}{C}_{e}\right) $ In the formula, BT is the Temmin constant (J/mol),
AT is the Temkin isotherm equilibrium binding
constant (L/mg).Non-uniform surface adsorption [43] Table 1.
Adsorption equilibrium process equations.
-
Applications Modified tea adsorbent Adsorbate structure Ref. Adsorption of hydrophobic organic compounds by Tea Tea powder Phenanthrene 
[40] Cd and cadmium by waste tea biochar Continuous high temperature pyrolysis of tea leaves to remove ash and make biochar CdCl2 
[33,45] Tea waste removal of heavy
metals in wastewaterPickling, alkali washing, vulcanization, etc. treat overnight wasteto deprotonate the surface of tea waste and activate the adsorption sites Pb, Cu, Zn, Cr, Ni [16] Tea-aluminum biosorbent Preparation of tea-aluminum biosorbent by chemical coprecipitation 
[15] Removal of methylene blue from aqueous solution by tea residue Deionized water is used to remove ash from the tea waste, and the pigment, the ophylline and caffeine in the tea waste are removed by boiling water, and finally dried. 
Methylene Blue[13,14] Adsorption of organic pollutants and polycyclic aromatic hydrocarbons (PAH)
by modified tea leavesTea industry waste is treated by Sohit extraction, saponification and acid hydrolysis PAH 
[34] Selective adsorption of radioactive cesium by crosslinked tea leaves Fresh tea leaves and discarded tea leaves are stirred in concentrated sulfuric acid at high temperature, and then washed with deionized water until it's neutral CsCl, CsOH [46] Removal of ciprofloxacin from aqueous solution by waste tea biochar Tea biochar was obtained from high temperature pyrolysis of tea by argon gas Ciprofloxacin 
[32] Table 2.
Applications of tea as adsorbent.
Figures
(3)
Tables
(2)