-
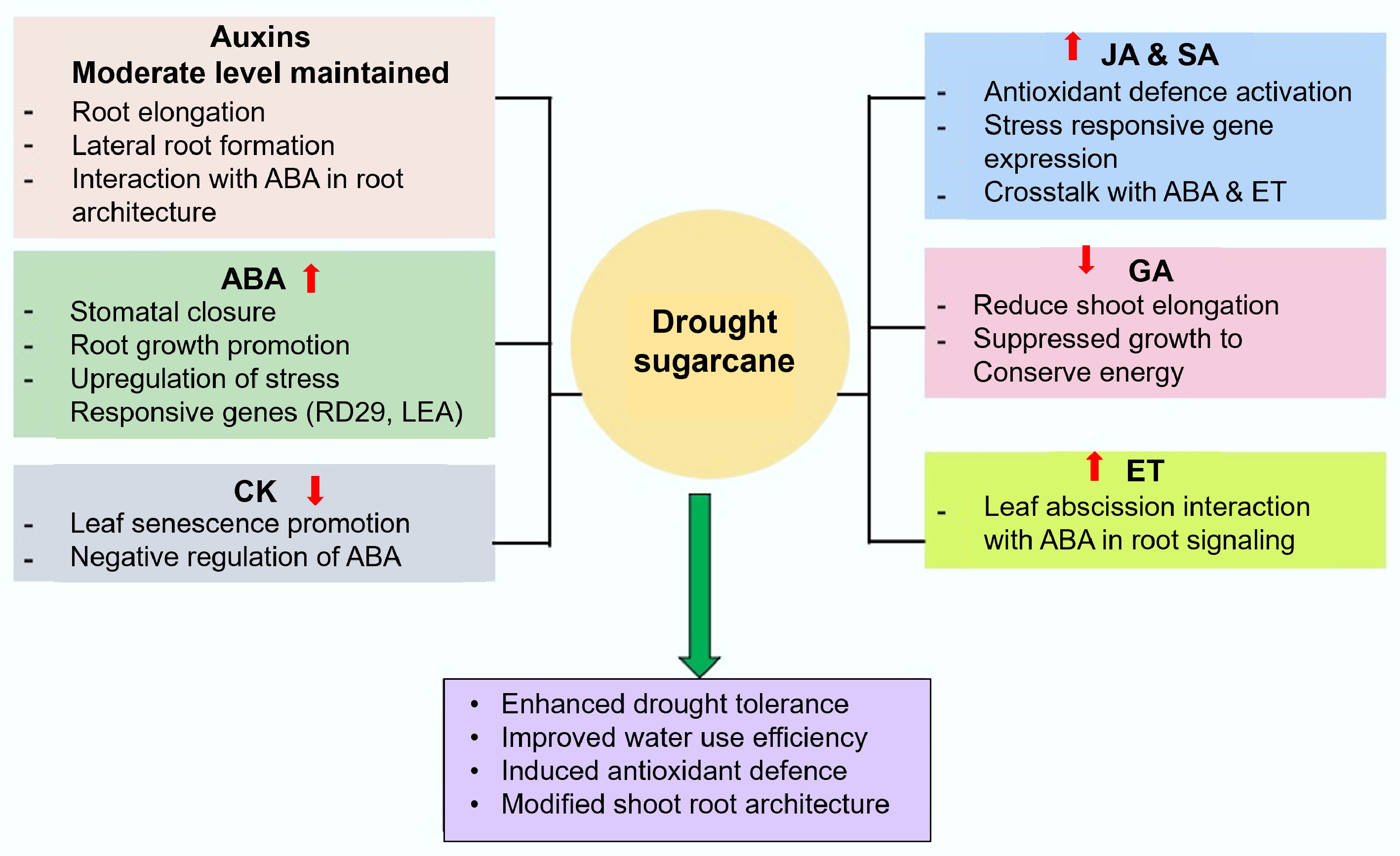
Figure 1.
Role of phytohormones in sugarcane under drought stress. JA: Jasmonic Acid; GA: Gibberellic Acid; ABA: Abscisic Acid; ET: Ethylene; CK: Cytokinin; SA: Salicylic Acid.
-
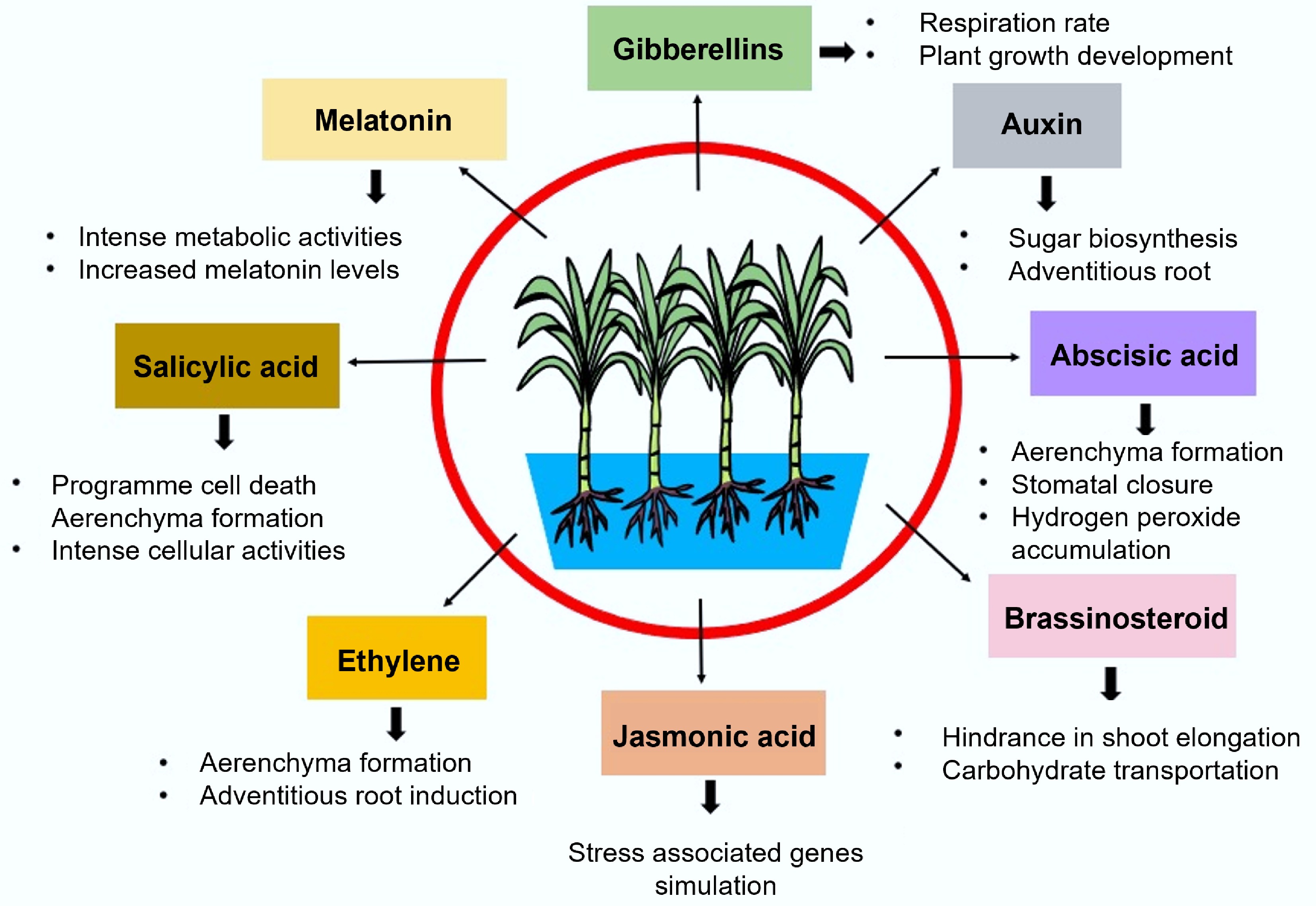
Figure 2.
Phytohormone induced waterlogging stress resistance in sugarcane.
-
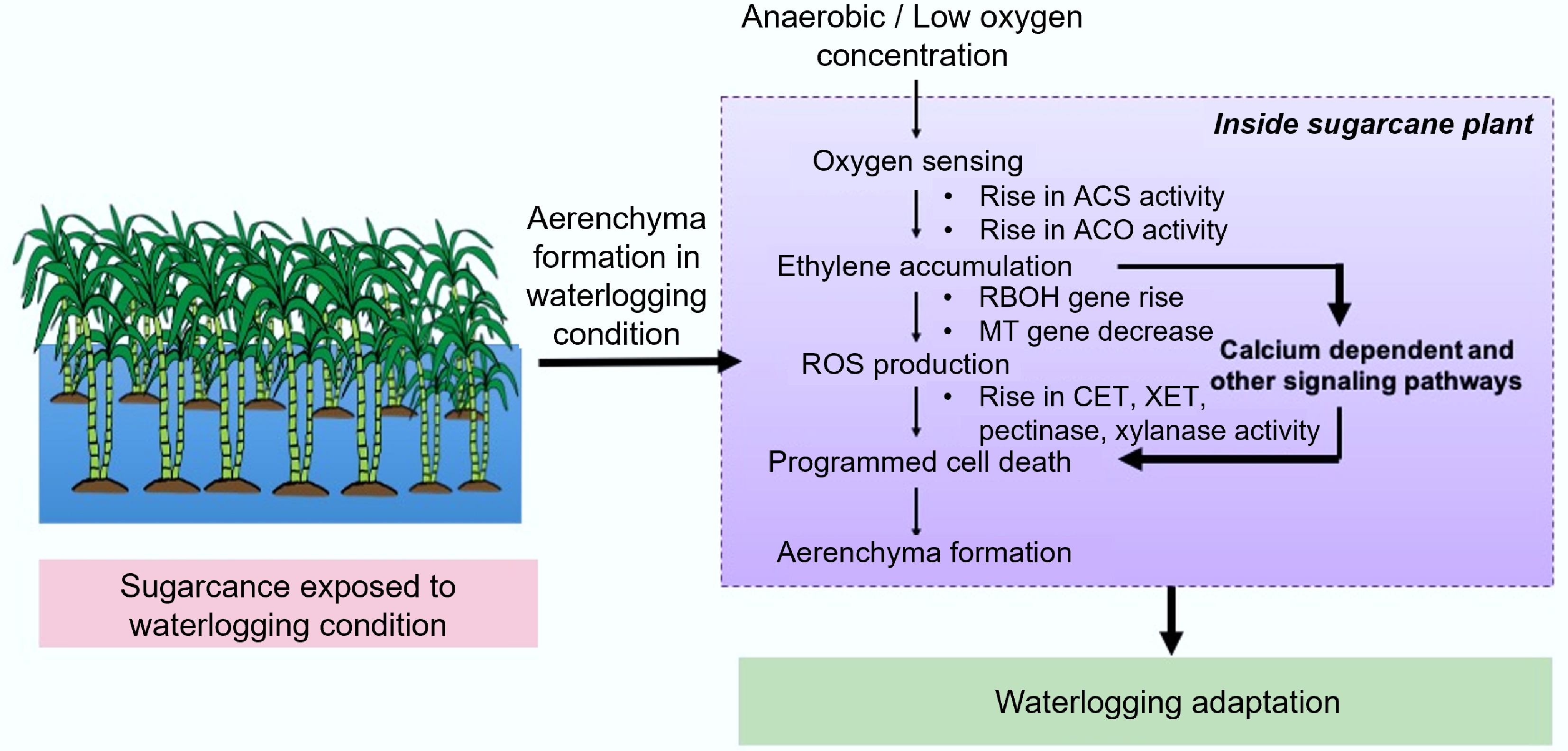
Figure 3.
Lysigenous aerenchyma formation and its pathway waterlogging conditions. ACS: Aminocyclopropane-1-carboxylix Acid Synthase; ACO: ACC Oxidase; CEL: Cellulase; XET: Xyloglucan Endotransglycosylase; MT: Metallothionein; RBOH: Respiratory Burst Oxidase Homolog.
-
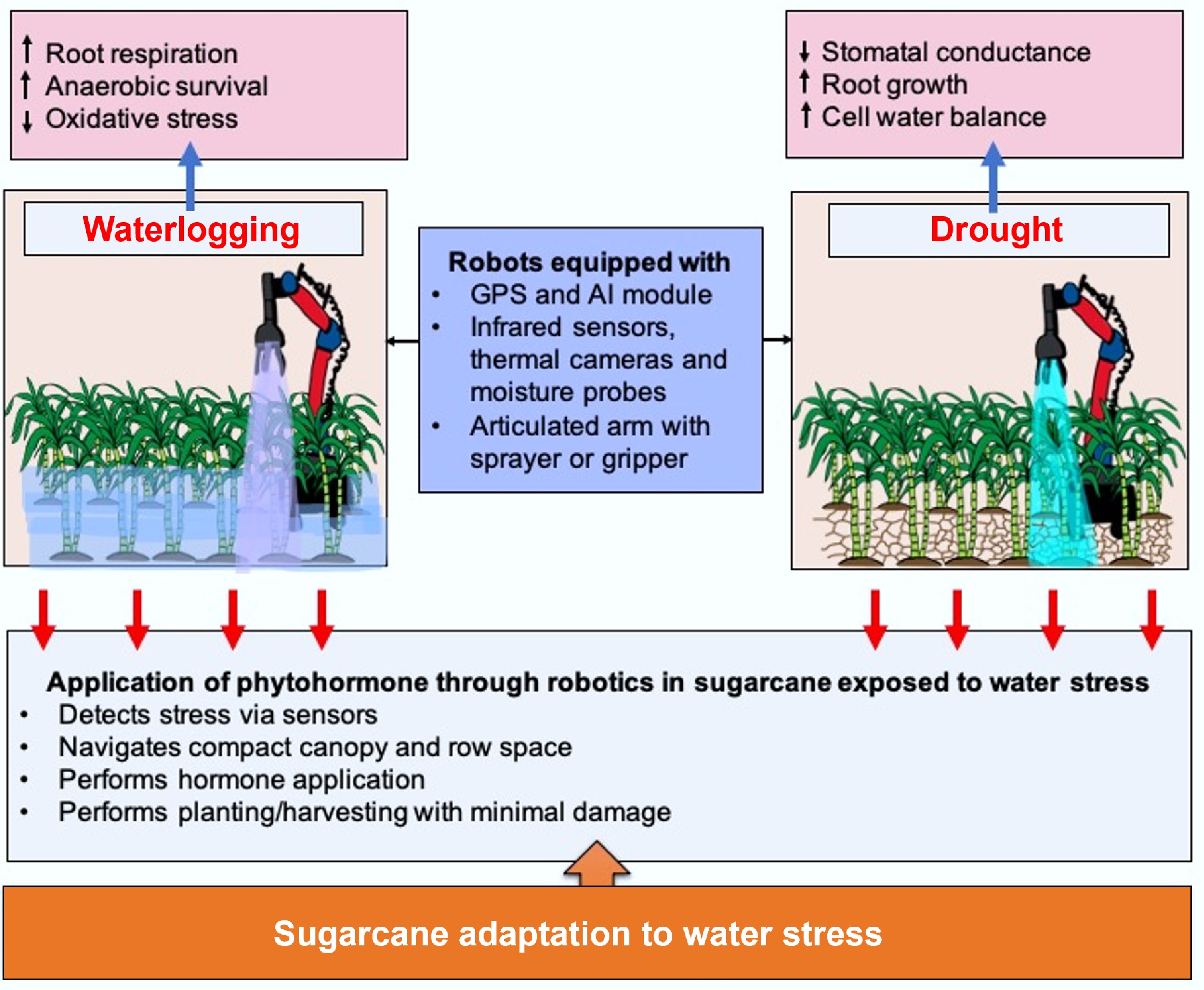
Figure 4.
Utilisation of robotics for phytohormone application on sugarcane for water stress resilience.
-
Phytohormones Endogenous phytohormone Exogenous phytohormone Origin Naturally produced within plants External application (e.g., foliar spray and root drench) Mode of action Promote internal signalling and physiological adaptations during stress Replicates internal signalling pathways for speed response Response timing Activation on stress perception, with slower onset Pre-condition to plant prior to stress, with faster onset Target sites Tends to act in site of synthesis or transmitted through phloem/xylem Can be targeted through discrete plant tissue for localized action Control Plant genetic and biochemical condition Externally by concentration, application method and timing Constrains May not react quickly under acute or intensive stress May pose phytotoxicity risks or ineffectiveness on excessive dosage or poorly schedule dosage Table 1.
Comparison of exogenous and endogenous phytohormone mechanisms in sugarcane for water stress regulation
-
Phytohormones Effect of drought Effect of waterlogging Ref. Abscisic acid (ABA) Promotes stomatal closure, osmotic adjustment, root growth, enhancement in antioxidant activity; increase in hydrogen peroxide
for 5 d after treatment; Activation of enzymes;
high ROS production; high relative water contentNo direct evidence available [22,93,104] Gibberellins (GAs) Rise in water demand; restoration of RWC; cell elongation increase;
rise in chlorophyll content; lower biomass; rise in shoot dry matter[111] Auxins (IAA, NAA) No direct evidence available Enhancement in nutrient uptake under anaerobic soil; adventitious rooting growth promotion but no significant changes across different developmental stages; no significant impact on juice quality [99] Table 2.
Comparative effect of exogenous phytohormones in sugarcane under water stress
-
Crops Encapsulated phytohormones Mechanism of stress resilience Encapsulation method and capsule material used Effects recorded Ref. Maize
(Zea mays)AB, SA, IAA Stomatal closure regulation; antioxidant defence control; osmoprotection and growth promotion, ROS lowering − Higher drought tolerance, better grain filling and lower oxidative stress [152,153] Arabidopsis thaliana ABA Intracellular glutathione concentrations increased (as happens during drought stress), the
glutathione cleaved the disulfide bond, removing the decanethiol gatekeeper and enabling ABA release. Slower, more sustained release, providing longer-lasting ABA signalling within plant tissues during stress responsesSol gel encapsulation; amorphous silica through transpiration. Provides resistance against drought stress [137] Arabidopsis thaliana ABA Triggers stomatal closure, reduce transpiration In situ polymerisation;
ligninEncapsulated ABA retained >75% after 60 h UV; free ABA degraded rapidly;
High drought resistance[138] Rice (Oryza sativa) ABA − In situ polymerisation;
ligninRegulate plant stomata;
High drought resistance[138] Table 3.
Nanoencapsulation phytohormone application in other crops related to sugarcane under water stress conditions
Figures
(4)
Tables
(3)