-
Pastures play an important role in cattle production in Brazil due, among other factors, to their high productive potential and low production cost[1] despite this, most of these areas are degraded or in a state of degradation[2]. Intensive cultivation and the trampling of animals, the excessive use of agricultural machinery and implements, or even the incorrect use of the soil, results in a decrease in the volume of unsaturated soils[1].
To circumvent the processes and causes of pasture degradation, the search for methods to recover and leverage productivity, are essential to maintain agribusiness and preserve the environment[3]. The physical structure and fertility of the soil are considerable factors for good formation and maintenance of pastures.
The quality of a forage plant is the combination of chemical composition, digestibility and voluntary consumption by the animal. Thus, it is important to know the levels of crude protein, neutral detergent fiber, acid detergent fiber and dry matter, in order to achieve the benefits that a quality forage will bring to animal feed[4].
Nitrogen is the nutrient that has the greatest and fastest effect on vegetative development, related to shoot tillering and root system development[3].
Grasses of the genus Urochloa, due to their high productive potential and quality, are considered an essential food support in raising cattle for beef or milk production[5]. Additionally, grasses in this genus exhibit a root system with a superior capacity to penetrate compacted soil layers when compared to other grasses. The positive effects observed in improving the physical quality of the soil tend to be more long-lasting compared to mechanical subsoiling interventions[6−8].
Forage plants of the Megathyrsus species have stood out due to the high productivity of the aerial part, large size, good quality and acceptability by animals[9]. The root system of Megathyrsus also contributes to improving the physical structure of the soil, particularly favoring the establishment of upcoming crops due to the formation of stable aggregates and macropores, which act as aeration channels[10].
Although the literature presents data, the lack of information for some foragers is notable. Thus, an important new step would be the characterization of widely cultivated forage grasses subjected to different degrees of soil compaction and different doses of nitrogen fertilizers. In this work, we hypothesize that soil compaction can induce different responses among the three tropical grasses. In addition, nitrogen fertilization can mitigate the effects of this stress through better development of the root system. Thus, in the present study, we characterized the morphology and chemical responses of three important forage grasses: Urochloa brizantha cv. MG-5 Vitória, Urochloa ruzizienses and Megathyrsus maximum cv. Mombaça.
-
The experiment was carried out in a greenhouse at Universidade Federal dos Vales do Jequitinhonha e Mucuri (UFVJM), campus JK, located at an altitude of 1,365 m in the city of Diamantina - MG, Brasil (18°12' south and 43°35' west). For the study, three forage species were selected: U. brizantha cv. MG-5 Vitória, U. ruziziensis, and M. maximum cv. Mombaça. The seeds originated from the Mato Grosso region, 2013/2014 crop.
At the Seed Laboratory of the Department of Agronomy (UFVJM), the profile of seed lots was characterized by the germination test. The germination test was conducted using four replicates of 50 seeds per species on germitest paper substrate, moistened with an amount of water equivalent to 2.5 times the dry weight of the substrate in acrylic gerbox containers. The containers were kept in a B.O.D. germinator with an alternating temperature of 20−35 °C and a photoperiod of 16−8 h. Evaluations were concluded at 21 d after installation for U. brizantha cv. MG-5 Vitória and U. ruzizienses species, and at 28 d for M. maximum cv. Mombaça. The results were expressed as a percentage of normal seedlings[11]. For the characterization of grasses under the effects of treatments, a randomized block design was used in a double factorial scheme (4 × 4), with four degrees of compaction being tested (65%, 75%, 85% and 95%) and four doses of nitrogen (N) fertilizer (0, 200, 250, 300 kg·ha−1 of N) using ammonium sulfate as a source, and four replications.
Normal Proctor assay
-
Approximately 5 kg of soil samples were collected from the AB horizon of an Oxisol Red Yellow[12], to carry out the normal Proctor test. To obtain the soil compaction curve, at least five specimens were compacted with increasing moisture content. The compaction of the specimens took place in three layers of soil, which received 25 blows from the hammer used in the normal Proctor test[13], weighing the specimen with known volume. In each specimen, a sample was collected for moisture determination. With the values of moisture and soil density, the points were plotted, obtaining, through Excel for Windows® software, the regressions that best fit these points determined at the maximum point of the function, obtaining the maximum soil density (DsMax) and the optimum humidity (UÓt) for compaction through the expressions, DsMax = −B/2A and UÓt = −(B2 − 4AC)/4a, where A, B and C are the coefficients of fitting the equations.
The degree of compaction (DC) is the ratio between the natural Density of the soil or the desired one and the maximum Density, obtained by the normal Proctor test, multiplied by 100, that is:
$\rm DC = \dfrac{Soil\; density\; in\; the\; field}{Max\;proctor\;density}\times 100 $ Once the degree of compaction was stipulated, knowing the maximum soil density and the pot volume, it was possible to calculate the soil mass to be placed inside the pots. The pots used obtained a known volume of 8 L. After filling the pots, and compacting the soil until reaching the weight for each degree of compaction, sowing was carried out.
Three independent experiments were set up with the respective forages: U. brizantha cv. MG-5 Vitória, U. ruzizienses and M.maximum cv. Mombaça. The N doses were applied in coverage at 30 and 60 d after planting. Sowing was carried out, and after emergence, thinning was carried out, leaving the two seedlings. Irrigation was carried out according to the humidity at field capacity, considering a water retention potential of 6 Kpa, and managed with the aid of an electronic soil moisture meter, brand FALKER 2030, operated according to the manufacturer's instructions[14].
Biometric analysis of forage species
-
At 90 d after sowing, the height of the plant was measured with the aid of a metal measuring tape, measuring from the base to the curvature of the leaves of each plant. Subsequently, cuts were made at a height of 20 cm from ground level with the aid of scissors. The samples were placed in identified paper bags and sent to the laboratory to obtain the green mass (GM) and dry mass (DM) of the aerial part.
The samples were weighed, obtaining the weight of the GM. Then, they were taken to the forced air circulation oven at 55 °C until constant weight for determination of the air dry material (ASA), weighed and ground in an analytical mill model Q298A and stored in sealed plastic pots, for analysis of the bromatological composition. Root volume (RV) was obtained by displacing the volume of water in a graduated cylinder.
Bromatological analysis
-
Crude protein (CP) analysis was performed by obtaining the total N content by the elemental analyzer. With this value, the calculation was made by multiplying the N content found by the factor 6.25[15].
Neutral detergent fiber (NDF) was obtained by the difference in weight after digestion with neutral detergent solution in non-woven fabric (TNT) bags taken to an autoclave following the methodology described by Detmann et al.[16]. After obtaining the NDF value, with the same samples the acid detergent fiber content (ADF) was obtained, which consisted of placing the samples in an acid detergent solution following the methodology described by Detmann et al.[16]. Through the difference, the ADF value was found for the respective treatments. After the sequential determination of the NDF and ADF fraction, the TNT bags containing the samples were placed in plastic bottles, where a 72% sulfuric acid solution was added to determine the lignin content in acid detergent, following the methodology described by Monzani[17]. Then, the CP, NDF, ADF and lignin contents were corrected for the dry matter basis.
Data analysis
-
The data obtained from the experimental units were submitted to analysis of variance to determine differences between treatments for each of the evaluated variables and, when significant differences were found, the Tukey test was used at the 5% level to compare means. Regression studies were performed for nitrogen doses. Analyzes were performed using the SISVAR® statistical program[18].
-
The forage seeds U. brizantha presented a germination percentage of 73%, while M. maximum and U. ruziziensis corresponded to 61% and 56% respectively. In the case of U. brizantha and M. maximum, a germination percentage higher than the minimum standard recommended for seed production and commercialization was observed, in accordance with Normative Instruction n° 30/2008[19] and Normative Instruction n° 33/2010[20]. The recommended minimum is 60% for Urochloa and 50% for Megathyrsus. However, for U. ruziziensis, the germination percentage was below the recommended minimum for Urochloa. The lower germination of U. ruziziensis can be attributed to the reduced viability of its seeds, possibly due to the fact that the batch in question was one year older compared to the others. Therefore, special attention was given to this cultivar during seeding, ensuring an ideal quantity of seeds to guarantee a uniform stand. With this purpose, 15 seeds of U. ruzizienses were planted in pots, while 10 seeds were used for U. brizantha and M. maximum. After germination, thinning was performed, leaving only two seedlings per pot for each species.
Biometric evaluation of forage species subjected to N doses and degrees of compaction
-
Table 1 presents the analysis of variance for the average levels of green mass (GM) and dry mass (DM), production of the aerial part height (H), root volume (RV), crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber (ADF) and lignina (LIG). No significant difference was observed for any of the variables evaluated in the different degrees of compaction for forage grasses U. brizantha cv. MG-5 Vitória, M. maximum cv. Mombaça and U. ruziziensis. And neither significant interaction was found for the different degrees of compaction and nitrogen fertilization for the forage M. maximum cv. Mombaça and for U. brizantha cv. MG-5 Vitória.
Table 1. Analyses of variance for the average contents of GM and DM production of the aerial part, H, RV, CP, NDF, ADF and LIG for the forage plants Megathyrsus maximum cv. Mombaça, Urochloa brizantha cv. MG-5 Vitória and Urochloa ruziziensis. UFVJM, Diamantina, 2016.
Pr > Fc VF DF GM (%) DM (%) H (cm) RV (cm3) CP (% DM) NDF (% DM) ADF (% DM) LIG (% DM) Megathyrsus maximum cv. Mombaça Block 3 0,03* 0,054ns 0,48ns 0,89ns 0,00* 0,29ns 0,37ns 0,23ns ND 3 0,00* 0,00* 0,00* 0,00* 0,00* 0,00* 0,00* 0,18ns DC 3 0,64ns 0,38ns 0,74ns 0,11ns 0,66ns 0,60ns 0,58ns 0,90ns ND × DC 9 0,45ns 0,59ns 0,82ns 0,46ns 0,33ns 0,91ns 0,59ns 0,25ns Waste 45 Urochloa brizantha cv. MG-5 Vitória Block 3 0,32ns 0,70ns 0,07ns 0,81ns 0,00* 0,84ns 0,80ns 0,45ns ND 3 0,00* 0,00* 0,00* 0,00* 0,00* 0,00* 0,00* 0,23ns DC 3 0,49ns 0,26ns 0,72ns 0,56ns 0,31ns 0,69ns 0,78ns 0,97ns ND × DC 9 0,46ns 0,26ns 0,76ns 0,63ns 0,77ns 0,31ns 0,45ns 0,38ns Waste 45 Urochloa ruziziensis Block 3 0,56ns 0,36ns 0,83ns 0,64ns 0,02* 0,64ns 0,85ns 0,82ns ND 3 0,00* 0,00* 0,23ns 0,00* 0,00* 0,00* 0,00* 0,88ns DC 3 0,76ns 0,22ns 0,71ns 0,10ns 0,52ns 0,63ns 0,35ns 0,33ns ND × DC 9 0,34ns 0,18ns 0,95ns 0,04* 0,63ns 0,66ns 0,55ns 0,55ns Waste 45 * Significant and ns (not significant) at 5% probability. ND = Nitrogen Dose; DC = Degree of Compaction; VF = Variation Fator; DF = Degree of Freedom. Each nitrogen dose was subject to compaction levels (65%, 75%, 85%, and 95%), but the compaction levels did not interfere with any of the variables analyzed for the studied species. In other words, even at high compaction levels that could potentially hinder plant growth, the studied species excelled by appropriately utilizing available resources, resulting in satisfactory growth, except for the RV in U. ruziziensis.
Tables 2 & 3 display the mean values of GM and DM production, respectively from the aerial part of the forage plants M. maximum cv. Mombaça, U. brizantha cv. MG-5 Vitória, and U. ruziziensis based on N doses. It can be observed that the GM and DM production from the aerial part increased when N doses were increased to the respective forage plants. This is attributed to the positive influence that this nutrient has on the forage grasses.
Table 2. Average values of green mass (GM) in g·plant−1 for the forage plants Megathyrsus maximum cv. Mombaça, Urochloa brizantha cv. MG-5 Vitória and Urochloa ruziziensis under different doses of nitrogen (N) kg·ha−1 in UFVJM, Diamantina, 2016.
Forage species Doses of N (kg·ha−1) 0 200 250 300 Megathyrsus maximum cv. Mombaça 7.51b 26.84a 27.19a 28.83a Urochloa brizantha cv. MG-5 Vitória 10.79b 35.79a 38.09a 38.46a Urochloa ruzizienses 10.53c 32.21b 33.80b 41.71a Averages followed by the same lowercase letter in the row do not differ by the Tukey test at 5% probability. Table 3. Average dry mass (DM) values in g·plant−1 for the forage plants Megathyrsus maximum cv. Mombaça, Urochloa brizantha cv. MG-5 Vitória and Urochloa ruziziensis under different doses of nitrogen (N) kg·ha−1 in UFVJM, Diamantina, 2016.
Forage species Doses of N (kg·ha−1) 0 200 250 300 Megathyrsus maximum cv. Mombaça 1.79b 5.53a 5.45a 5.65a Urochloa brizantha cv. MG-5 Vitória 2.59b 7.83a 8.26a 8.51a Urochloa ruzizienses 2.09c 6.53b 6.69b 8.35a Averages followed by the same lowercase letter in the row do not differ by the Tukey test at 5% probability. As can be observed in Table 4, when applying higher N doses to the studied forage crops, there was an increase in plant height, which is directly related to the increase in DM and GM of the aerial part. This behavior demonstrates that nitrogen availability in the soil and its absorption by plants reflect the growth of grasses. For the M. maximum, without nitrogen fertilization, the average height was found to be 66.70 cm, while at the highest dose (300 kg·ha−1 of N), the average height was 94.71 cm. Regarding U. brizantha cv. MG-5 Vitória, at the nitrogen dose of 200 kg·ha−1, the forage gained greater height. For the U. ruziziensis, no significant differences were observed between the control and the applied nitrogen doses.
Table 4. Average plant height values (H) in cm for the forage plants Megathyrsus maximum cv. Mombaça, Urochloa brizantha cv. MG-5 Vitória and Urochloa ruziziensis under different doses of nitrogen (N) kg·ha−1 in UFVJM, Diamantina, 2016.
Forage species Doses of N (kg·ha−1) 0 200 250 300 Megathyrsus maximum cv. Mombaça 66.76b 91.53a 89.74a 94.71a Urochloa brizantha cv. MG-5 Vitória 76.16b 103.68a 91.36a 101.17a Urochloa ruzizienses 62.81a 72.63a 70.71a 74.06a Averages followed by the same lowercase letter in the row do not differ by the Tukey test at 5% probability. Table 5 presents the average values of RV based on N doses for the three studied forage species. It is noteworthy that the maximum value of RV in the forage plant M. maximum cv. Mombaça was achieved with the application of nitrogen fertilizer at the dose of 250 kg·ha−1, below which the RV continuously increased; then there was a decrease at the highest dose of 300 kg·ha−1. For the species U. brizantha cv. MG-5 Vitória, as the N dose to the plant increased, the RV also increased. In the case of U. ruziziensis, the RV reached its maximum when the N fertilizer dose was 200 kg·ha−1, before which the root volume showed a growing trend and then decreased at the dose of 250 kg·ha−1 of N.
Table 5. Average values of root volume (RV) in cm3 for the forage plants Megathyrsus maximum cv. Mombaça, Urochloa brizantha cv. MG-5 Vitória and Urochloa ruziziensis under different doses of nitrogen (N) kg·ha−1 in UFVJM, Diamantina, 2016.
Forage species Doses of N (kg·ha−1) 0 200 250 300 Megathyrsus maximum cv. Mombaça 7.44b 13.63a 14.13a 11.56a Urochloa brizantha cv. MG-5 Vitória 9.31b 16.56a 17.25a 17.75a Urochloa ruzizienses 6.69b 16.50a 14.13a 15.50a Averages followed by the same lowercase letter in the row do not differ by the Tukey test at 5% probability. An interaction between N dose and DC is observed in the RV of U. ruziziensis (Fig. 1). For the 65% DC, as the N dose increases, the RV increases considerably. As for the 75%, 85% and 95% DC, it is observed that there is an increase in the RV up to the dose of 250 kg·ha−1 of N and a decrease in the RV above that dose.
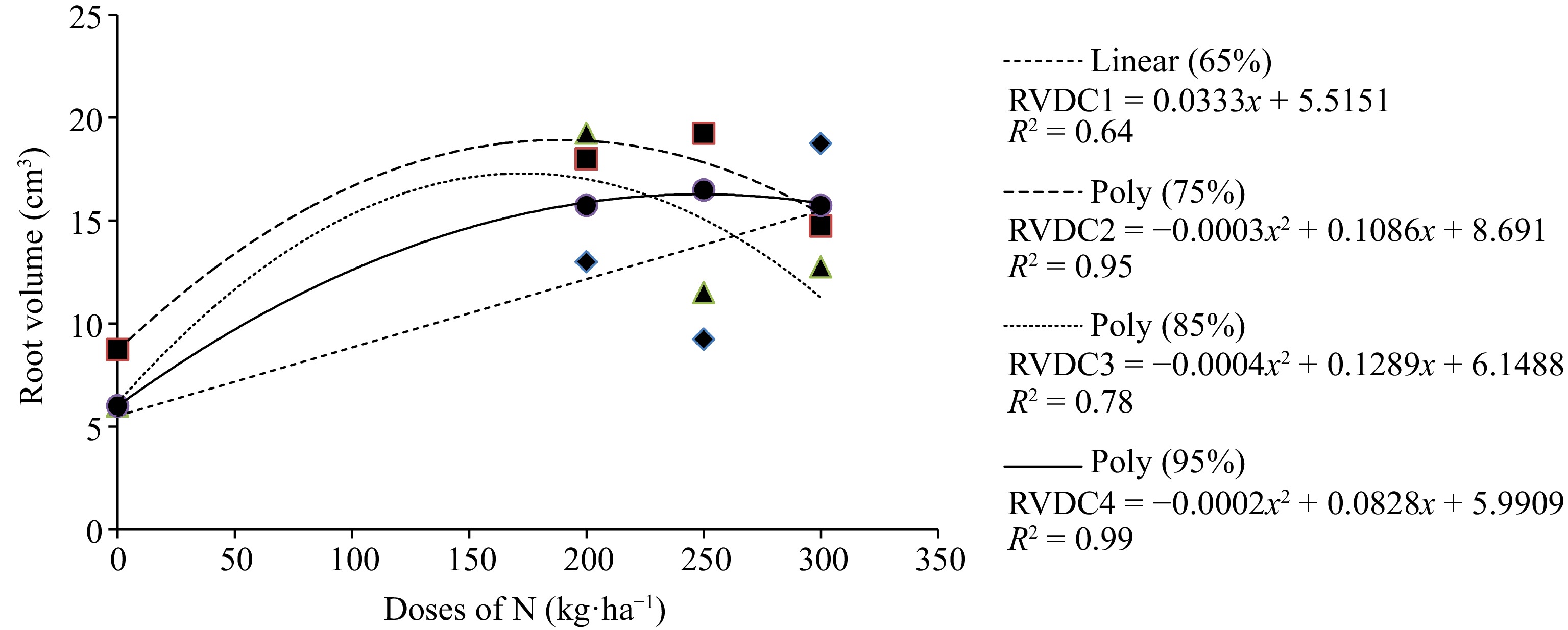
Figure 1.
Average value of RV in Urochloa ruziziensis under increasing doses of N and different DC. DC1 ♦ = 65%; DC2 ■ = 75%; DC3 ▲ = 85% and DC4 ● = 95%.
The average values of RV for U. ruziziensis fertilized with increasing N doses under different compaction levels are presented in Table 6. It is noticeable that there is a difference between the average values only for the N application dose of 250 kg·ha−1 at the respective compaction levels. This indicates that at the compaction level of 75% with the N dose of 250 kg·ha−1, the plant obtained the highest average RV.
Table 6. Average value of root volume in cm3 for the forage plant Urochloa ruziziensis as affected by nitrogen (N) fertilization under different degrees of compression (DC).
DC (%) Doses of N (kg·ha−1) 0 200 250 300 65 6,0a 13,0a 9,3b 18,8a 75 8,8a 18,0a 19,3a 14,8a 85 6,0a 19,3a 11,5ab 12,8a 95 6,0a 15,8a 16,5ab 15,8a Means followed by the same lowercase letter in the column do not differ by Tukey's test at 5% probability. DC (%) = (Soil density in the field/Max proctor density) × 100. -
Table 7 shows the average contents of crude protein (CP) of the forage species M. maximum cv. Mombaça, U. brizantha cv. MG-5 Vitória and U. ruziziensis. It is observed for the three forage species that as the N dose increased, the CP value increased. The average CP values for the forage M. maximum cv. Mombaça, ranged from 7.36% to 21.76%, showing that even in the control (zero dose), this cultivar obtained a CP value of over 7%. This value is considered the minimum for the plant to have good digestibility by the animals.
Table 7. Average crude protein (CP) values in % DM for the forage plants Megathyrsus maximum cv. Mombaça, Urochloa brizantha cv. MG-5 Vitória and Urochloa ruziziensis under different doses of nitrogen (N) kg·ha−1 in UFVJM, Diamantina, 2016.
Forage species Doses of N (kg·ha−1) 0 200 250 300 Megathyrsus maximum cv. Mombaça 7.36b 20.29a 21.08a 21.76a Urochloa brizantha cv. MG-5 Vitória 4.73c 17.40b 18.93ab 21.29a Urochloa ruzizienses 6.23c 23.14b 25.14ab 26.45a Averages followed by the same lowercase letter in the row do not differ by the Tukey test at 5% probability. The forage U. brizantha cv. MG-5 Vitória showed an increase in CP content as N doses increased. It is observed, in the control (zero dose), at CP content of 4.73%. For the other doses, this value reached was greater than 7%, reaching 21.29% when the dose of 300 kg·ha−1 was applied (Table 7). There was an effect of N doses on CP levels for U. ruziziensis, with a linear increase as N doses increased, reaching 26.45% when the dose was applied of 300 kg·ha−1 of N (Table 7).
NDF and ADF
-
Tables 8 & 9 show the average values of NDF and ADF in relation to N doses for the forage plants M. maximum cv. Mombaça, U. brizantha cv. Vitória, and U. ruziziensis. The NDF and ADF levels were reduced with higher N doses. Despite the decrease in NDF levels for the analyzed forages, these values were still higher than the critical range of 55%−60%[21]. This indicates that these forages may not be highly palatable to animals, meaning the plant becomes less attractive to the animal. As for ADF values, all analyzed species showed levels below 40%, suggesting that these forages have good digestibility.
Table 8. Average values of neutral detergent fiber (NDF) in % DM for the forage plants Megathyrsus maximum cv. Mombaça, Urochloa brizantha cv. MG-5 Vitória and Urochloa ruziziensis under different doses of nitrogen (N) kg·ha−1 in UFVJM, Diamantina, 2016.
Forage species Doses of N (kg·ha−1) 0 200 250 300 Megathyrsus maximum cv. Mombaça 73.72a 66.21b 64.07b 64.13b Urochloa brizantha cv. MG-5 Vitória 75.18a 67.41b 64.56b 64.34b Urochloa ruzizienses 66.55a 55.32b 55.75b 56.56b Averages followed by the same lowercase letter in the row do not differ by the Tukey test at 5% probability. Table 9. Average values of acid detergent fiber (ADF) in % DM for the forage plants Megathyrsus maximum cv. Mombaça, Urochloa brizantha cv. MG-5 Vitória and Urochloa ruziziensis under different doses of nitrogen (N) kg·ha−1 in UFVJM, Diamantina, 2016.
Forage species Doses of N (kg·ha−1) 0 200 250 300 Megathyrsus maximum cv. Mombaça 36.06a 31.71b 30.09b 30.67b Urochloa brizantha cv. MG-5 Vitória 36.59a 32.97b 30.58b 30.81b Urochloa ruzizienses 31.36a 24.37b 24.07b 25.22b Averages followed by the same lowercase letter in the row do not differ by the Tukey test at 5% probability. Lignin
-
Table 10 presents the average values of lignin in relation to N doses for the analyzed forage plants. There was no significant difference in lignin content among different N doses for any of the studied forage species.
Table 10. Average values of lignin in % DM for the forage plants Megathyrsus maximum cv. Mombaça, Urochloa brizantha cv. MG-5 Vitória and Urochloa ruziziensis under different doses of nitrogen (N) kg·ha−1 in UFVJM, Diamantina, 2016.
Forage species Doses of N (kg·ha−1) 0 200 250 300 Megathyrsus maximum cv. Mombaça 4.50a 3.96a 3.08a 3.17a Urochloa brizantha cv. MG-5 Vitória 3.11a 4.61a 3.51a 5.29a Urochloa ruzizienses 2.70a 3.01a 2.67a 3.08a Averages followed by the same lowercase letter in the row do not differ by the Tukey test at 5% probability. The compaction levels did not affect the bromatological analyses. Despite the high compression, at 95%, the plant was able to absorb the available nutrient.
-
The low germination rate of forage seeds is considered one of the main obstacles to the establishment of pastures. However, for U. brizantha and for M. maximum, a percentage of germination was higher than the minimum standard recommended for production and commercialization of seeds according to Normative Instruction n° 30/2008[19] and Normative Instruction n° 33/2010[20], which corresponds to 60% for species of the genus Urochloa and 50% for Megathyrsus. As for U. ruziziensis, the percentage of germination was lower than the recommended minimum, which is 60%. In this way, care was taken with this genotype at the time of sowing, sowing an ideal amount of seeds was conducted to ensure a uniform stand.
The low germination rate of U. ruziziensis may be caused by a physiological phenomenon known as dormancy, which is common in tropical pastures. Freshly harvested seeds exhibit low germination rates in the early stages of storage and show deep dormancy during this period. As the storage time increases, this dormancy tends to decrease[22, 23]. However, care must be taken when storing the seeds. Seeds stored for long periods may lose their vigor[24]. This loss of vigor is due to the deterioration of the metabolic system caused by this long storage time, leaving these seeds with slow development or prevented from germinating, leading to the development of poorly formed seedlings and a reduction in the number of seeds that germinate. Therefore, satisfactory seed storage is based on slowing down the normal metabolism of seeds as much as possible, causing as little damage as possible[25].
Biometric evaluation of forage species subjected to N doses and degrees of compaction
-
Considering the biometric evaluations carried out in forage species. The direct relationship that exists between the addition of N via fertilization with the increase in shoot GM and DM and consequently in forage height in M. maximum cv. Mombaça, U. brizantha cv. MG-5 Vitória and U. ruziziensis demonstrates the positive effect that N fertilization promotes on growth, increase in the number of tillers and expansion of the aerial part of the plant[26]. Nitrogen has a rapid effect on plant development. With the addition of N doses, there is an increase in shoot tillering, which results in efficient vegetative development[3]. When N is made available in the soil, it is soon absorbed by the forage, which is associated with the carbon chains, promoting an increase in cellular constituents and, consequently, a significant increase in the vigor and total production of dry matter of the plants[27]. Furthermore, the accumulation of carbohydrates, amino acids, enzymes and phytohormones is affected, thus promoting an increase in biomass[28]. The use of N fertilizer is considered a promising alternative to be implemented in the field, in which it promotes an increase in total productivity from 102% to 269%, which leads to greater availability of biomass for food and greater stocking capacity animal in the area[29].
In the root system of the forage M. maximum cv. Mombaça, there was a positive response with an increase in the doses of N fertilizer up to a dose of 250 kg·ha−1 of N, promoting a growth of this system, with a decrease in the dose of 300 kg·ha−1 of N. With the application of N, the aerial part of the plants responds more significantly than the root system[30]. In a study evaluating the effect of increasing doses of N (0, 150, 300 and 450 kg·ha−1) on the root system of M. maximum subjected to rotational stocking, the researchers found a decrease in root length of 3.3% with N fertilization close to 300 kg·ha−1[31]. For U. brizantha vc. MG-5 Vitória as the doses of N fertilizer increased, there was a positive linear growth in the root system of this forage. When the plants are not using reserve compounds for shoot growth, these can be used by the root system, favoring its growth[32]. As for the forage U. ruziziensis, an apex of root development was observed in the application of the N fertilizer dose of 200 kg·ha−1, with an increasing behavior of the RV up to that point and then there was a decrease in the dose of 250 kg·ha−1 of N.
However, it was possible to observe an interaction between N fertilizer dose and DC in the RV of U. ruziziensis. For the 65% DC, as the N dose increased, the RV increased considerably. This is due to the soil structure being looser, providing better absorption of this nutrient by the roots, resulting in a better development of the root system.
For compaction degrees 75%, 85% and 95%, there was an increase in RV up to the application of 250 kg·ha−1 of N. The root system of plants needs a large amount of oxygen due to its high respiration rate. With the reduction of pore spaces between soil particles, there will be a reduction in the respiration of this system, harming its development[30]. For N fertilization to be efficient for the plant, it needs large volumes of water, which favors the production of roots, promoting the absorption of nutrients[33]. When the soil is compacted, there is a decrease in macro and microporosity, which makes it difficult for water and nutrients to infiltrate the soil profile, limiting its availability to the plant, resulting in poor development of the root system, which reflects on the production of aerial part.
Forage quality composition
-
Nitrogen fertilizer promoted a considerable increase in CP in forage M. maximum cv. Mombaça, U. brizantha cv. MG-5 Vitória and U. ruziziensis. This value is higher than what is considered the minimum when it comes to plant digestibility. CP contents of less than 7% limit DM consumption and reduce the digestibility of the plant[34], since the inadequate levels of N cause the population of microorganisms in the rumen to decrease and, in response, digestibility and intake of dry mass are reduced[21], causing the animal to consume less feed[34]. The absence or low dose of N fertilization in pastures reduces the levels of CP and rumen degradable protein, which can limit the performance of ruminants with higher protein requirements[35].
The doses of N fertilizer promoted a decrease in NDF and ADF values for the three forage grasses studied. However, even with a decrease in NDF values, they were higher than what is considered critical. Since the NDF is considered a more limiting factor in the consumption of roughage, it is related to the consumption of the plant by the animal, and when it is higher than 55%−60% of dry mass, they are negatively correlated[21], showing that it is not a very attractive forage for the animal. NDF values found in the present work were higher than the critical value of 55% for the three studied forages, thus the voluntary consumption of these forages in grazing could be limited, resulting in loss of animal gain.
ADF is negatively correlated with plant digestibility. ADF levels found in forage grasses above 40% are considered limiting for the digestibility of dry mass, compromising animal performance[21]. It is observed that, with the increasing application of N doses in the soil, average values of ADF below 40% were found for the three studied forages. Thus, it can be stated that M. maximum cv. Mombaça, U. brizantha cv. MG-5 Vitória and U. ruziziensis, proved to be forages with good chemical composition in ADF, being considered plants with good digestibility.
-
The increase in N doses improves the nutritional quality of the evaluated species, since it promotes decreases in NDF and ADF contents and increases in green mass, shoot dry mass, height and protein contents. It is of great importance to study the cost-benefit ratio so that the appropriate dosage of N can be recommended.
Even in compacted soil, the development of the forage root system U. brizantha cv. MG-5 Vitória responded gradually to N doses, proving to be a forage resistant to soils with reduced pore spaces.
-
The authors confirm their contribution to the article as follows: conception and elaboration of the research: Pires de Sousa Baracho I, Nery MC, Rocha WW; setting up, conducting the experiment and collecting the data: Pires de Sousa Baracho I, Valeriano FR, Maria da Cruz Bento B, de Souza Rocha A, de Melo Farnezi MM; analysis and interpretation of results: Pires de Sousa Baracho I, Nery MC, Rocha WW, de Melo Farnezi MM, de Cássia Ribeiro Carvalho R; preparation of the draft manuscript: Pires de Sousa Baracho I, Valeriano FR. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The Universidade Federal dos Vales do Jequitinhonha e Mucuri (UFVJM), where he took the postgraduate course in plant production, in which he led this study. The Fundação de Amparo à pesquisa do Estado de Minas Gerais (Fapemig), for granting the research grant.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Pires de Sousa-Baracho I, Nery MC, Rocha WW, de Melo Farzeni MM, Valeriano FR, et al. 2024. Assessment for forage grass quality submitted to compaction degrees and nitrogen doses. Grass Research 4: e008 doi: 10.48130/grares-0024-0004
Assessment for forage grass quality submitted to compaction degrees and nitrogen doses
- Received: 01 July 2023
- Revised: 28 February 2024
- Accepted: 01 March 2024
- Published online: 09 April 2024
Abstract: The objective of this study was to evaluate the productivity and bromatologic composition of forage species Urochloa brizantha cv. MG-5 Vitória, Urochloa ruzizienses and Megathyrsus maximum cv. Mombaça, submitted to degress of compaction, and increasing doses of nitrogen fertilization. To perform the normal Proctor test, representative samples were collected, determining the density and soil humidity. The design utilized was in randomized blocks in factorial 4 × 4, being 65%, 75%, 85% and 95% of compaction and 0, 200, 250, 300 kg·ha−1 of doses of nitrogen, with four replications, for each forage species. The application of nitrogen linearly increased the production of green mass, dry mass of shoot, height of plants and crude protein content of the respective forages. As you increase nitrogen doses, the contents of neutral and acid detergent fibers decreased in the three studied species. For the root volume, the cultivar that responded linearly and positively to the increasing doses of nitrogen in the compacted soil, was the Urochloa brizantha cv. MG-5 Vitória, showing to be more efficient in compacted soils than Urochloa ruziziensis and Megathyrsus maximum cv. Mombaça.
-
Key words:
- Bromatologic composition /
- Megathyrsus maximum /
- Urochloa sp.












