-
Floral volatiles emitted from angiosperm plants, primarily comprising with terpenoids, phenylpropanoids/benzenoids, fatty acid derivatives, and nitrogenous compounds, play vital ecological roles in attracting pollinators and florivores defense[1,2]. As for the ornamental plant species, various floral colors and shapes are generally the primary targeted traits during the breeding and cultivation with neglecting of the scent trait. It has been extensively accepted that the domestication of ornamental cultivars of different species could lead to the loss of floral scent characteristics due to the complex cross-interaction of the metabolism pathway between floral volatile compounds and pigments[3−5]. Therefore, it is of great significance to regain the lost fragrance in the cultivars by crossing with scented wild species or genetic manipulation.
The Clematis genus comprises approximately 300 wild species and numerous garden hybrids, which is one of the most popular perennial climbing ornamental plants worldwide[6,7]. Based on the morphology of the perianth, species in Clematis genus have been classically subdivided into three infrageneric groups, including spreading sepals in a flat disc shape, bell-like with ascending sepals, and tubular with erect sepals[7]. Among the species of the genus Clematis, C. florida and C. viorna have been subjected to intensive breeding and selection, which is the primary source of the currently available Clematis cultivars.
Although various wild Clematis are scented, the majority of cultivars have no detectable scent. It will be intriguing to introduce fragrances into Clematis by using different wild species as breeding materials. Therefore, the evaluation and effective use of the fragrant characteristics in a greater number of wild species and cultivars is essential for the producing a wide range of fragrant interspecific hybrids. The qualitative and quantitative variation of floral volatile composition between cultivars and species has been widely observed in other ornamental species[8−10]. However, no comparative study has been performed between species and cultivars in Clematis genus.
In this context, we investigated the diversity and compared the variation of floral scent of representative aromatic wild species and horticultural cultivars in Clematis genus. We expect to encounter significant variation in their floral volatile profiles. To evaluate and utilize the valuable aromatic germplasm resources in Clematis, the floral volatile compounds of four species and five cultivars with varied aroma intensity screened by initial sensory evaluation were identified by dynamic headspace collection coupled with gas chromatography-mass spectrometry (GC-MS) analysis. Our study could provide a theoretical and practical basis for the breeding and cultivation of aromatic Clematis varieties.
-
The flowers of the wild Clematis species and cultivars at the stage of full blooming were grown at the resource nursery in the Institute of Subtropical Crops of Zhejiang Province (120°37'51.1” E, 28°0' 7.34” N), China. All the plants were cultivated under the same conditions of fertilization, irrigation, disease prevention, and pesticide application. The flowers of different wild species and cultivars were collected for sensory evaluation and volatile collection in 22−28, April, 2018.
Screening the aromatic species and cultivars by sensory evaluation
-
The aroma intensity of 29 available Clematis species and 42 cultivars were determined initially by the survey of the panel composed of 10 people (five males and five females) aging from 23−54. In this study, approximately 5−7 g of flowers at full-openness stages of each species/cultivars were collected and carefully placed in clean water within a semi-open room with proper air circulation, which allows them to acclimate for a period of 1 h. After completing the olfactory test, all participants evaluated the aroma intensity of each sample. The aroma intensity of all Clematis species/cultivars were classified as three degrees of strong, medium and weak[11]. Finally, four wild species (W1−W4) and five cultivars (C1−C5) of Clematis were screened out as aromatic Clematis species/cultivars for the following volatile collection and identification (Table 1).
Table 1. Information and classification of the selected Clematis wild species and their cultivars.
Code Accession Classification Corolla
typesCollection
localityAroma
intensityAbbreviation 1 C. henryi Wild species (WS) Bell-shaped Wenzhou, Zhejiang, China Medium W1 2 C. finetiana Wild species spreading Wenzhou, Zhejiang, China Strong W2 3 C. courtoisii Wild species spreading Hangzhou, Zhejiang, China Medium W3 4 C. owatarii Wild species spreading Pingdong, Taiwan, China Weak W4 5 C. florida 'Diana's Delight' Cultivars (CS) spreading England Weak C1 6 C. florida 'Duchess of Edinburgh' Cultivars double petals Poland Weak C2 7 C. florida 'Marie Boisselot' Cultivars spreading French Strong C3 8 C. viorna 'Jade' Cultivars Bell-shaped Japan Medium C4 9 C. florida 'Bees Jubilee' Cultivars spreading Poland Weak C5 Floral volatile collection by dynamic headspace system
-
Volatiles emitted from intact flowers of screened Clematis were collected in an open headspace sampling system (Analytical Research Systems, Gainesville, FL, USA) as previously reported with minor modification[8]. A single detached inflorescence in a single flask with 150 mL of distilled water were placed in a glass chamber (20 cm diameter, 50 cm tall) covered with a removable lid. Charcoal-purified air entered the chamber at a flow rate of 1.3 L/min from the top through a Teflon hose. Volatiles were collected for 4 h by pumping air from the chamber through a Super Q volatile collection trap (Analytical Research Systems) and eluted using 100 µL methylene chloride containing nonyl acetate (0.003% w/v) as an internal standard. The semi-quantification relative to the internal standard (peak area) was applied for the quantification of floral volatiles.
Floral volatiles identification by GC-MS
-
Floral volatiles were analyzed by a gas chromatograph (Agilent-9000, Agilent Technologies, Santa Clara, CA, USA) coupled to a quadrupole mass selective detector (Agilent-7000D). Separation was performed on an Agilent HP 5 MS capillary column (30 m × 0.25 mm) with helium as carrier gas (5 ml/min of flow rate). A splitless injection (injection temperature 250 °C) was used, and a temperature gradient of 6 °C/min from 60 °C (3-min hold) to 300 °C was applied. Products were identified using the National Institute of Standards and Technology (NIST) mass spectral database. Semi-quantification was performed by comparing the peak area of individual compounds to that of the internal standard. Kovat's retention indices were calculated by injecting the hydrocarbon standard of C7 to C40 (Sigma-Aldrich, St. Louis, MO, USA) to GC-MS.
Principal component analysis (PCA)
-
Principal component analysis (PCA) using SPSS 22.0 software was conducted to determine relationships among the four wild species and five cultivars in their chemical composition based on the emissions of individual floral volatile compounds.
Statistical analysis
-
Differences in emission rates of volatile compounds among different species and cultivars were analyzed by one-way analysis of variance (ANOVA). The analyses were conducted with SAS V8 software (Version 8.02. SAS Institute, Cary, NC, USA) and all statistical effects were considered significant at p < 0.05. All p-values for multiple comparisons have been corrected by Bonferroni correction.
-
To classify the aroma intensity of Clematis species/cultivars, we conducted a sensory evaluation of the fragrant characteristics among 71 accessions. And the final nine aromatic samples consist of two corolla types: W1 and C4 belonging to the ascending sepals in bell-like shape, while others to the spreading sepals in a flat disc shape (Table 1, Fig. 1). Based on the tentative results of floral scent, the spreading sepals shaped C. finetiana (W2) and the C. florida 'Marie Boisselot' (C3) of nine odorous Clematis species/cultivars exhibit a strong degree of flower fragrance. And the medium degree of aroma intensity was found in the bell-like C. henryi (W1), C. viorna 'Jade' (C4) and the C. courtoisii with the spreading sepals (W3). The remaining samples indicated the weak degree of floral odors (Table 1). Due to the intraspecific variances occurred in fragrant intensity evaluation among nine Clematis species/cultivars, it is necessary to further detect the qualitative and quantitative variation in floral volatiles.
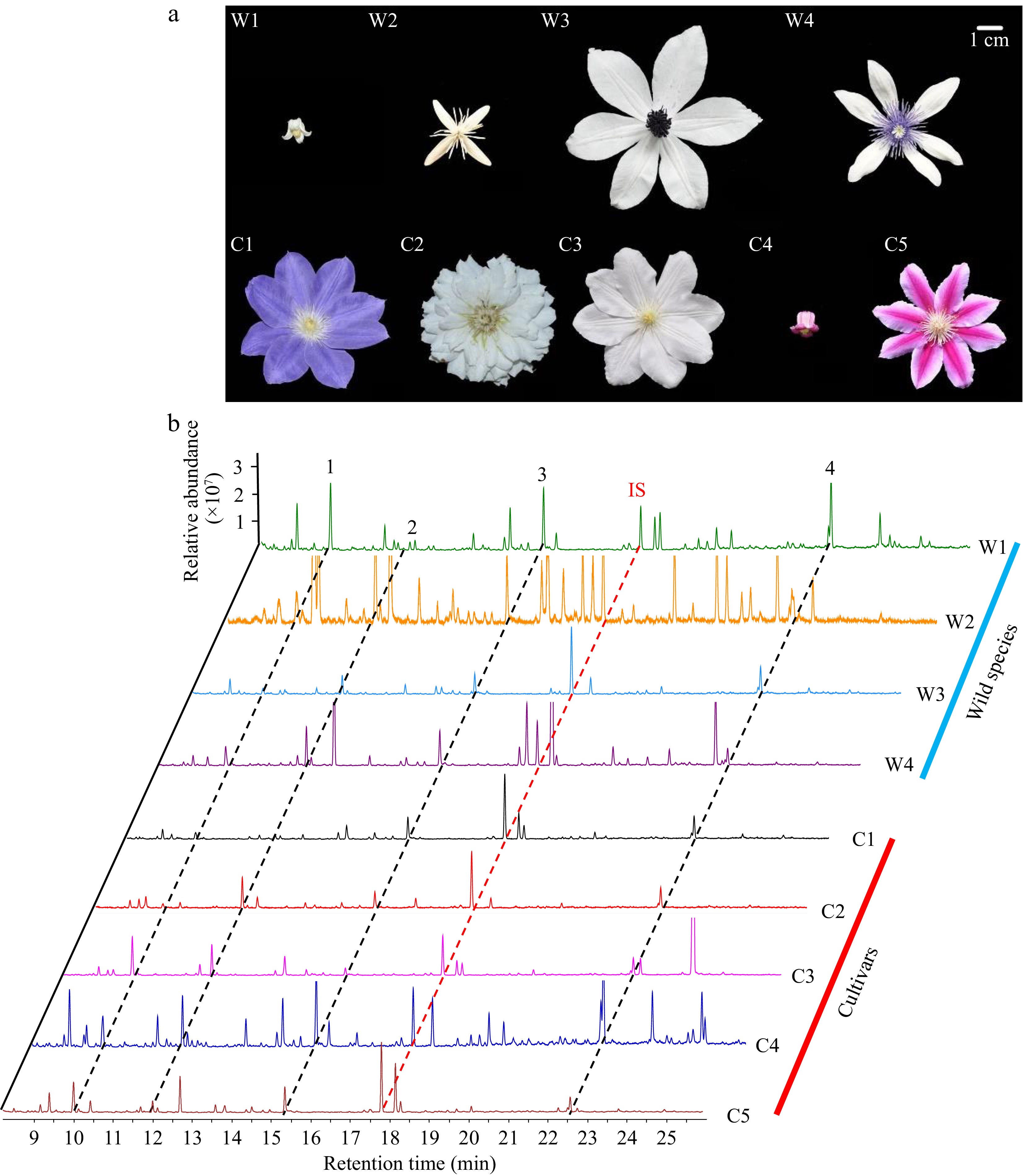
Figure 1.
(a) Morphology of the selected Clematis wild species and cultivars flowers during the full-openness stage. (b) The corresponding gas chromatography mass spectrometry of floral volatiles of Clematis species/cultivars. W1−W4 indicates the four wild species, and C1−C5 represents the five cultivars. The abbreviations of sample names and their classification are listed in Table 1. All peaks were identified by reference to the NIST 2017 library. IS means the internal standard. The peaks of representative volatile compounds were displayed as benzyl alcohol (1), linalool (2), 2-phenoxyethanol (3), α-farnesene (4).
Diversity of floral volatiles among the various Clematis wild species and cultivars
-
The flowers in full-openness stage of nine samples were selected for the floral scent collection and analysis (Fig. 1a). In brief, a total of 162 volatile compounds were successively identified with the headspace collection (relative values > 1% of total amount). The GC-MS results revealed that the interspecific diversity in volatile profiles exists among the nine Clematis species/cultivars, except for the common floral components, such as the benzyl alcohol of benzenoids (1), the linalool (2), 2-phenoxyethanol (3) and α-farnesene (4) belonging to terpenoids (Fig. 1b).
Among the nine Clematis samples, W2 showed the highest total emission rate of floral volatiles with 50.34 μg/g/h, which is consistent with the result of the strong degree by sensory evaluation. Whereas C1, C2 and C5 released the lower amounts of floral volatiles with accordance with their aroma intensity of the weak degree (Table 1, Fig. 2a). Furthermore, the floral volatile components in these nine Clematis species/cultivars are classified into four categories, which constituted 21 terpenoids, 24 benzenoids, 93 fatty acid derivatives and 24 N-containing compounds. Fatty acid derivatives were accounted for more than 50% of total detected compounds in all samples except for W2 and C3. C3 emitted a greater proportion of terpene volatiles than other species/cultivars (Fig. 2b). In accordance with the two corolla types, it is worth noting that the flowers with bell-shaped sepals (W1 and C4) typically showed higher total emission rates than the groups with spreading sepals. In addition, W2 exhibits a higher emission rate in all four categories of volatiles (Fig. 2c−f). Therefore, the unique wild Clematis germplasm resource could play a significant impact on the history of modern breeding due to its rich fragrance.

Figure 2.
Variation of floral volatile emissions among the Clematis wild species and cultivars. (a) Total emission rate (ng/g/h) of the floral scent from the nine samples. (b) Relative percentage (%) of terpenoids, benzenoids, fatty acid derivatives and N-containing compounds. (c)−(f) The corresponding emission rate of the four categories of volatile compounds. Data are presented as the mean value with standard error (mean ± SEM). The different letters above the columns represent the significance among the average emission rates in different groups according to ANOVA analysis (p < 0.05).
In terms of the major terpenoid compounds, it was observed that the emission rates of limonene, α-terpinene, linalool, α-farnesene and E-nerolidol in the C3 with spreading sepals were significantly higher than the other samples (Fig. 3a, c−f). Besides, the statistical results of methylated benzenoids displayed that the largest emission of methoxyanisole was detected in the C1, while the trace amount of methyl salicylate were found to emit only from W1 and W3 (Fig. 3g & h). As for the fatty acid derivatives, the higher emission rates of methyl octanoate and methyl decanoate were released from the flowers of W4 (Fig. 3i & j).
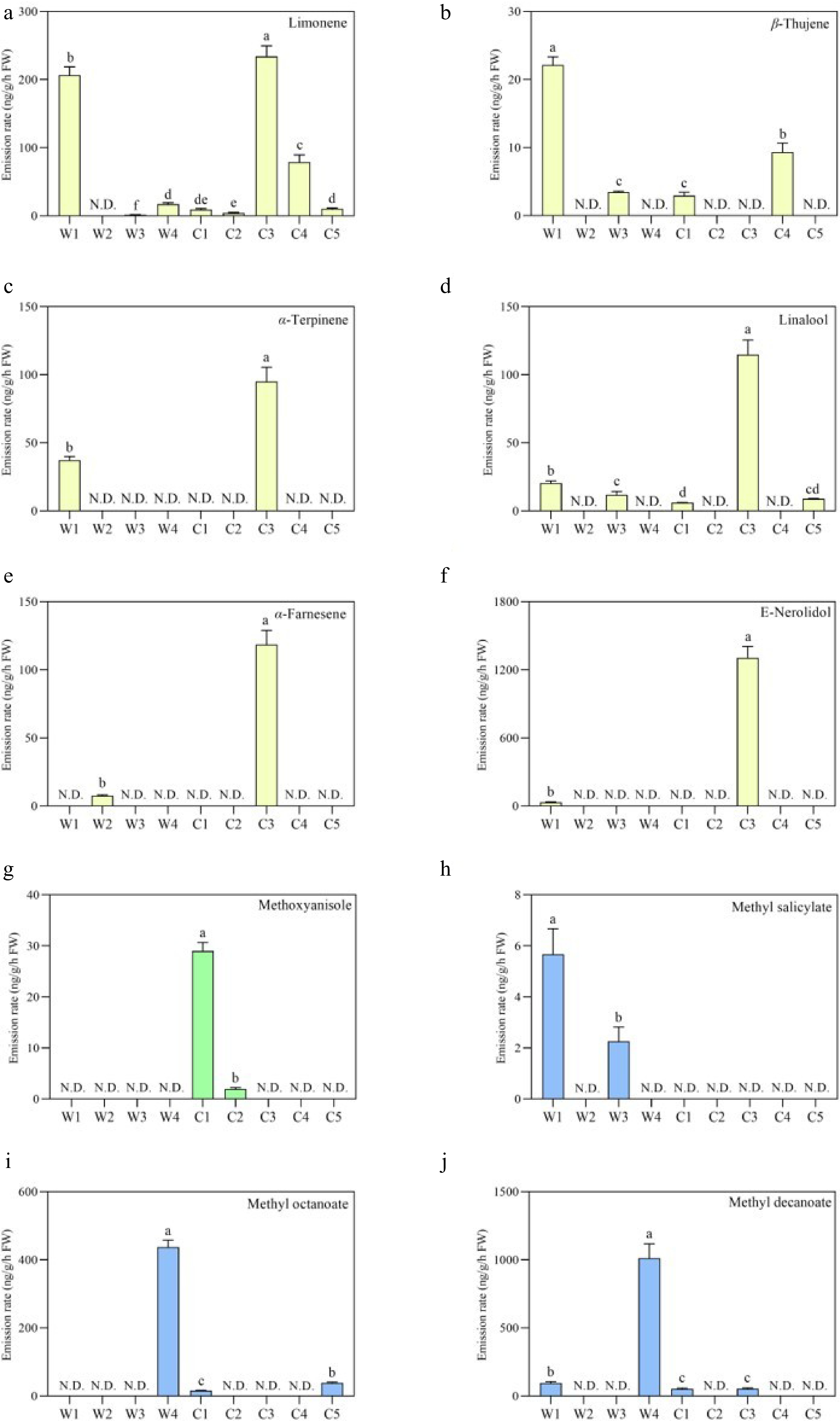
Figure 3.
Comparison of the major volatile components released from the Clematis wild species and cultivars flowers. (a)−(f) The emission rate (ng/g/h) of monoterpenoids and sesquiterpenoids, including limonene, β-thujune, α-terpinene, linalool, α-farnesene and E-nerolidol. (g)−(j) The emission rate (ng/g/h) of the methyl esters of benzenoids and fatty acid derivatives, including methoxyanisole, methyl salicylate, methyl octanoate and methyl decanoate. Data are presented as the mean value with standard error (mean ± SEM). The different letters in the column represent the significance among the average amount in different groups according to ANOVA analysis (p < 0.05).
Heatmap and cluster analysis of individual volatile compounds
-
To generalize the information on the potential biochemical pathways of different categories of volatiles in the Clematis species/cultivars, the identified 162 volatiles were subjected to cluster analysis. The heatmaps implicating the emission pattern of terpenoids, benzenoids, fatty acid derivatives and N-containing compounds were displayed (Fig. 4a−d). The results demonstrated that the selected nine Clematis samples were mainly divided into two groups, and the W2 with spreading-shape sepals was distinctly separated from the other Clematis species/cultivars.
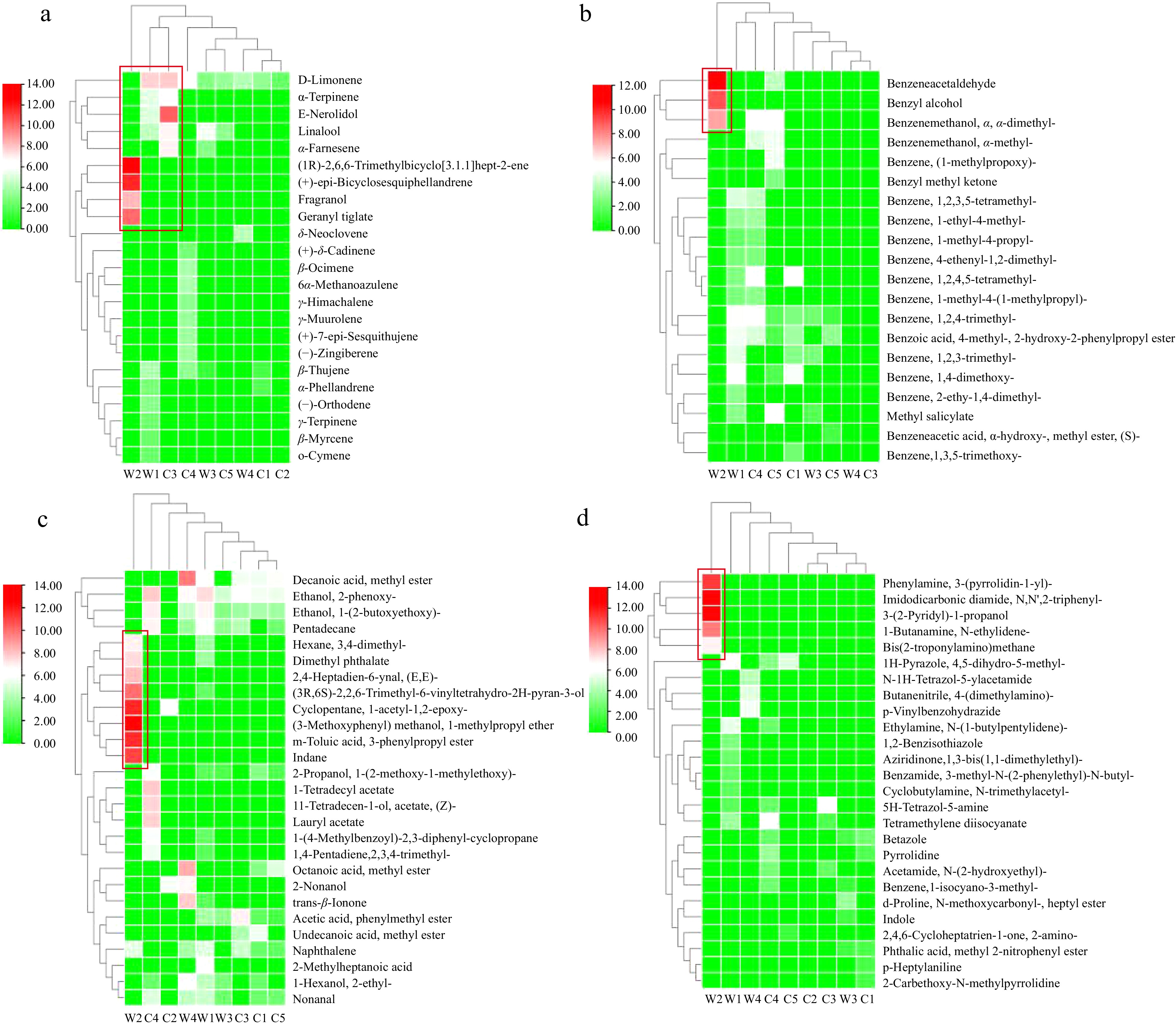
Figure 4.
Clustering heatmap of the emission rate of individual components distinguished with four categories of floral volatiles. The color scale ranging from red to green represents the relevant value of the emission rate of volatiles, respectively. All values of volatiles were normalized by log2 transformation. The red squares indicate the major components of each group of floral volatiles.
Moreover, the group of terpenoids showed that the flowers of nine Clematis species/cultivars mainly consisted of five monoterpenoids (D-limonene, α-terpinene, linalool, (1R)-2,6,6-trimethylbicyclo [3.1.1] hept-2-ene and fragranol) and four sesquiterpenoids (E-nerolidol, (+)-epi-bicyclosesquiphellandrene, α-farnesene and geranyl tiglate) (Fig. 4a). In contrast, volatile benzenoids, such as phenylacetaldehyde, benzyl alcohol and α-dimethyl-benzenemethanol were specifically emitted from the flowers of W2 (Fig. 4b). Likewise, the larger amount of fatty acid derivatives and N-containing compounds emitted from the flowers of W2 were identified as the key components that caused the discrepancy of floral volatile composition with other eight Clematis species/cultivars (Fig. 4c & d).
Principal component analysis (PCA) of the major volatile components
-
To further demonstrate the discrepancy in the emission patterns between the Clematis wild species and cultivars, PCA was carried out on account of detected 56 major volatile components in the clustering heatmap of Fig. 4. The top two principal components, PC1 and PC2, accounted for 39.27 % and 20.06 % of the variation, respectively (Fig. 5a). The 2D score chart and the eigenvector load plot were established for exploring the influence of the floral scent on the differentiation of the nine accessions. C19 (methyl octanoate), C24 (ethanol, 2-phenoxy-) and C30 (methyl decanoate) with the highest positive scores in PC1 were separately clustered with other volatile components (Fig. 5a). Moreover, the principal component score plot showed the wild species and cultivars in clematis could be basically distinguished as two groups based on the emission pattern of floral volatiles (Fig. 5b). 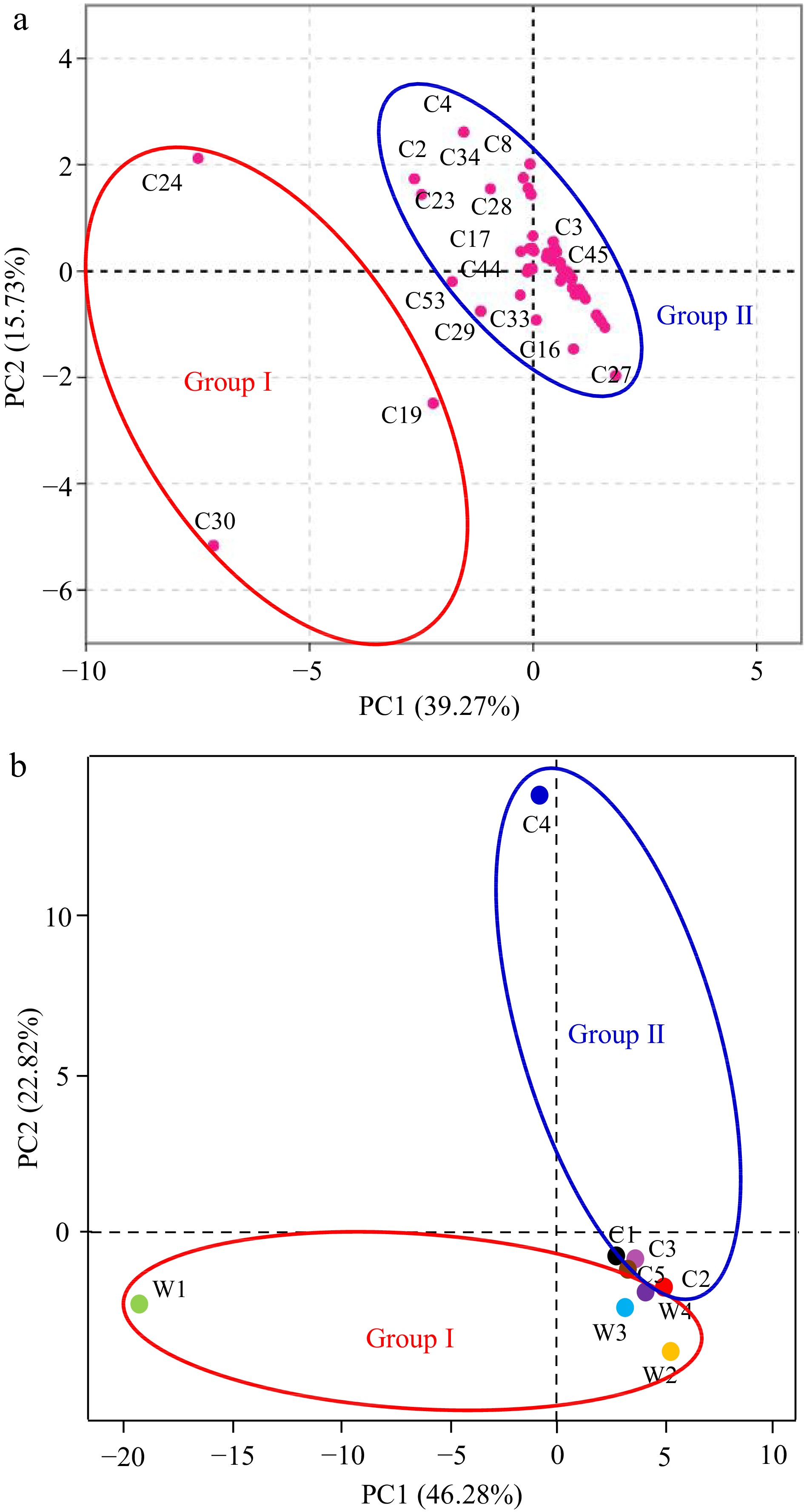
Figure 5.
Principal component analysis (PCA) of the emission rate of major volatile compounds. (a) Eigenvector loading plot of 56 major volatiles corresponding to more than 1% of total amount. (b) Principal component score plot of the emission rate in the nine samples. The distinguished compounds were designated with D-limonene (C2), α-terpinene (C3), linalool (C4), α-farnesene (C8), 2-nonanol (C16), nonanal (C17), octanoic acid, methyl ester (C19), ethanol, 1-(2-butoxyethoxy)- (C23), 2-phenoxyethanol (C24), cyclopentane, 1-acetyl-1, 2-epoxy- (C27), 2-methylheptanoic acid (C28), undecanoic acid, methyl ester (C29), decanoic acid, methyl ester (C30), trans-β-ionone (C33), pentadecane (C34), benzene, 1, 4-dimethoxy- (C44), methyl salicylate (C45), 1H-pyrazole, 4,5-dihydro-5-methyl- (C53), respectively.
-
On the basis of the initial sensory survey, this study aimed to explore the interspecific discrepancy of volatile compositions in the nine fragrant Clematis species/cultivars. The results demonstrated that a total of 162 volatiles were collectively emitted from the flowers of nine Clematis wild species and cultivars at the full-openness stage. The floral volatiles profiling consists of four primary chemical classes, including terpenoids, benzenoids, fatty acid derivatives and nitrogenous compounds, which aligns with the typical chemical makeup of floral scents[12]. Moreover, further analysis revealed that the terpenoid components of Clematis species/cultivars flowers were primarily attributed by D-limonene, linalool and E-nerolidol (Fig. 4a). Likewise, previous studies have reported that the three floral terpenoid volatiles were verified to mainly be emitted from the sepals and ovaries of C. florida 'Kaiser'[13]. On the other hand, the phenylacetaldehyde and benzyl alcohol belonging to benzenoids and the nonanal of fatty acid derivatives detected in the W2, C2 and C4 also exhibited consistent compositions with the floral scent of C. florida 'Kaiser'. In addition, W2 was clustered into a separated group based on the volatile emission in four categories. Our results indicated that the complexity and diversity of the wild Clematis species could provide the potential for screening the germplasm resources for the breeding of aromatic Clematis cultivars[13]. Nevertheless, it is worth noting that fatty acid derivatives accounted for 40%−80% of the total emission in the eight Clematis species/cultivars, except for releasing the higher proportion of terpenoids in the C3 (Fig. 2b). It suggests that multiple genes of crossed biosynthetic pathways were involved in the production of floral volatiles in Clematis[14].
The deciphering of the fragrant profiling could shed light on the identification to the various potential key synthases for the biosynthesis[15]. Generally, the biosynthesis of floral volatiles originates from a limited number of metabolic pathways[12]. Likewise, the aroma compositions emitted from the wild Clematis species and cultivars could also be classified into four groups due to their different biosynthetic origins (Fig. 2b), including (1) the mevalonic acid (MVA) and methylerythritol phosphate (MEP) pathways, (2) shikimate/phenylalanine pathways, (3) fatty acid-derived volatiles biosynthesis pathways and (4) branched-chain amino acids pathways[12]. In terms of the biosynthetic pathways of terpenoids, three key terpene synthases (TPSs) in the flowers of C. florida 'Kaiser' have been functionally characterized[13]. As shown in the Fig. 6, linalool and E-nerolidol were confirmed to be emitted from the flowers of the wild species (W1, W3) and cultivars (C1, C3, C5), suggesting that the TPSs with homology with those of C. florida 'Kaiser' may also be present in other Clematis species. Besides TPSs, key synthases in shikimate/phenylalanine pathways and fatty acid-derived volatile biosynthesis pathways, such as O-methyltransferase (OMT) and SABATH methyltransferases could also contribute to the complexity and diversity of the floral volatiles in Clematis[16]. In our study, four products of methylation including methoxyanisole, methyl salicylate, methyl octanoate and methyl decanoate were detected in the flowers of the wild Clematis species (W3 and W4) and cultivars (C1, C3 and C5), which indicated that the OMTs and SABATHs enzymes could also be involved in the modification of benzenoid and fatty acid derivative products[17]. Therefore, identifying and characterizing OMTs and SABATH enzymes would be a valuable focus for future research.
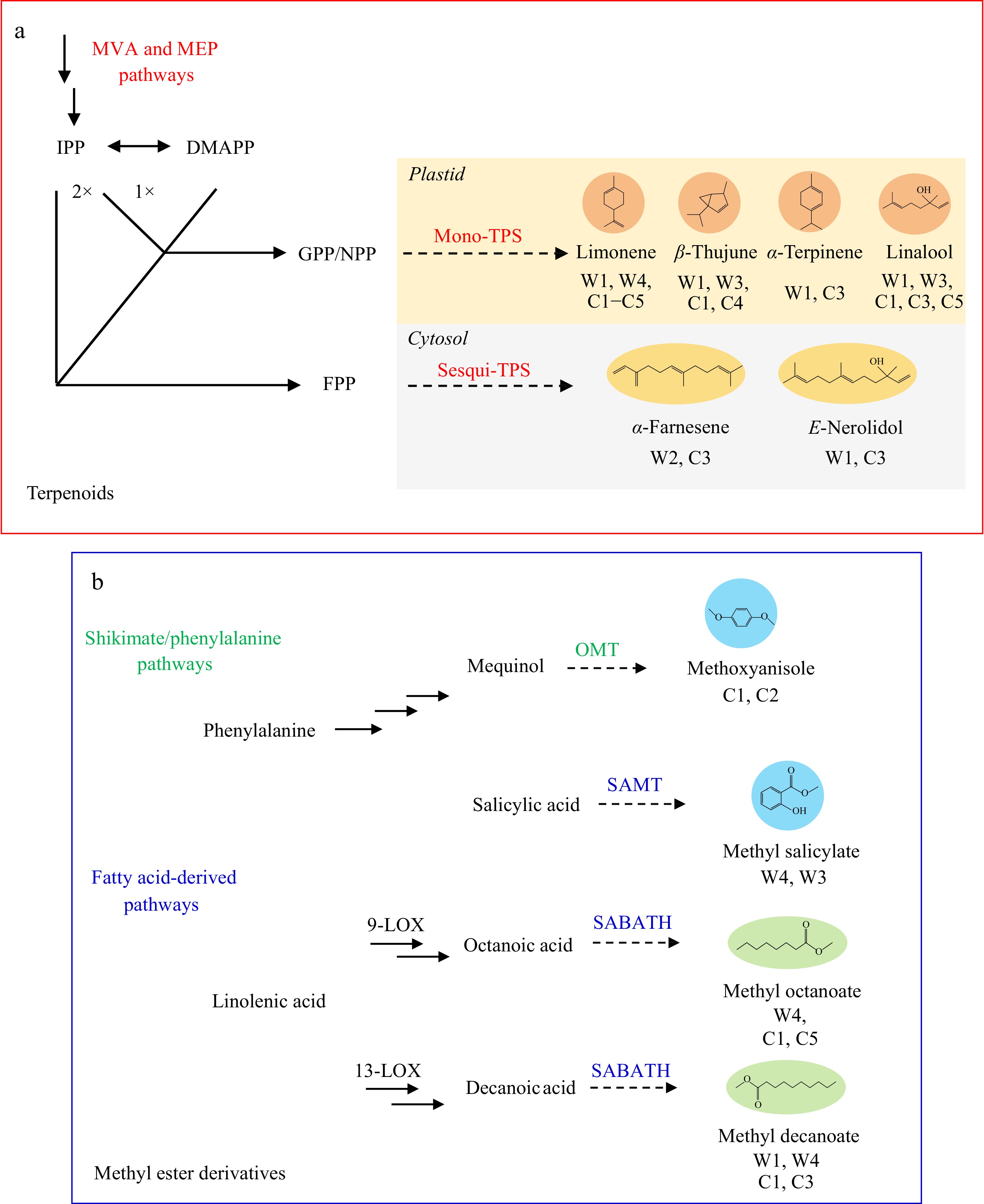
Figure 6.
Summary diagram of putative biosynthetic pathways of major floral volatiles among the intergeneric comparison. The proposed biosynthetic pathway of terpenoids and methyl esters emitted from Clematis flowers according to the identified volatile profiling. The dotted lines with arrows represent the verified the catalytic steps of terpenoids and methyl ester derivatives. (a) The corresponding prenyl-diphosphate substrates (IPP, isopentenyl diphosphate; DMAPP, dimethylallyl diphosphate; GPP, geranyl diphosphate; FPP, farnesyl diphosphate) and the mono- or sesquiterpenoid products are marked with black, respectively. (b) The underlying methyl ester biosynthesis pathways involved in the shikimate/phenylalanine and the fatty acid-derived volatiles are also indicated, respectively.
Floral scent not only serves the roles in attracting pollinators for sexual reproduction, but also helps in safeguarding plants against attacks from pathogens, parasites, and herbivores[18,19]. Furthermore, the characteristic floral volatile profiling could be an indicator for the pollinator types visiting the flowers[1]. For instance, the significant quantities of benzenoids and, the trace amount of terpenoids and nitrogen-containing compounds emitted from the plants could affect the behavior of pollinating moth insects[1]. The abundant emission of linalool, d-limonene, phenylacetaldehyde, phenylamine, 3-(pyrrolidin-1-yl)- and 1H-pyrazole, 4,5-dihydro-5-methyl- identified in our study have been verified to determine the orientation of specific pollinators in several plant species, including Penstemon digitalis, Magnolia liliflora[20,21]. Furthermore, the statistic results of volatiles indicated that the shape of Clematis flowers could be correlated to the emission rate of floral scent, especially in the type of bell-like corollas. Therefore, although the sterile flowers were observed in several domesticated Clematis cultivars such as 'Kaiser', we speculate that the floral volatiles emitted from the majority of Clematis species/cultivars could still serve as an olfactory cue for the frequently visited pollination insects, including the Apis mellifera (bees) and Bombus (bumblebees)[22,23]. Thus, it will be tempting to clarify the role of individual volatile constituents emitted from the different corolla shapes of the wild Clematis species and cultivars in attracting insects. In brief, four fragrant wild species and five fragrant cultivars were categorized into three categories based on the intensity of sensory evaluation. Additionally, the diversity and intraspecific variation of floral volatiles in the wild Clematis species and cultivars were systematically summarized, which also lays the groundwork for the development of new Clematis germplasm with specific aroma types through accelerated breeding techniques.
-
The current study highlighted the qualitative and quantitative variation of floral volatile constituents released from various fragrant Clematis species/cultivars on the basis of the preliminary sensory evaluation. A total of 162 volatile compositions, including terpenoids, benzenoids, fatty acid derivatives and N-containing compounds, were detected successively. The qualitative and quantitative statistic data confirmed the interspecific discrepancy of floral volatiles underpinning the terpenoids and methyl ester compounds was occurred in the wild Clematis species and cultivars. The C. finetiana of wild species possess the characteristic releasing pattern distinguishing with other species/cultivars. In conclusion, the investigation of the interspecific discrepancy of floral terpenoids and methyl esters between species and cultivars will facilitate the breeding of aromatic cultivars of Clematis by grasping the aromatic germplasm resources and developing novel breeding strategies.
-
The authors confirm contribution to the paper as follows: conception and design: Jiang Y, Qian R; experiment conducting: Ma X, Zhang W, Hu Q, Zheng J; analysis and interpretation of results: Jiang Y, Zhang W, Qian R; draft manuscript preparation: Jiang Y, Zhang W, Qian R. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This work was supported by Utilization of Resource Plants Zhejiang Science and Technology Major Program on Agricultural New Variety Breeding (2021C02071-6); Open Project of Wenzhou Key Laboratory for Innovative Utilization of Resource Plants (2022-01); Wenzhou Major Scientific and Technological Innovation Projects (ZS2020002).
-
The authors declare that they have no conflict of interest.
-
# These authors contributed equally: Yifan Jiang, Xiaohua Ma
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Jiang Y, Ma X, Zhang W, Hu Q, Zheng J, et al. 2023. Diversity and intraspecific variation of floral volatile compounds underscoring the terpenoids and methyl esters among the wild Clematis species and their cultivars. Ornamental Plant Research 3:20 doi: 10.48130/OPR-2023-0020
Diversity and intraspecific variation of floral volatile compounds underscoring the terpenoids and methyl esters among the wild Clematis species and their cultivars
- Received: 14 September 2023
- Accepted: 21 November 2023
- Published online: 29 November 2023
Abstract: Floral scent is a crucial characteristic that greatly affects the reproductive process and also the market value of numerous ornamental crops. As the pivotal species in basal dicotyledonous, Clematis is widely favored as an ornamental vine plant due to its varied colors and shapes of flowers. However, the diversity and complexity of the floral fragrance in Clematis is largely unexplored. Thus, on the basis of the initial sensory survey, four fragrant wild Clematis species and five cultivars were selected to investigate the variation of the floral volatile profiling between the wild Clematis species and their cultivars. Our results demonstrated that a total of 162 volatile compositions, including terpenoids, benzenoids, fatty acid derivatives, and N-containing compounds, were successively detected by the combination of dynamic headspace collection and GC-MS analysis. The qualitative and quantitative comparison combined with clustering and PCA analysis indicated the interspecific discrepancy in floral volatiles between the wild Clematis species and cultivars. C. finetiana exhibited a higher emission with the characteristic releasing pattern being distinct from other species. In conclusion, the exploitation of the emission patterns, biosynthesis, and potential biological functions of floral scent, especially for terpenoids and methyl esters, provides a comprehensive understanding of volatile metabolism in fragrant Clematis species and their cultivars. These findings can be useful for aromatic resources screening and breeding strategies developing to create new aromatic cultivars of Clematis.
-
Key words:
- Clematis /
- Interspecific variation /
- Floral scent /
- Terpene /
- Methyl ester /
- Pollinator attraction












