-
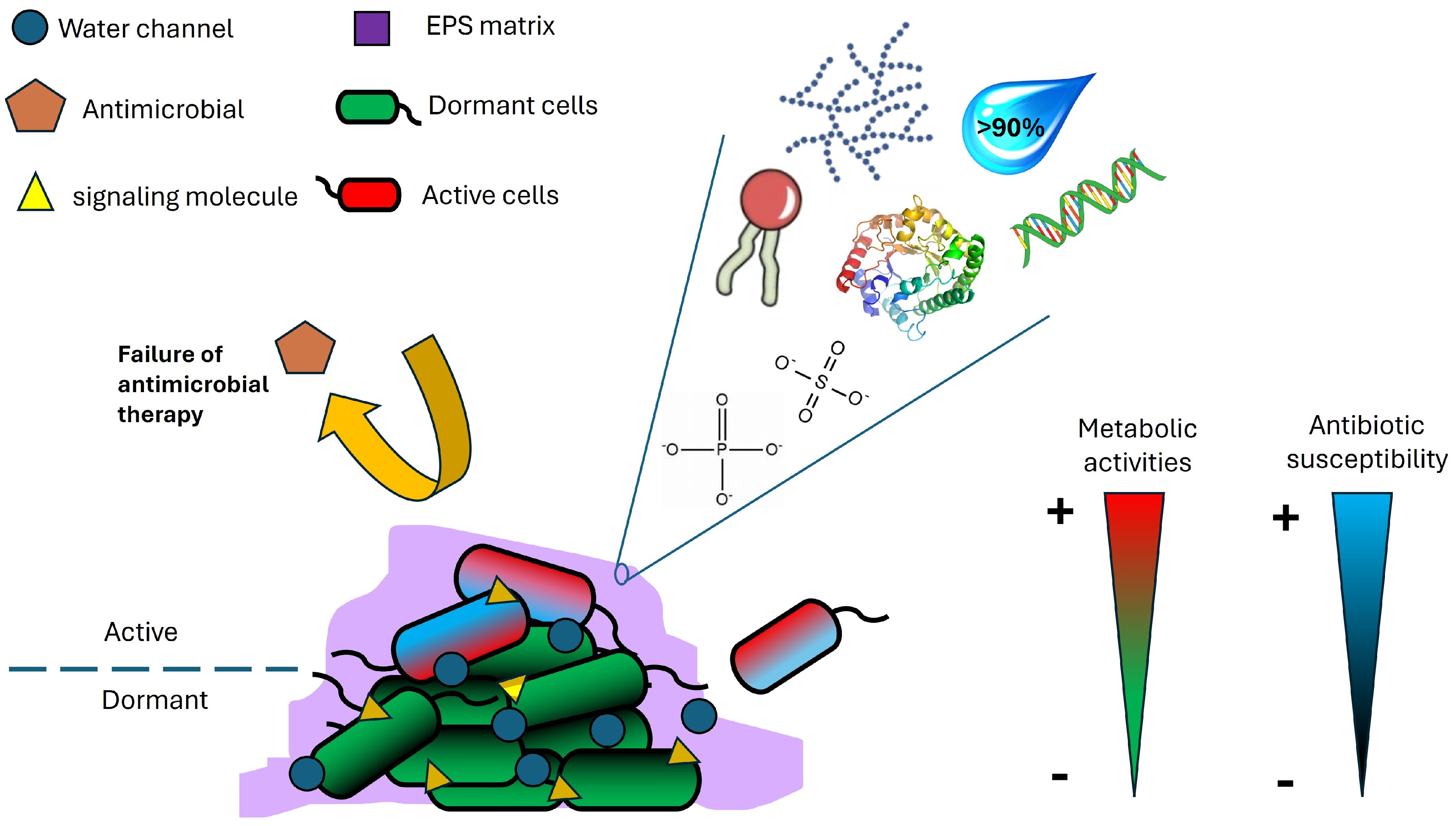
Figure 1.
Characteristics of microbial biofilms.
-
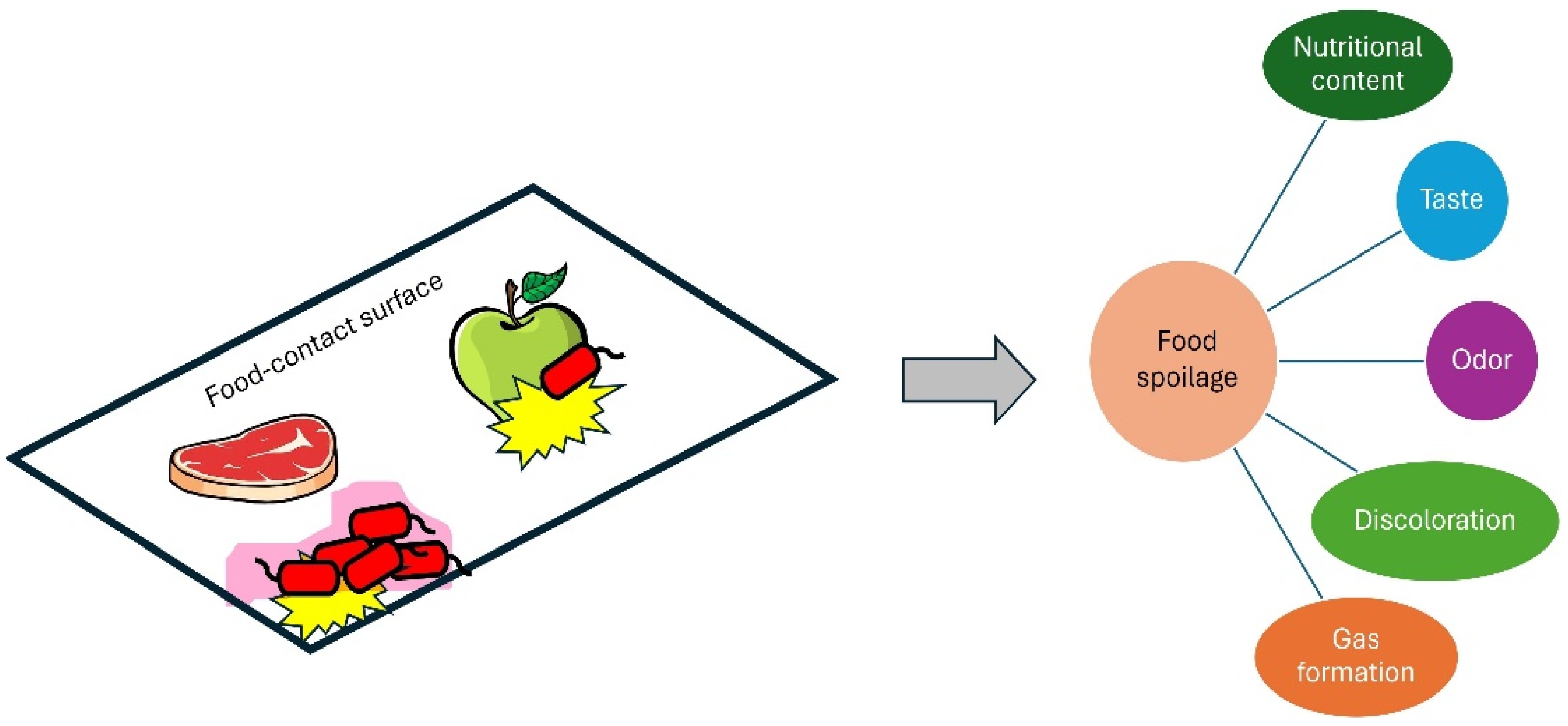
Figure 2.
Negative impacts of microbial biofilms on foods.
-
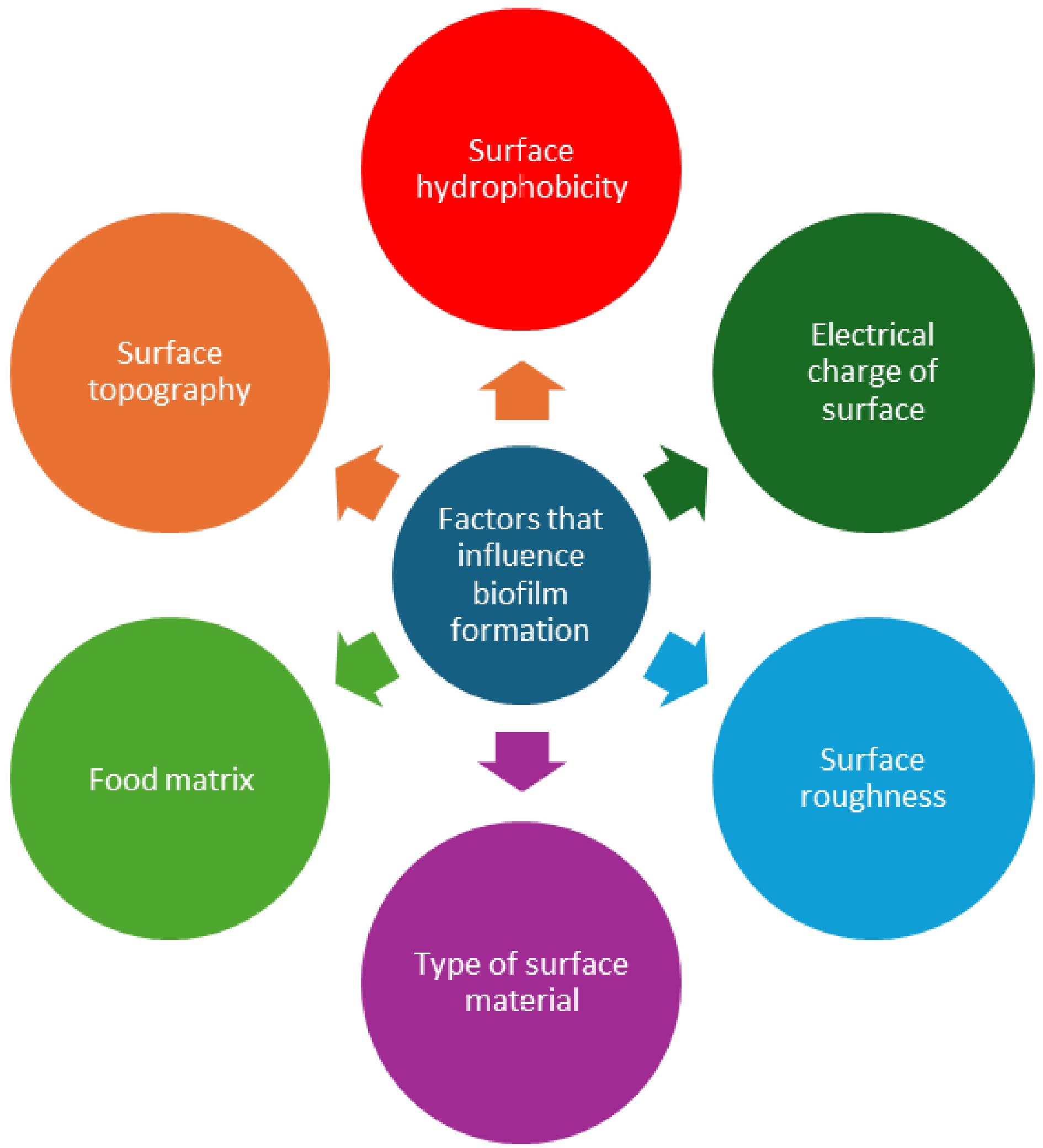
Figure 3.
Various factors that influence biofilm formation on food-contact surfaces.
-
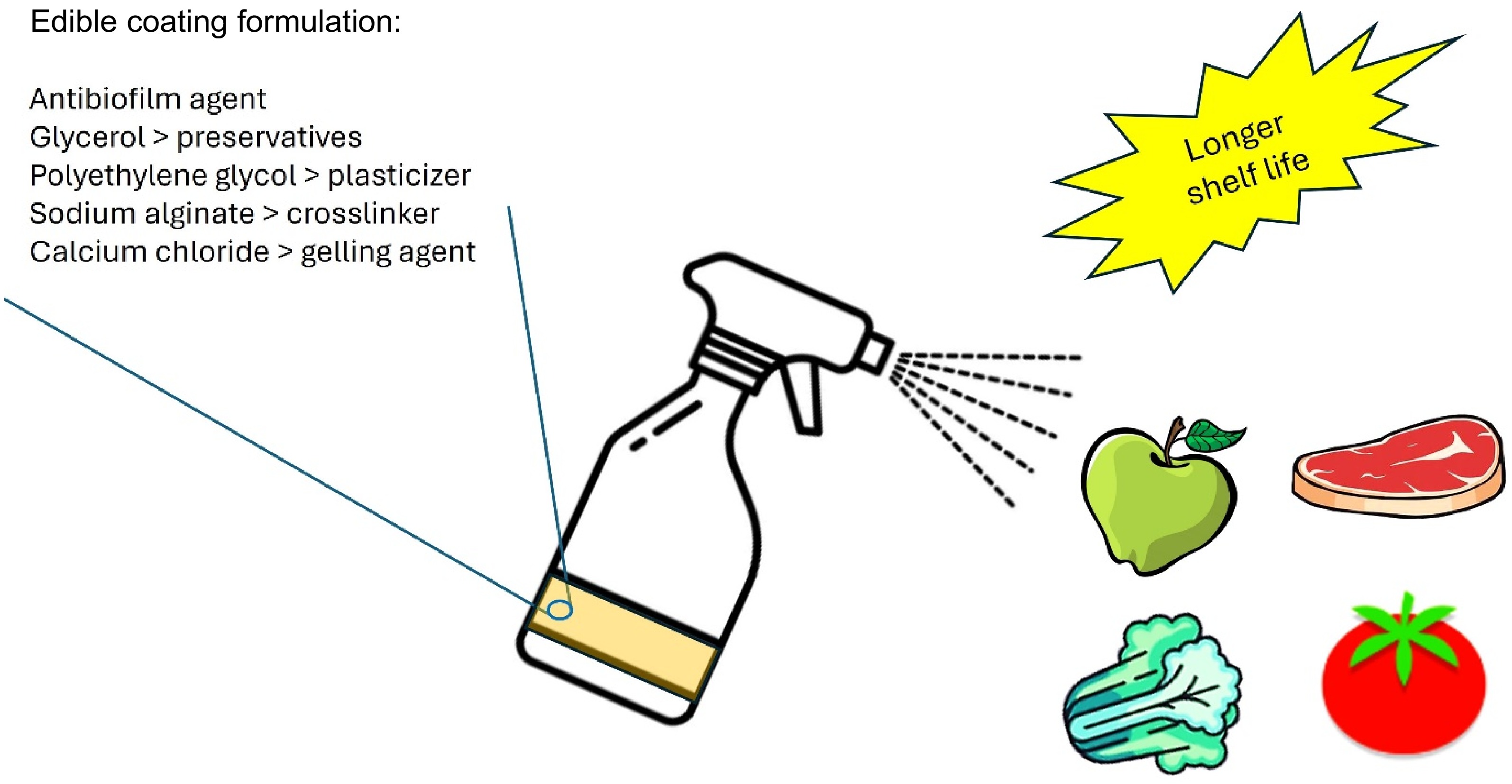
Figure 4.
Spraying edible coatings can prolong the shelf life of foods.
-
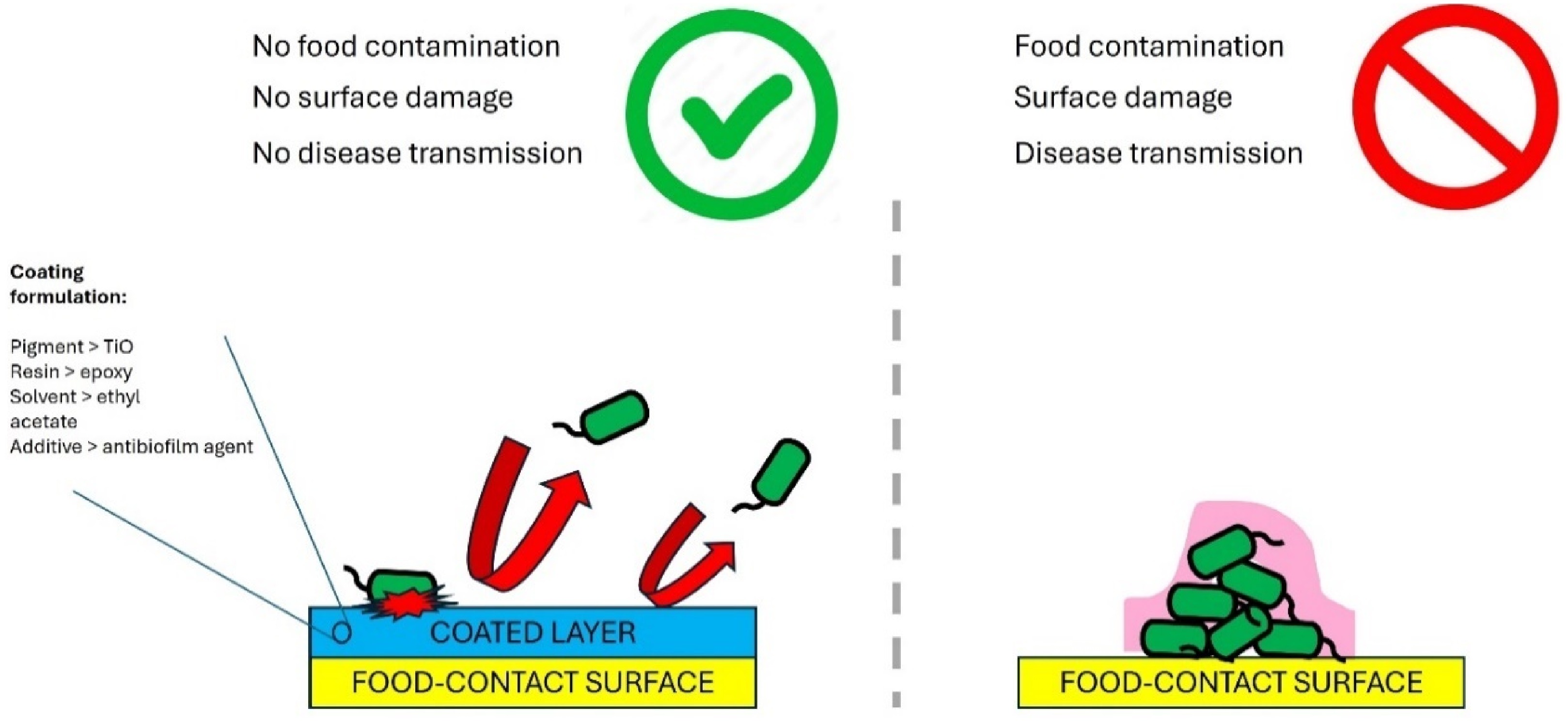
Figure 5.
Antibiofilm coated food contact surfaces prevents biofilm formation.
-
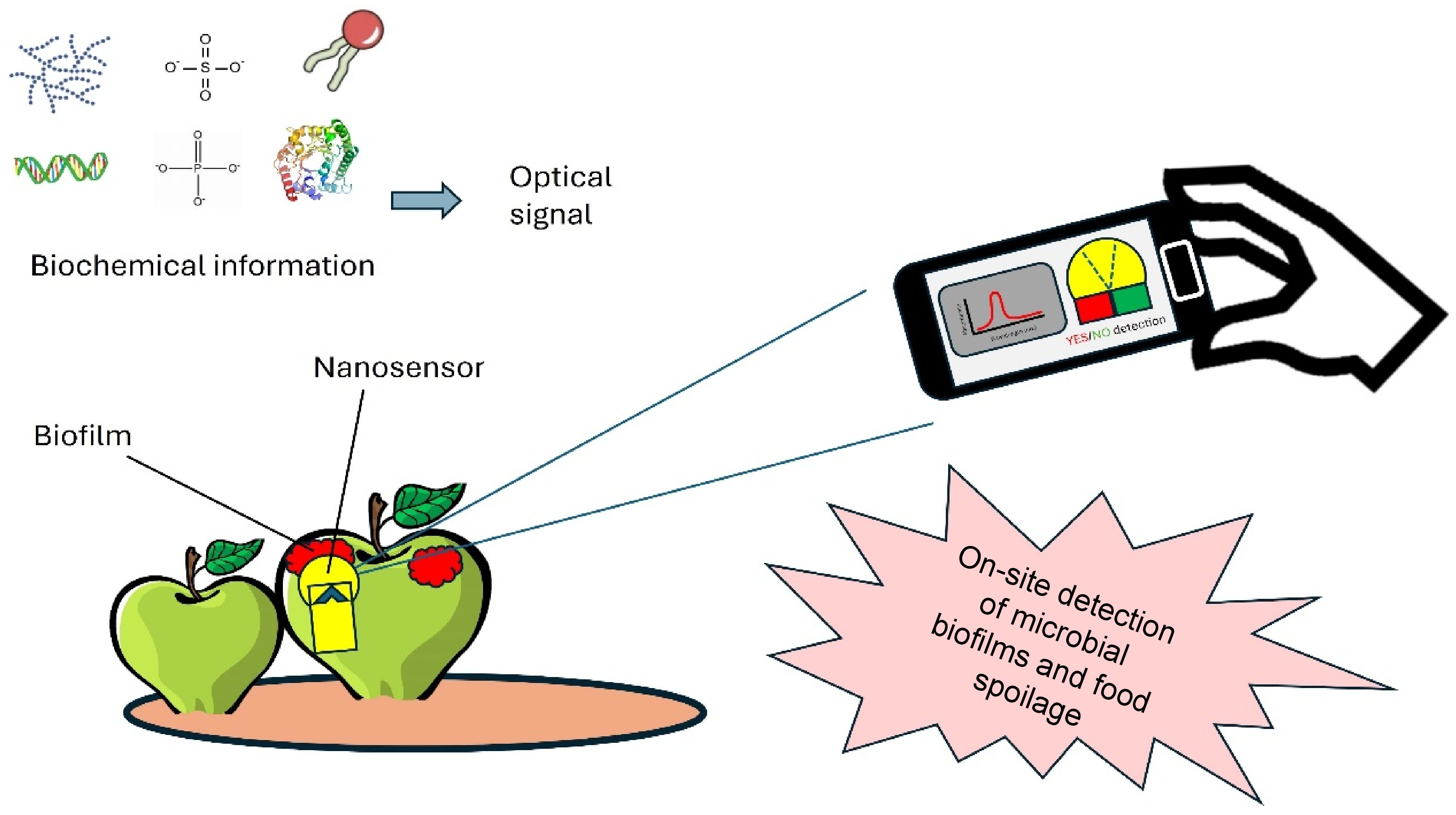
Figure 6.
Application of nanosensors for rapid detection of biofilms in foods.
-
Antibiofilm agents Edible coatings Foods Efficacy Authors Quercetin Pectin Apples The prepared edible coatings form a protective barrier over the surface of apples, effectively resisting bacterial infection and extending shelf life to 10 d while maintaining good commercial quality Du et al.[36] Pectin Corn starch Mozzarella cheese The pectin coating over mozzarella cheese increases its shelf life from 7 to 21 d. Tripathi & Mishra[37] ZnO nanoparticles Chitosan/gum
arabic (CH/GA)Banana CH/GA/ZnO coating maintains freshness of banana for more than 17 d in comparison with the less than 13 d for the control banana at 35 °C and 54% relative humidity. La et al.[38] Lytic bacteriophages Chitosan Tomato Approximately 3 log differences in microbial levels between the control and the treatment samples. Amarillas et al.[39] 3% thyme and oregano essential oil Soy protein Fresh beef The coatings with 3% thyme and oregano EOs exhibits 2.86 and 2.59, 1.97 and 1.90, and 1.87 and 1.83 log CFU/g reductions in S. aureus, L. monocytogenes, and E. coli populations, respectively, as compared with the control. Yemiş & Candoğan[40] Table 1.
Summary of previous works related to edible coatings containing antibiofilm agents.
-
Antimicrobial agents Surface materials Efficacy Authors Microfibrillated cellulose Glass Inhibits the growth of both Gram-negative and Gram-positive bacteria (E. coli and S. epidermidis) due to the intrinsic porosity and hydrophilicity. Qi et al.[48] Gold nanoparticles Latex Reduces biofilm formation by E. coli, 369 to 9.87 ± 5.57% at a AuR NP concentration of 0.5 ng mL−1; and at a dose of 25 ng mL−1 no bacterial growth can be detected. Piktel et al.[49] Zinc oxide and silver oxide nanoparticles Polyester Polyester surfaces embedded with zinc oxide and silver oxide nanoparticles show sufficient, controlled levels of nanoparticles released to avoid bacterial adhesion. Fontecha-Umaña et al.[50] Copper nanoparticles Mussel-inspired dendritic polyglycerol
(MI-dPG)Cu NP-incorporated MI-dPG surface coating shows efficient long-term antibacterial properties against E. coli, S. aureus, and kanamycin-resistant E. coli through an 'attract–kill–release' strategy. This coating also inhibits biofilm formation and shows good compatibility to eukaryotic cells. Li et al.[51] Curcumin-liposome-type polydiacetylene/phosholipid nanovesicles Silanized glass Incubation of E. coli and Bacillus cereus with nanovesicle-coated glass results in a 2.5 log reduction in their counts. Dogra et al.[52] Table 2.
Summary of previous studies related to antibiofilm coated food-contact surfaces.
-
Methods Materials Analyte Detection Authors Fluorescence Polystyrene nanoparticles pH in biofilms A temperature-dependent decrease in pH over a 4-h period caused by the acidifying glucose metabolism of E. coli Kromer et al.[64]. Fluorescence Graphene oxide nanoparticles DNA aptamers in biofilms The fluorescence intensity increases from 83.97 to 139.6 (a.u.) for the GO–APT1 mixed solution and from 85.07 to 172.6 (a.u.) for the GO–APT2 mixed solution. Wang et al.[65] Field-efect-transistor (FET) array Silicon nanowires Glucose metabolites in biofilms A high correlation between glucose signals by the redox-reactive SiNW FET device to the solution’s transparency measurements Yeor-Davidi et al.[66] Localized surface plasmon resonance (LSPR) Gold nanoparticles Biofilm growth kinetics After 2 h, the Au NM LSPR substrate is completely covered with either bacteria or conditioning biomolecules, preventing further shifts in the local dielectric constant, and thus the LSPR signal starts to exhibit blue shifts. Funari et al.[67] Fluorescence Gold nanoparticles Overall biofilm physicochemical properties The sensor is composed of AuNP-fluorescent protein conjugates that are disrupted in the presence of biofilms. This disruption turns on the fluorescence and results in different colored fluorescence patterns for biofilm identification. Li et al.[68] Table 3.
Summary of previous works on nanosensors for biofilm detection in foods.
Figures
(6)
Tables
(3)