-
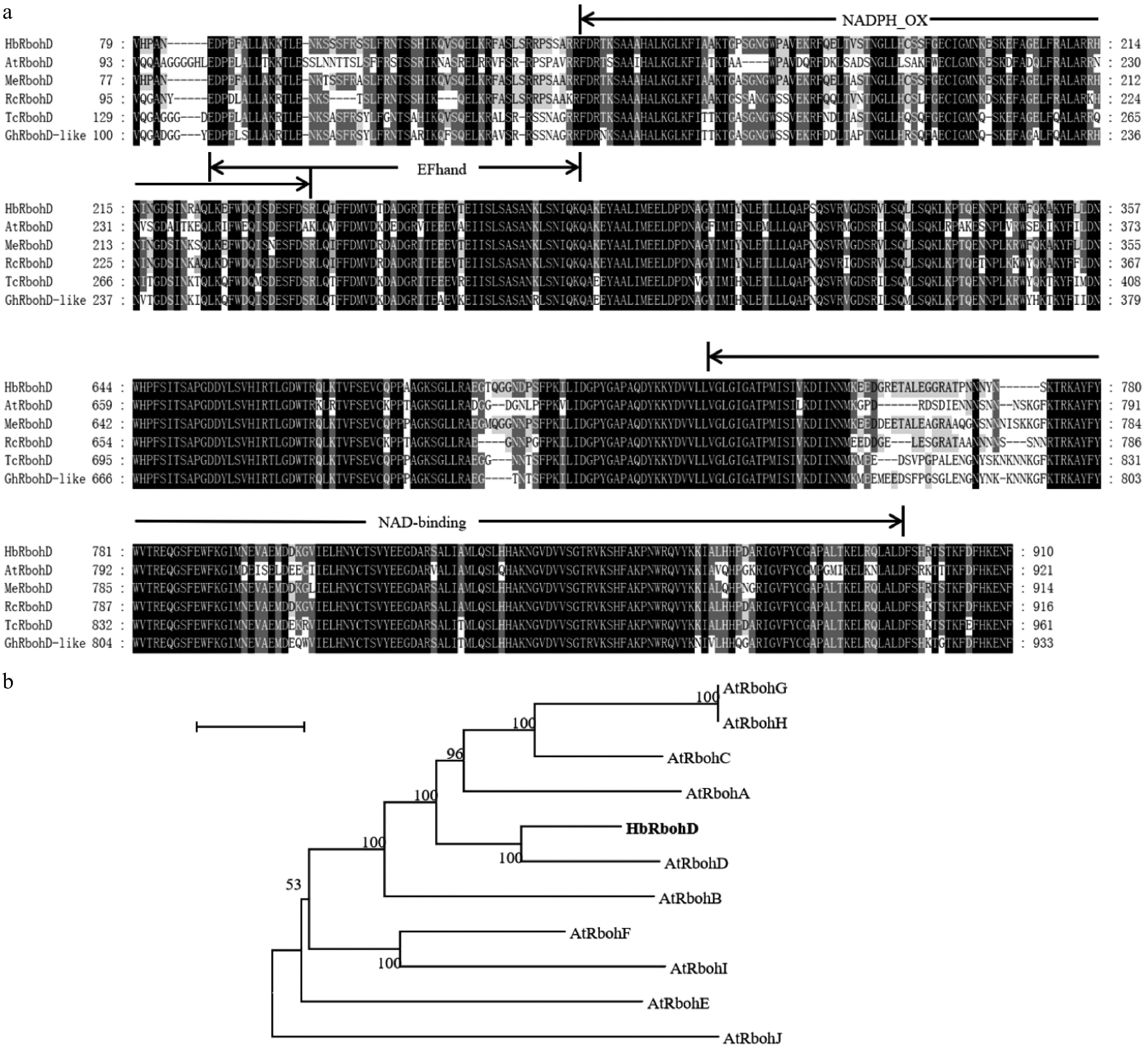
Figure 1.
Multiple sequence alignment and phylogenetic analysis of HbRbohD. (a) Comparative analysis of RbohD homologs across diverse plant species. Conserved domains are highlighted, with regions identical in all examined sequences shown in black, while residues matching HbRbohD are denoted in gray. AtRbohD was from Arabidopsis thaliana (NP_199602), MeRbohD was from (XP_021624267), RcRbohD was from Ricinus communis (XP_015570593), TcRbohD was from (EOY14749), GhRbohD-like was from (XP_040943195). (b) Phylogenetic tree of HbRbohD with Rboh proteins from A. thaliana, including AtRbohA (NP_196356), AtRbohB (NP_172383), AtRbohC (NP_199919), AtRbohD (NP_199602), AtRbohE (NP_173357), AtRbohF (NP_564821), AtRbohG (NP_194239), AtRbohH (NP_200809), AtRbohI (NP_192862), AtRbohJ (NP_190167).
-
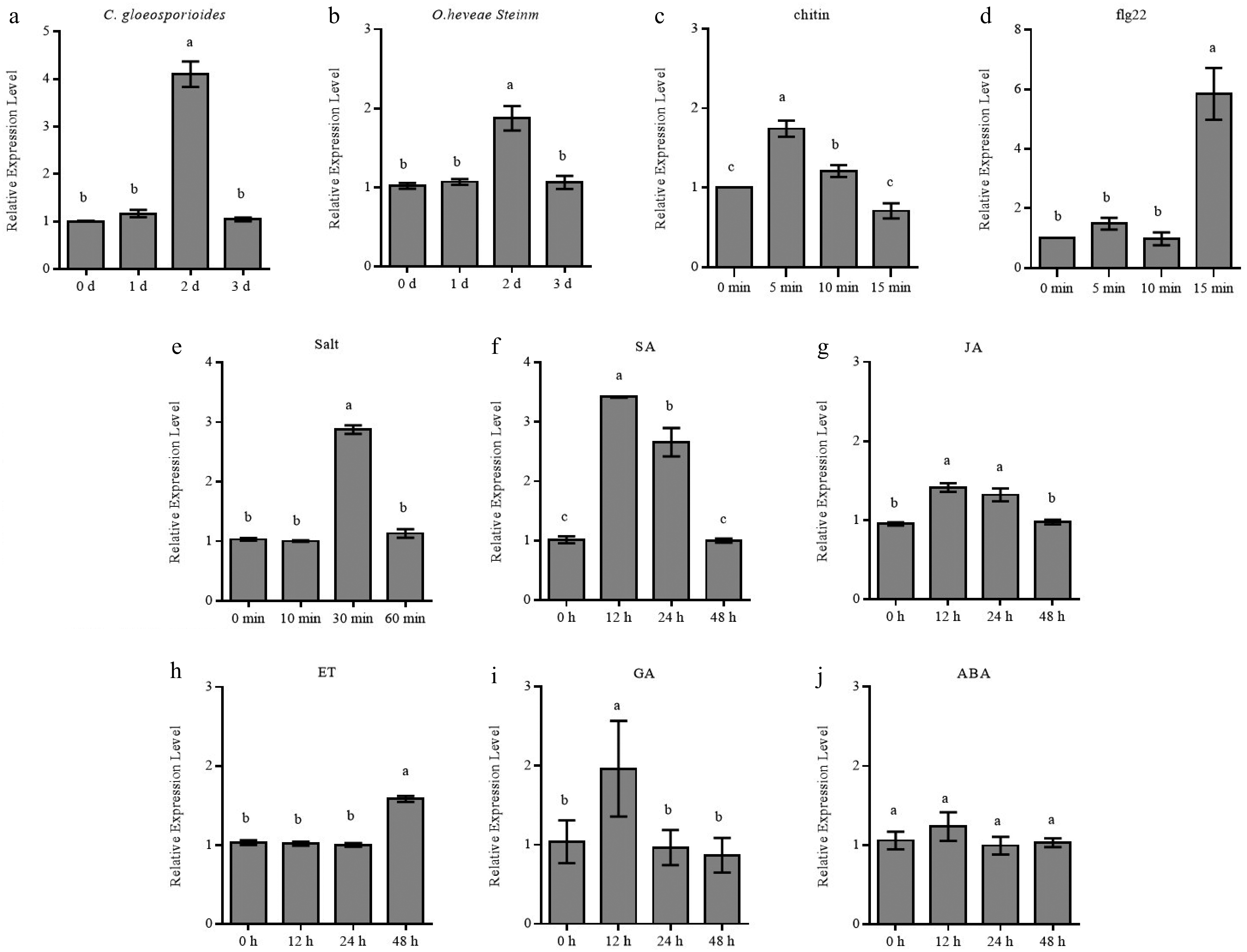
Figure 2.
Expression patterns of HbRbohD in response to stresses and phytohormone treatments. The expressions of HbRbohD in rubber tree leaves inoculation with (a) C. gloeosporioides, and (b) O. heveae Steinm, in rubber tree mesophyll protoplasm treated with (c) chitin, and (d) flg22, in rubber tree suspension callus cultures treated with (e) salt, and treatment with various phytohormone, such as (f) SA, (g) JA, (h) ET, (i) GA, and (j) ABA were quantitatively assessed through qRT-PCR analysis, with transcript abundance being normalized against baseline measurements obtained at day 0 (control). The expression level was normalized using actin as housekeeping gene. Data are shown as the means ± SD from three independent experiments and the mean values assigned dissimilar superscript letters differ significantly (p < 0.05).
-
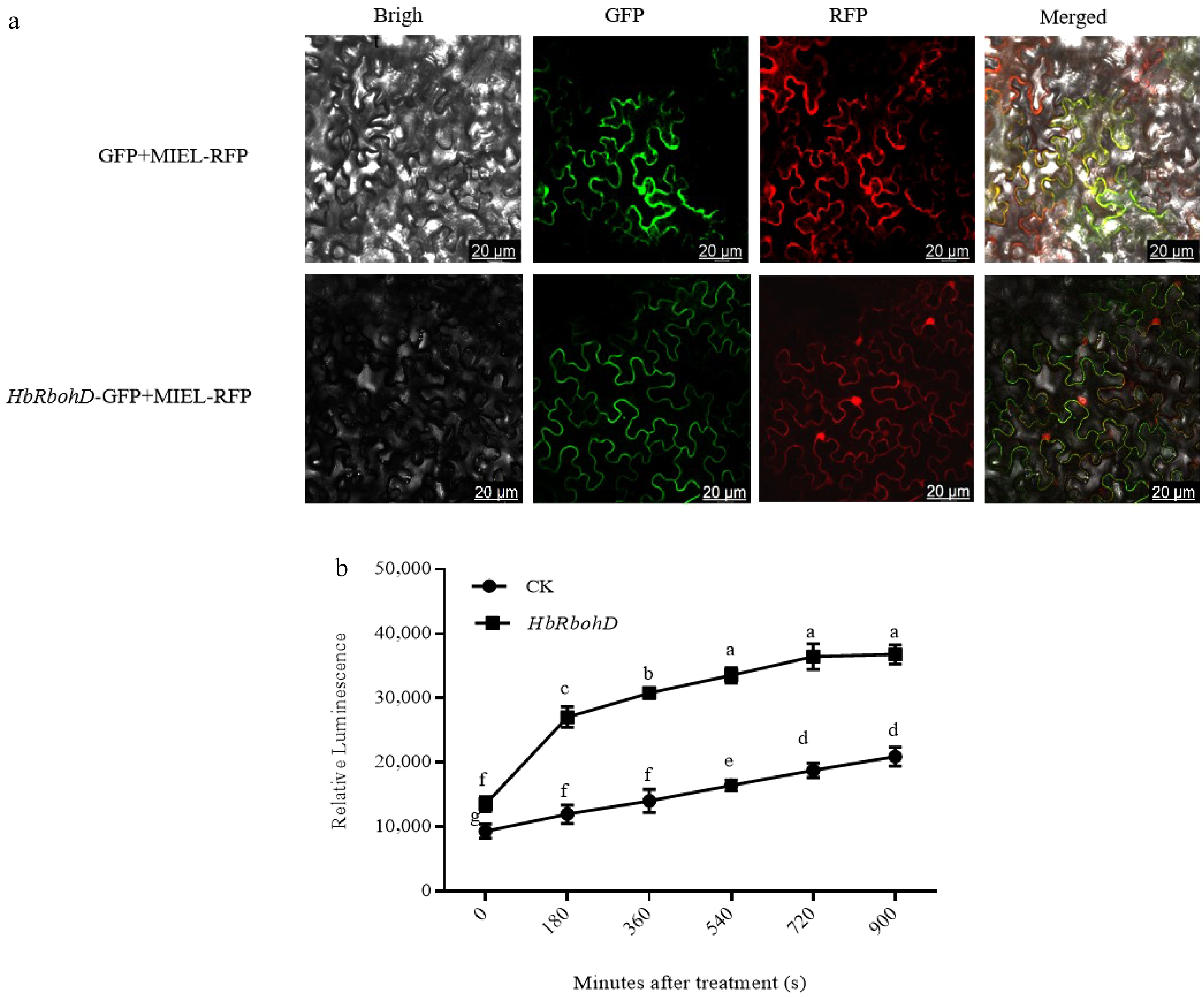
Figure 3.
Subcellular localization of HbRbohD and the function of HbRbohD on ROS production. (a) Subcellular localization of HbRbohD. Tobacco leaves co-expressing GFP/MIEL-RFP and HbRbohD-GFP/MIEL-RFP were viewed with fluorescent microscope 24 h after infiltration (bar, 20 μm). (b) The function of HbRbohD on ROS production. The oxidative degree in protoplasts was detected by dichlorofluorescin diacetate (DCFH-DA) and the fluorescence intensity was measured every 3 min for 15 min. Data presented are the mean ± SD from three independent experiments and the mean values assigned dissimilar superscript letters differ significantly (p < 0.05).
-
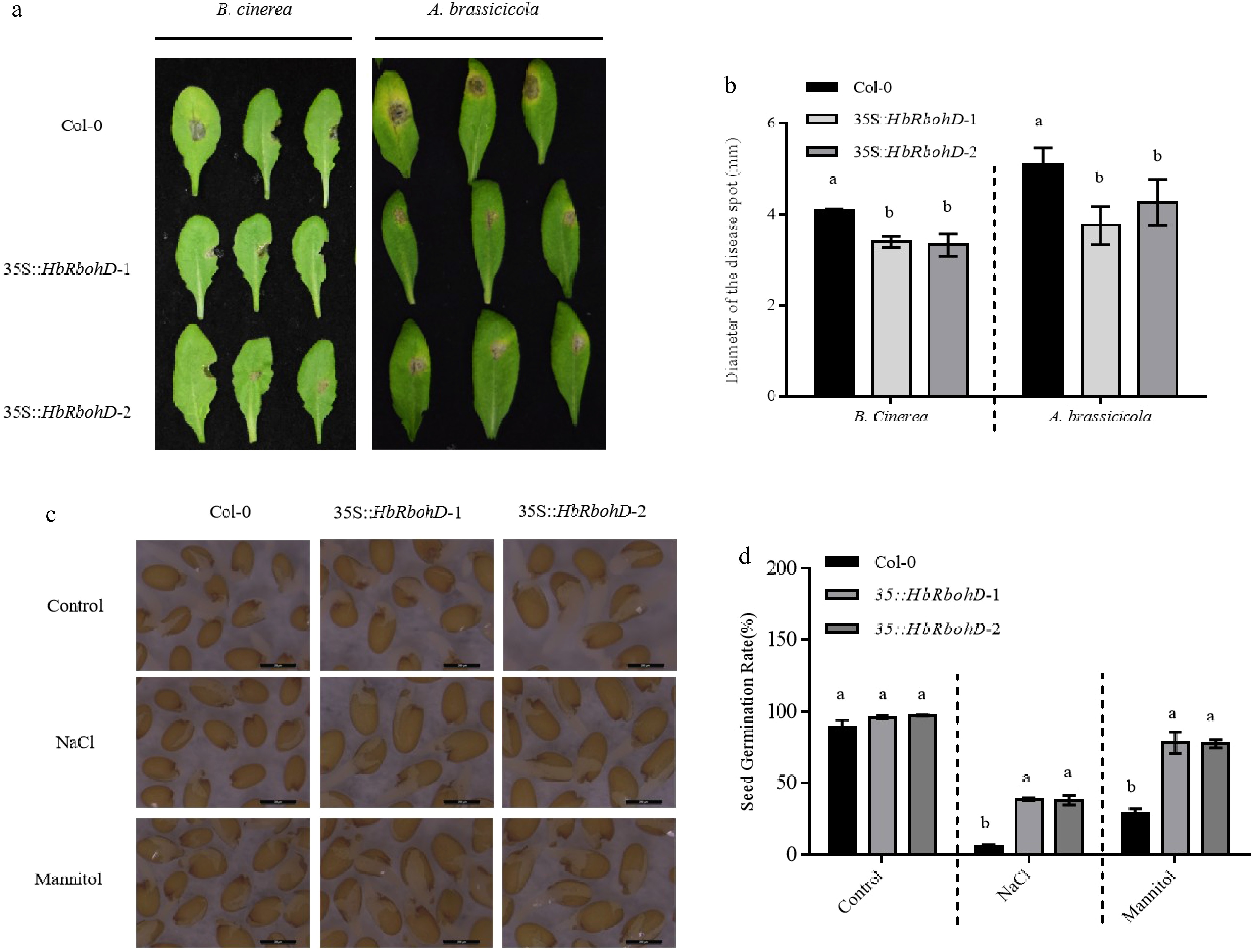
Figure 4.
Disease and seed osmotic tolerance assay of Col-0 and 35S::HbRbohD. (a) Disease symptoms of A. thaliana Col-0 and 35S::HbRbohD to B. cinerea and A. brassicicola. (b) Statistical analysis of necrotic lesion in WT and 35S::HbRbohD. The symptoms observation and the lesion measure were performed at 3 d post inoculation with B. cinerea and A. brassicicola, respectively. (c) Seed germination of Col-0 and 35S::HbRbohD on ½ MS medium with exogenous NaCl treatment (150 mM) and mannitol treatment (200 mM). (d) The quantitative analysis of germination rates of Col-0 and 35S::HbRbohD on ½ MS medium with exogenous NaCl treatment (150 mM), and mannitol treatment (200 mM). Data presented was the mean ± SD from three independent experiments and the mean values assigned dissimilar superscript letters differ significantly (p < 0.05).
-
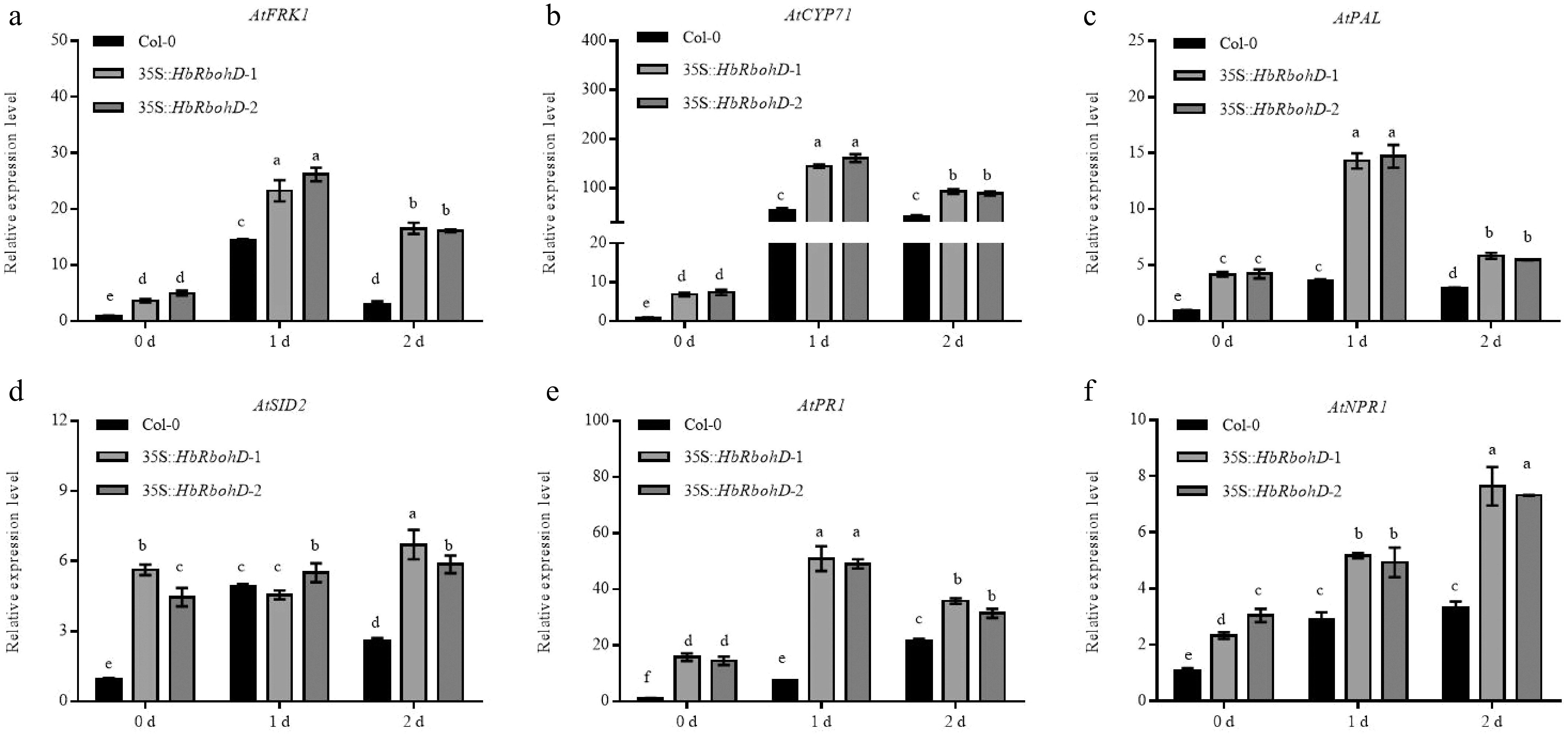
Figure 5.
Relative expression assay of defense related genes in Col-0 and 35S::HbRbohD inoculated with B. cinerea. The expression of defense related genes, such as (a) AtFRK1, (b) AtCYP71, (c) AtPAL, (d) AtSID2, (e) AtPR1, and (f) AtNPR1, were analyzed by qRT-PCR in Col-0 and 35S::HbRbohD inoculated with B. cinerea. Data are shown as the means ± SD from three independent experiments. Different letters above columns indicate a significant difference (p < 0.05).
-
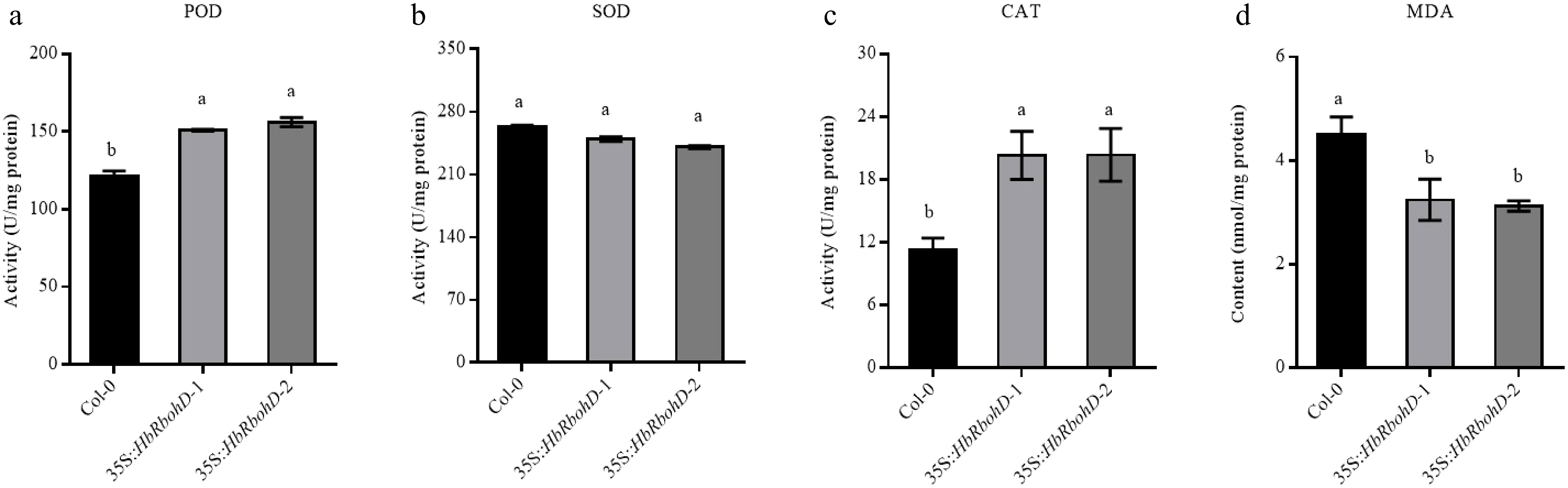
Figure 6.
Activity of antioxidant enzymes and MDA content in Col-0 and 35S::HbRbohD. The key antioxidant enzyme activities of (a) POD, (b) SOD, (c) CAT, and the content of (d) MDA were analyzed in Col-0 and 35S::HbRbohD, respectively. Data presented was the mean ± SD from three independent experiments and the mean values assigned dissimilar superscript letters differ significantly (p < 0.05).
-
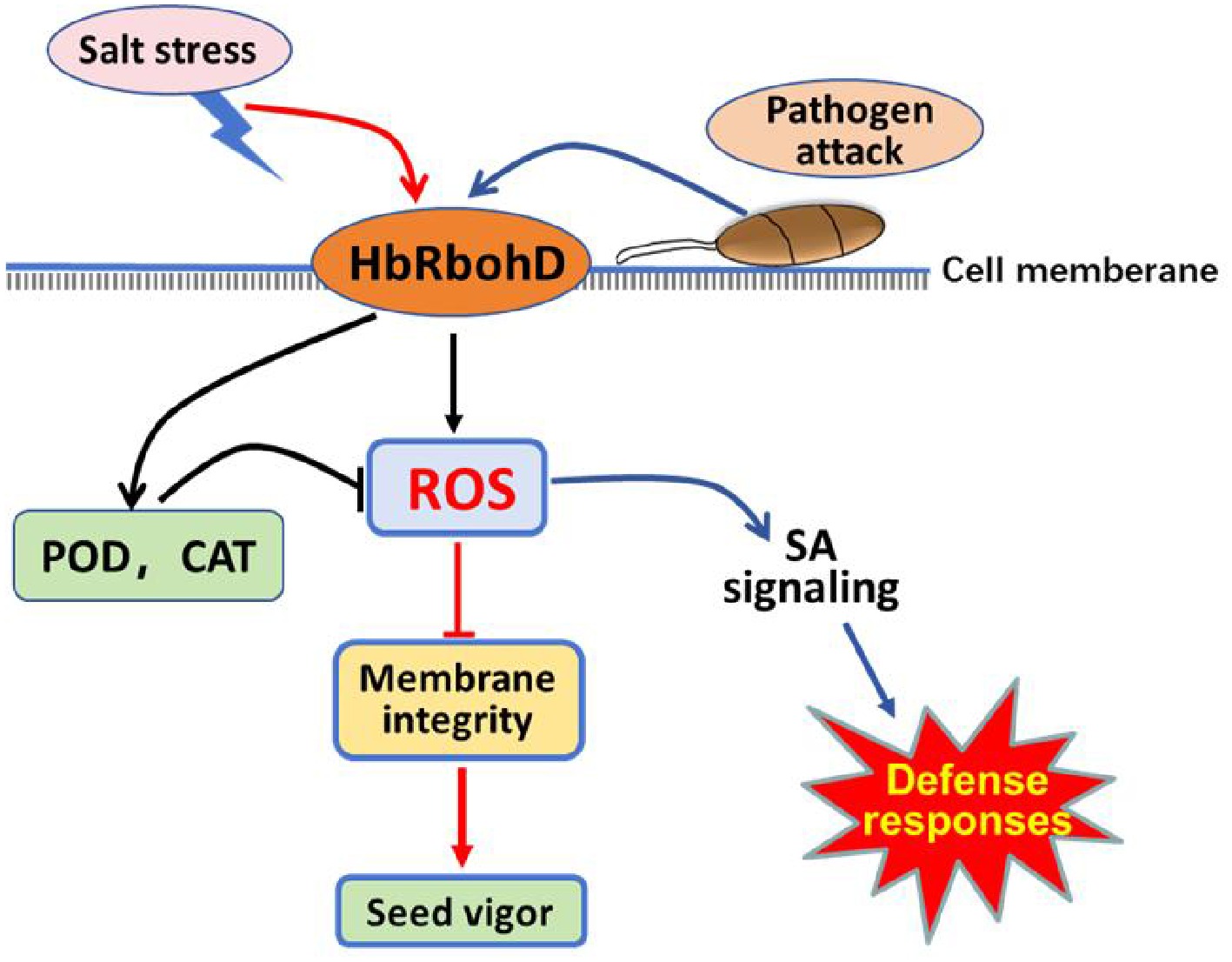
Figure 7.
Proposed model of respiratory burst oxidase homolog D of rubber tree (HbRbohD) signaling in response to biotic and abiotic stressed. HbRbohD regulates ROS homeostasis through antioxidant enzymes to regulate cell membrane integrity and seed vigor under salinity stress conditions (red arrows). HbRbohD also regulates the expression of defense-related genes,especial, enabling plants to respond to pathogen attack (blue arrows). The black arrows represent the common pathway shared by salt stress and pathogen attack.
Figures
(7)
Tables
(0)