-
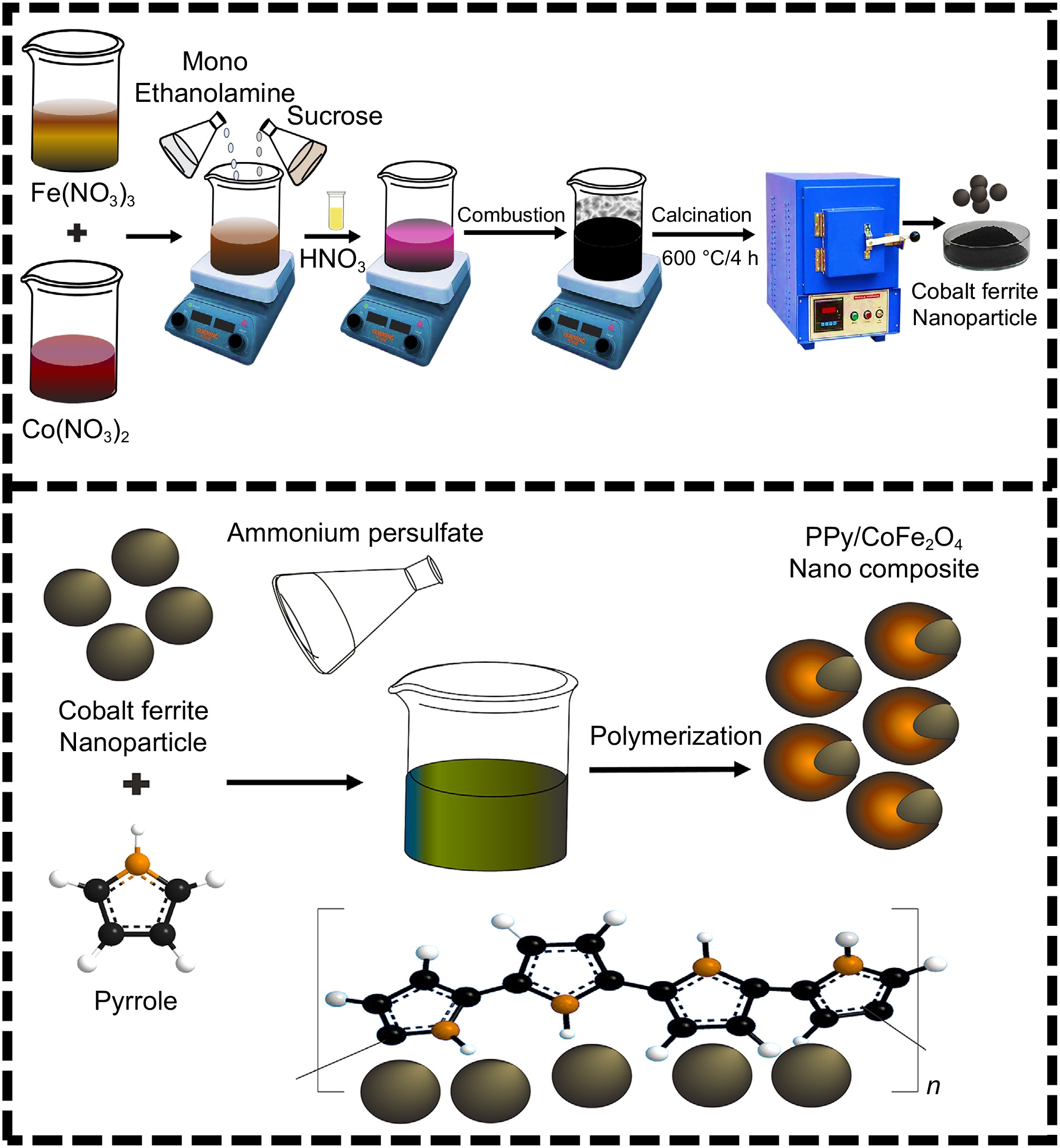
Figure 1.
Schematic for the fabrication of CoFe2O4 nanoparticles and PPy/CoFe2O4 nanocomposite.
-
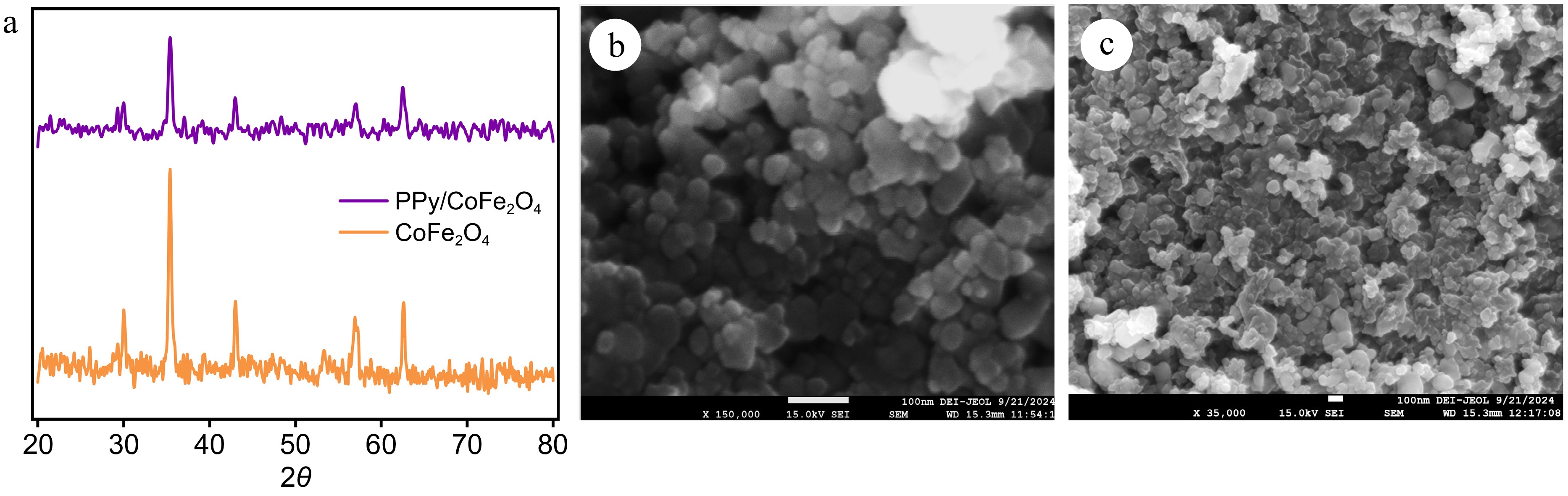
Figure 2.
(a) X-ray diffraction patterns of cobalt ferrite and polypyrrole coated cobalt ferrite nanocomposite. (b) FE-SEM image of cobalt ferrite. (c) FE-SEM image polypyrrole coated cobalt ferrite.
-
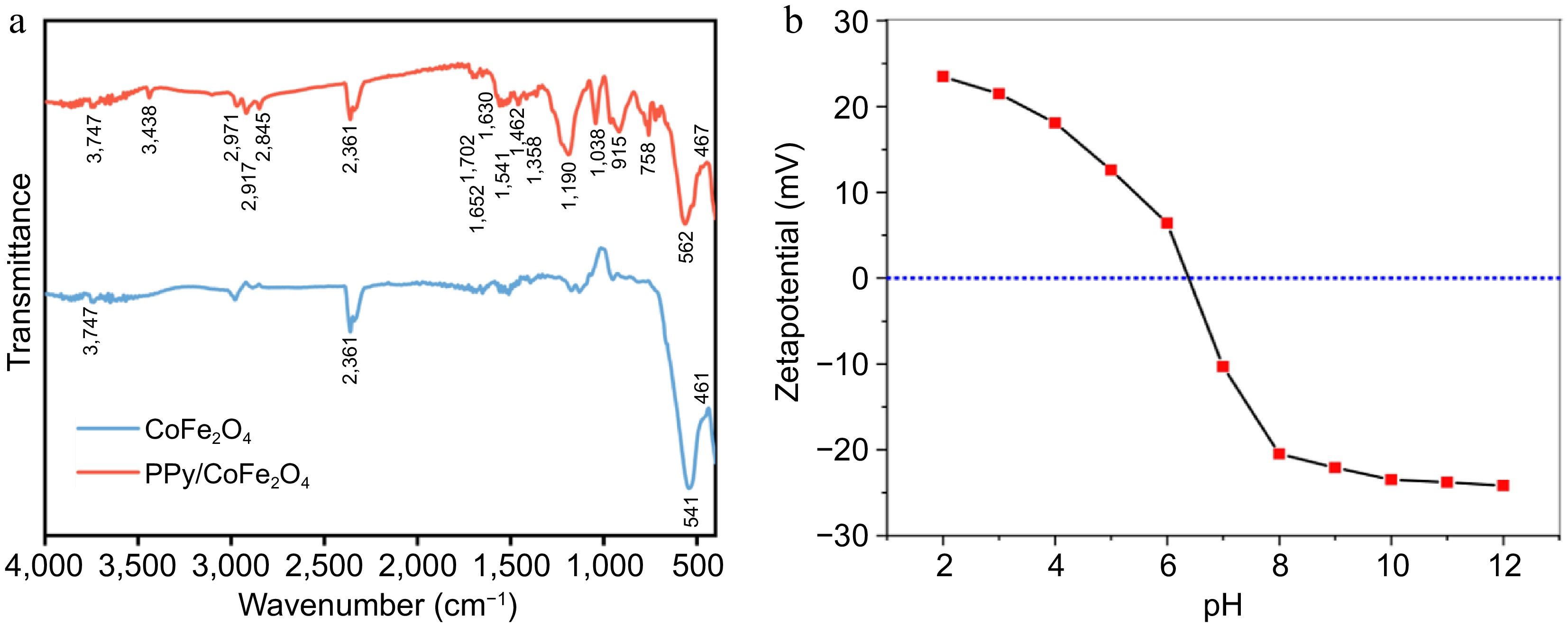
Figure 3.
(a) FTIR spectrum of synthesized PPy/CoFe2O4 and CoFe2O4. (b) pH's influence on PPy/CoFe2O4's zeta-potential.
-
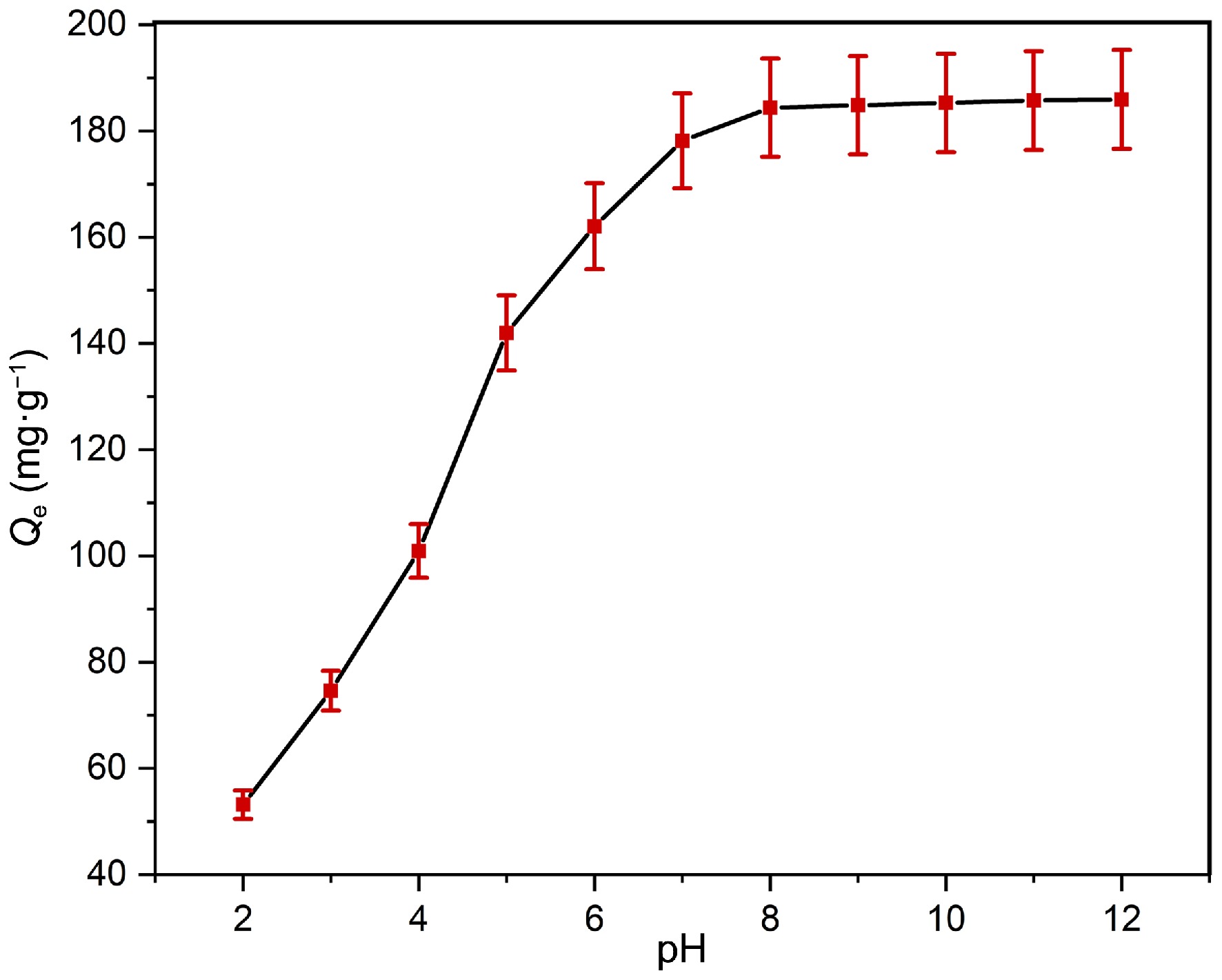
Figure 4.
Impact of pH on adsorption capability of Pb(II) at contact time = 120 min, adsorbent dose = 0.16 g·L−1, T = 303 K, and Pb(II)] = 30 mg·L−1.
-
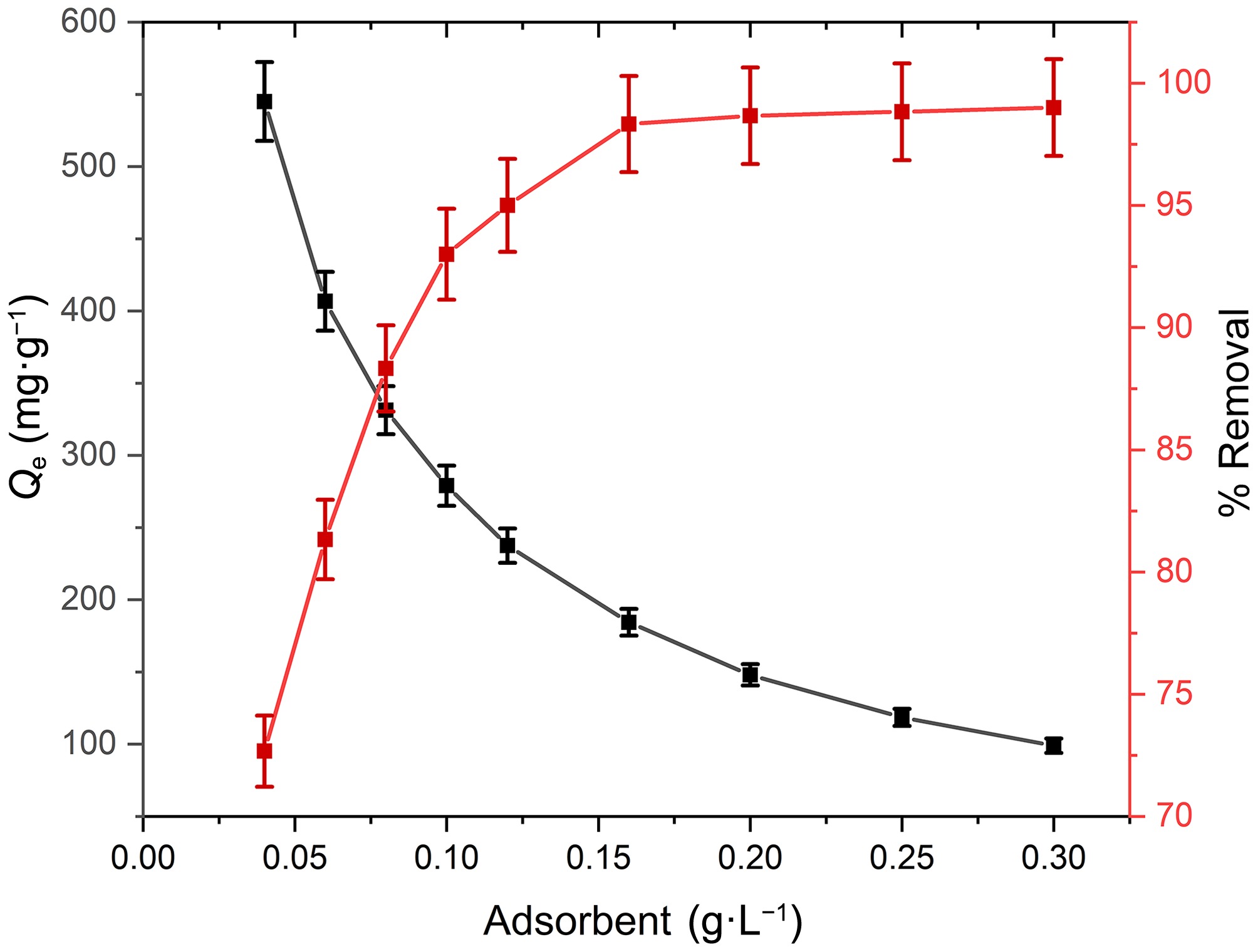
Figure 5.
Impact of adsorbent dose on adsorption capacity and % removal Pb(II) at contact time = 120 min, T = 303 K, pH = 8.0, and [Pb(II)] = 30 mg·L−1.
-
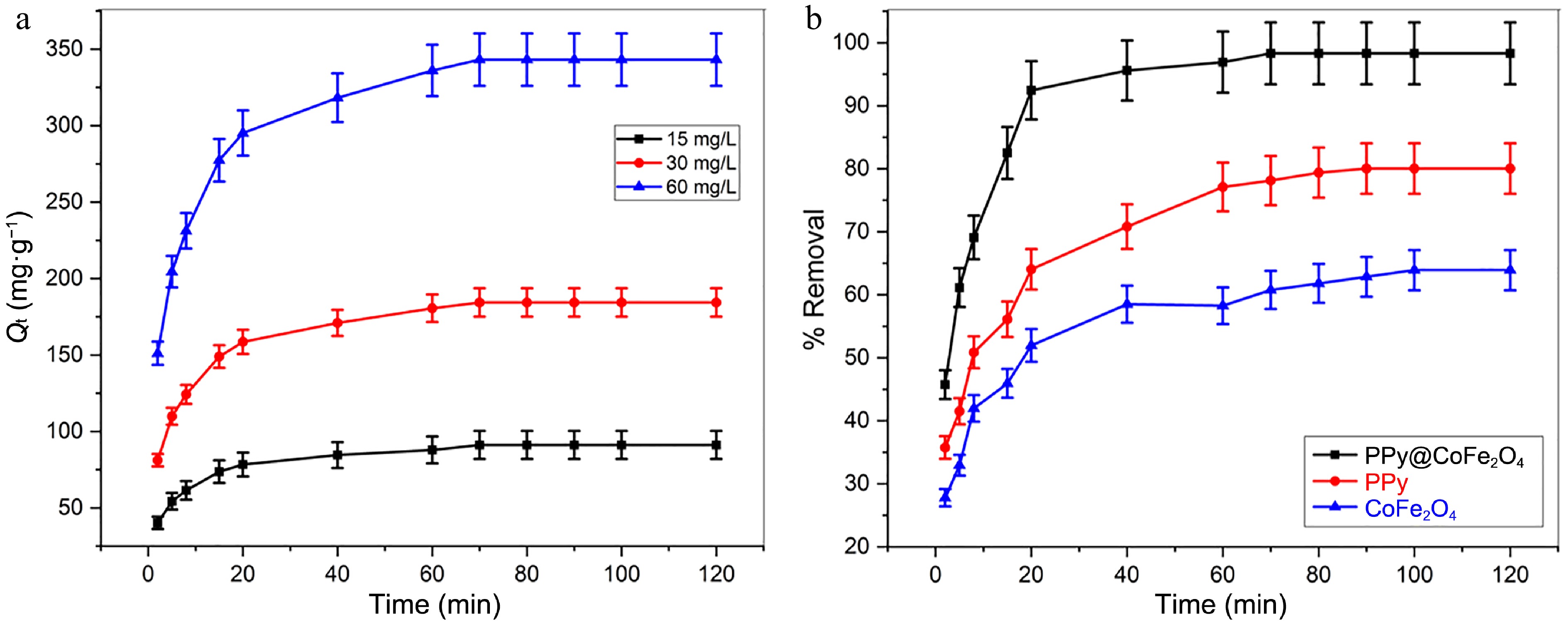
Figure 6.
(a) Impact of [Pb(II))] and contact duration on adsorption capability of Pb(II) at contact time = 120 min, T = 303 K, pH = 8.0, and adsorbent dose = 0.16 g·L−1. (b) Analysis of the efficacy of Pb(II) removal utilizing PPy/CoFe2O4, PPy, and CoFe2O4 at contact time = 120 min, adsorbent dose = 0.16 g·L−1, T = 303 K, pH = 8.0, and [Pb(II)] = 30 mg·L−1.
-
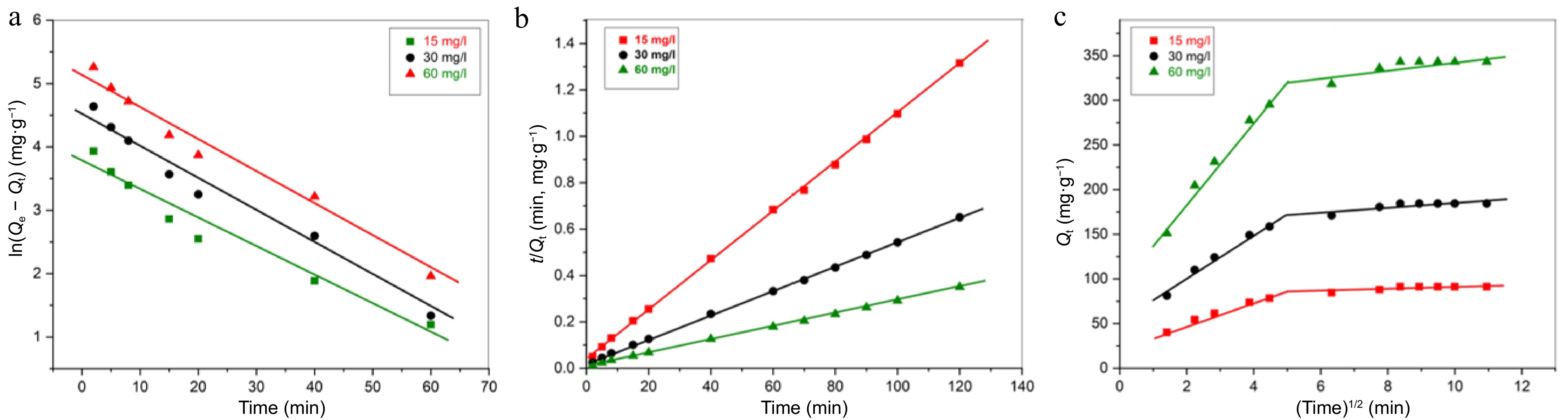
Figure 7.
(a) PFO kinetic study, (b) PSO kinetic study, (c) IPD diffusion kinetic study for adsorption of Pb(II) by PPy/CoFe2O4 at contact time = 120 min, T = 303 K, pH = 8.0, [Pb(II)] = 30 mg·L−1, and adsorbent dose = 0.16 g·L−1.
-
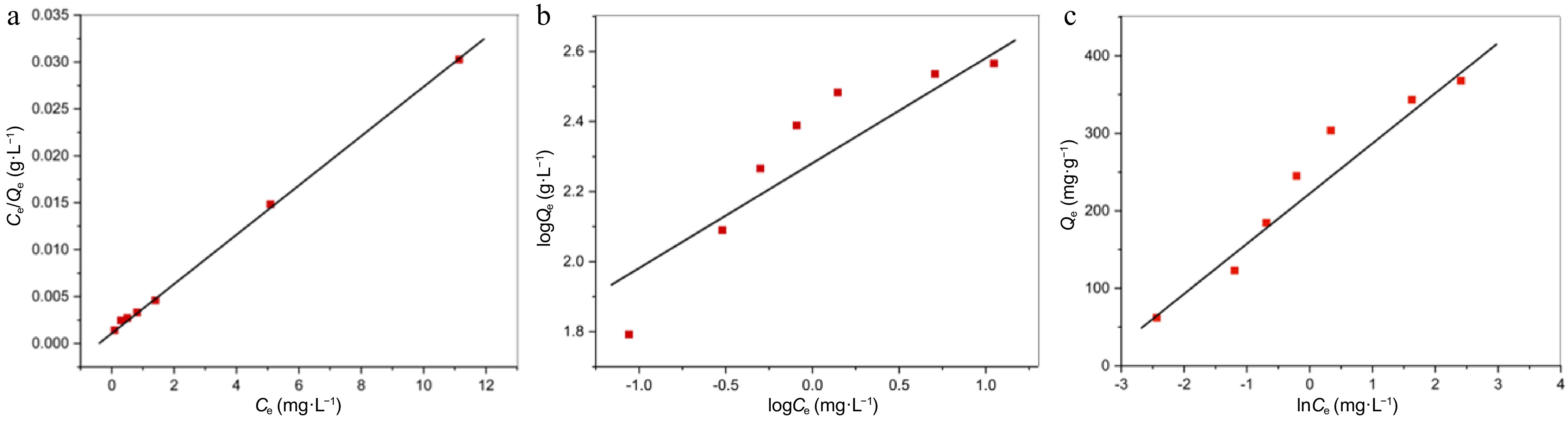
Figure 8.
(a) The relationship between Ce and Ce/Qe (Langmuir model). (b) The relationship between logCe and logQe (Freundlich model). (c) The relationship between lnCe and Qe (Temkin model).
-
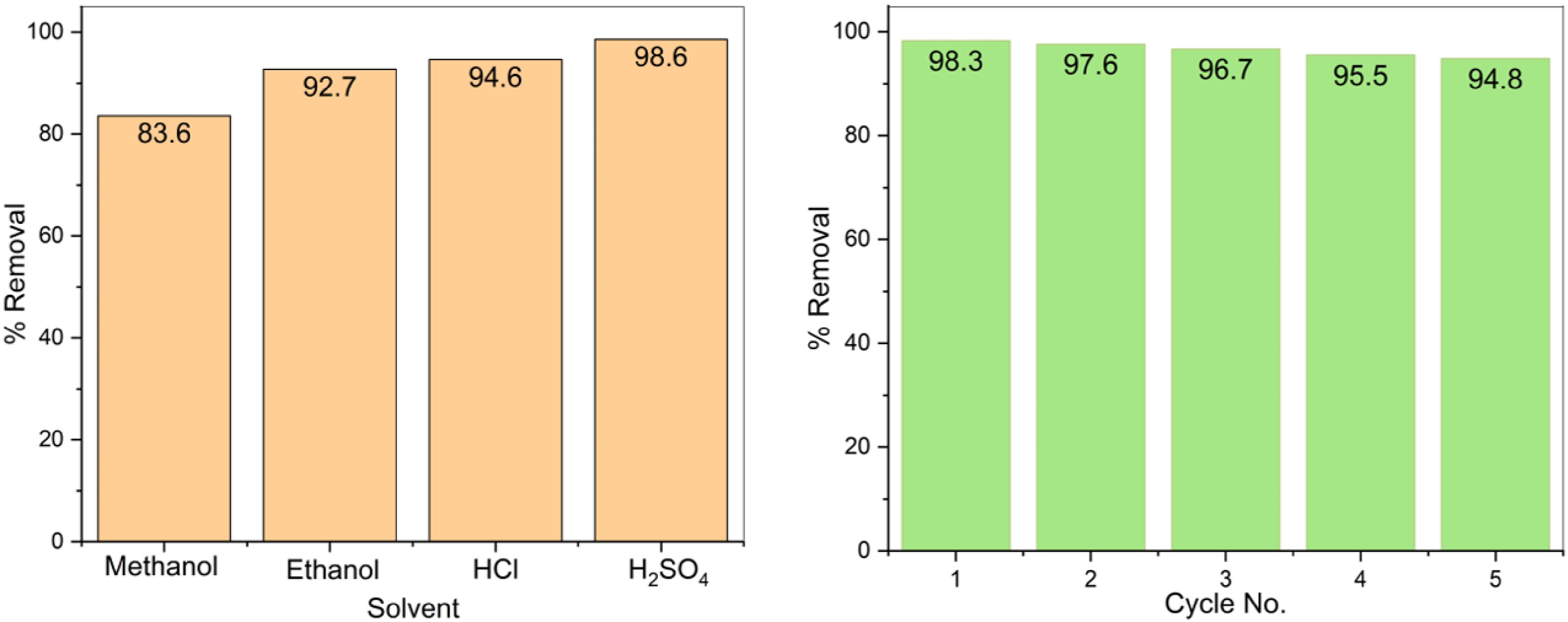
Figure 9.
(a) Effect of solvent on regeneration of PPy/CoFe2O4. (b) PPy/CoFe2O4 reusability.
-
Kinetic models Variables Initial Pb(II) content (mg·l−1) 15 30 60 Pseudo-first order kinetic Qe (mg·g−1) 42.26 93.60 173.27 R2 0.9582 0.9797 0.9672 k1 (min−1) 0.0552 0.0532 0.0531 Pseudo-second order kinetic k2 (g−1·mg−1·min−1) 3.07 × 10−3 1.43 × 10−3 7.54 × 10−4 Qe (mg·g−1) 92.59 188.68 357.14 R2 0.9995 0.9991 0.9989 Qe (mg·g−1) [Experimental] 91.2 184.4 343.2 Table 1.
Variables computed from the PSO, and PFO kinetic model.
-
Temp (K) ∆S# (kJ·mole−1) ∆H# (J·K−1 mole−1) ∆G# (kJ·mole−1) 298 −128.2 − 42.2 − 2.4 303 − 3.3 308 − 4.5 313 − 5.5 318 − 6.6 Table 2.
Thermodynamic variables for adsorptive removal of Pb(II) by PPy/CoFe2O4.
-
Isotherm Constant values Freundlich KF (mg·g−1 ) = 202.63; 1/n = 0.361; R2 = 0.8592 Langmuir RL = 0.044 − 0.007; R2 = 0.9995; KL (L·mg−1) = 2.15;
Qmax (mg·g−1) = 357.14Temkin B = 67.30; KT (L·g−1) = 32.46; R2 = 0.9415 Table 3.
Freundlich, Langmuir, and Temkin isotherm constants.
-
Adsorbent pH Contact time (min) Qmax
(mg·g−1)Ref. Polyaniline/Fe3O4 9.0 120 111.11 [45] Polythiophene-coated rice husk ash nanocomposite 4.0 240 34.48 [46] Polyaniline Sn(IV) tungstomolybdate nanocomposite 6.0 60 44.64 [48] Fig sawdust activated carbon 4.0 120 80.64 [50] Sulfonated magnetic nano particle 7.0 1440 108.93 [51] Poly(acrylic acid)/bentonite nanocomposite -- 1440 93.01 [52] Amino-functionalized magnetic nanoadsorbent 5.0 60 40.1 [53] Ti(IV) iodovanadate cation exchange 6.0 120 18.8 [54] Palygorskite-iron oxide nanocomposite 5.0 720 26.7 [55] Chitosan-starch composite 3.46 300 99.01 [56] PPy/CoFe2O4 8.0 120 357.14 This work Table 4.
Competitive assessments of the adsorption capabilities of Pb(II) onto diverse adsorbent materials.
Figures
(9)
Tables
(4)