-
Pear is one of the most significant fruit crops worldwide. 'Cuiguan' pear (Pyrus pyrifolia cv. 'Cuiguan') is a famous early ripening pear variety cultivated in southern China that is known for its pleasant appearance, aroma and taste; however, its shelf life is short, as the quality of the fruit at room temperature can rapidly decrease[1]. Blue mould rot (Penicillium expansum) is considered an important disease in the ripening and postharvest storage of pear fruit[2], as it is responsible for inducing serious economic losses to the pear industry in China. P. expansum produces patulin, a mycotoxin that can contaminate fruit and thus have deleterious effects on humans and food safety[3,4]. Fungicides are the most effective means for controlling this disease. However, the problems of pesticide residues and pathogen resistance caused by the long-term use of fungicides are becoming increasingly clear. There is thus an urgent need to develop an easy and effective alternative strategy for managing postharvest fruit disease. Chemical elicitors inducing host disease resistance have received increased attention in recent years[5].
Methyl jasmonate (MeJA) is a significant growth regulator of plants in induced resistance[6]. Salicylhydroxamic acid (SHAM) negatively regulates the physiological effects of endogenous jasmonic acid in plants by inhibiting the MeJA signalling pathway[7]. Previous experiments have shown that MeJA can be used as an exogenous signal elicitor to induce plant disease resistance in pineapple fruit[8], strawberry fruit[9] and kiwifruit[10]. Furthermore, MeJA can also act as an endogenous signalling molecule to transmit signals in plants to resist disease stress and improve the activity of defence enzymes, such as peroxidase (POD), polyphenol oxidase (PPO), superoxide dismutase (SOD), catalase (CAT), β-1,3 glucanase (GLU) and chitinase (CHI), inducing resistance against kiwifruit soft rot[10], Fragaria chiloensis fruit against grey mould decay[11] and avocado fruit against anthracnose[12]. SHAM could effectively restrain the alternative respiratory pathway and is widely used in a variety of cells or tissues[13].
MeJA can induce a stress tolerance response in plants not only by improving the activity of defence enzymes but also by stimulating the expression of resistance genes[14]. The regulation of resistance-related genes by MeJA in plant defence responses has been widely reported. Previous studies have shown that MeJA treatment enhanced the expression of CAT, POD and APX enzyme-related genes in peach fruit and improved resistance to chilling injury[15]. In addition, MeJA treatment improved the expression of the PR5 (pathogenesis-related gene) and reduced the incidence of green mould decay in citrus fruits[16]. MeJA treatment enhanced the gene expression of PAL, CAT, SOD, GLU and CHI in grape berries; reduced the rate of fruit rot and increased the resistance to grey mould[17]; and increased PR expression in tomato fruit against Botrytis cinerea[18].
We previously found that exogenous MeJA-primed defence responses improved resistance against Botryosphaeria dothidea in kiwifruit[10] and reduced blue mould decay incidence in pear fruit, although no direct inhibition of P. expansum was observed (data not published). However, the exact physiological and molecular mechanisms in pear fruit are not well understood. The aim of this study was to determine the effect of MeJA vapour exposure on the disease incidence of blue mould rot, defence-related enzyme activity and gene expression in artificially infected pear fruit.
-
The effects of MeJA treatment on the induction of pear fruit resistance are shown in Fig. 1 and Table 1. Lesion diameters of 100 μmol/L MeJA-treated fruits were obviously smaller than those of the other two groups, with induction effects up to 14.59%; however, the occurrence of blue mould rot was aggravated by 1,000 μmol/L MeJA and 100 μmol/L SHAM treatments. Treatment with 100 μmol/L MeJA for 12, 24, 36 and 48 h before inoculation enhanced the resistance of pear. Compared with the control (0 h), the lesion diameter of the MeJA treatment group was 19.45 mm at 36 h before inoculation, and the induction effect was highest at 15.23%, which was obviously higher than that of control and other MeJA treatments fruits (p < 0.05).
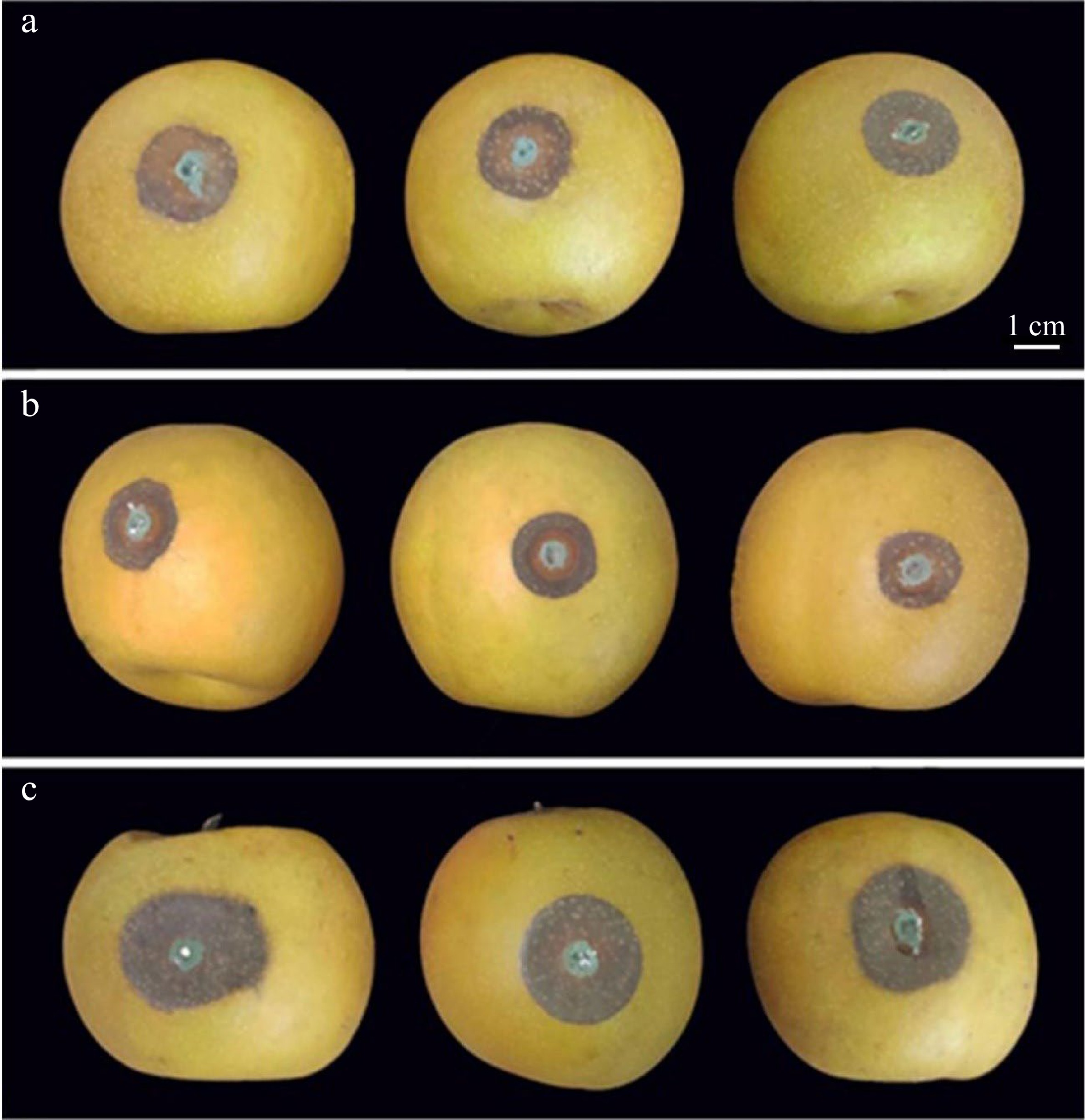
Figure 1.
Symptoms in pear fruit of methyl jasmonate (MeJA) and salicylhydroxamic acid (SHAM) treatment followed by inoculation with Penicillium expansum. (a) Control; (b) 100 μmol/L MeJA treatment; (c) 100 μmol/L SHAM treatment. For experimental details see the Materials and Methods.
Table 1. Effects of MeJA treatment on induction resistance in pear fruit inoculated with Penicillium expansum.
MeJA/SHAM Lesion diameter
(mm)Induction effect
(%)MeJA treatment
time (h)Lesion diameter
(mm)Induction effect
(%)CK 17.50 ± 0.59ab 0 0 22.95 ± 0.45a 0 10 μmol/L MeJA 16.89 ± 1.42b 3.46 ± 2.39b 12 21.26 ± 0.83a 7.34 ± 2.88b 100 μmol/L MeJA 14.94 ± 1.13c 14.59 ± 1.57a 24 21.52 ± 1.12a 6.26 ± 1.60b 1,000 μmol/L MeJA 20.09 ± 0.25a −14.80 ± 0.71c 36 19.45 ± 0.42b 15.23 ± 1.46a 100 μmol/L SHAM 21.45 ± 0.86a −22.58 ± 0.84d 48 20.84 ± 0.25a 9.20 ± 0.80b Values are the means of three replicates. Data in the table are mean ± SD. Different letters indicate significant difference (p < 0.05) between the treatments determined using the Duncan's multiple range test. Effects of MeJA on enzyme activities involved in the defence response
-
As shown in Fig. 2a, the MeJA treatment group exhibited a peak in PPO activity at 48 h after inoculation, which was significantly (1.45 and 1.78 times) higher than that of the control and SHAM groups, respectively. The POD activity was higher in the MeJA group fruits than in the control and SHAM group fruits from 48 h to 144 h after inoculation; specifically, the POD activity in the MeJA group fruits was as high as 1.17 and 5.25 times that in the control and SHAM groups at 96 h, respectively (Fig. 2b) (p < 0.05). The CAT activity of MeJA group fruits showed a slow increase reaching a peak after 96−120 h. In the MeJA treated fruits there was a significant increase over the control and the SHAM treated fruits (Fig. 2c). After 96 h of inoculation, the SOD activity reached its maximum (130.00 U/g FW), which was significantly higher than that of the control (121.07 U/g FW) and SHAM groups (105.82 U/g FW) (Fig. 2d) (p < 0.05).
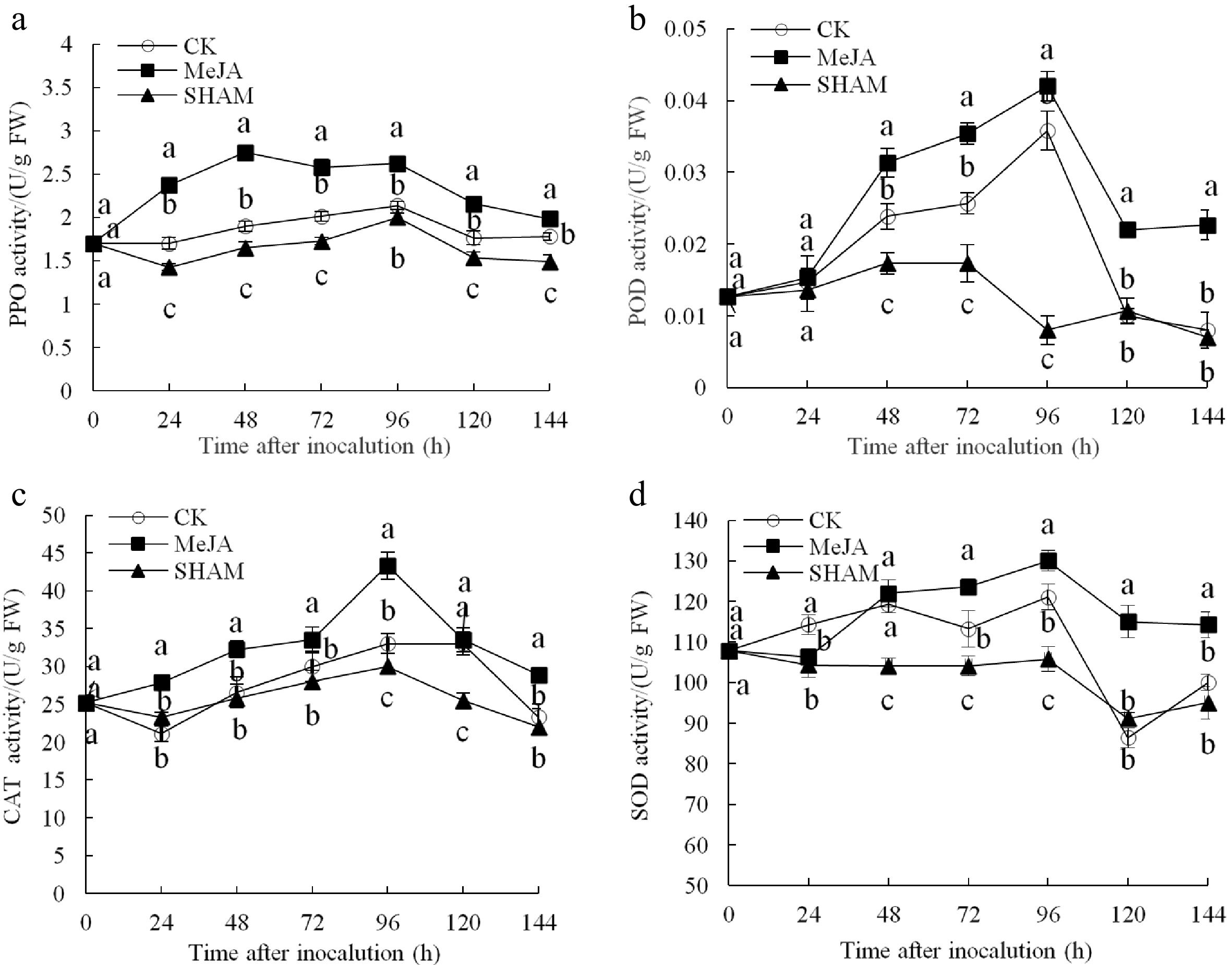
Figure 2.
Changes in the activities of PPO (a), POD (b), CAT (c) and SOD (d) in pear fruit inoculated with Penicillium expansum after 100 μmol/L MeJA treatment for 36 h. Values are the means of three replicates and vertical bars indicate the standard errors.
Effects of MeJA on disease-related protein
-
The CHI activity of MeJA pear fruit was obviously higher than that of the other two groups, except at 24 h within 144 h after inoculation (Fig. 3a). The highest CHI activity observed was 232.72 U/g FW at 72 h after inoculation, which was 1.38 and 1.65 times that of the control and SHAM groups, respectively (p < 0.05). The GLU activity change is shown in Fig. 3b. The activity of GLU in the MeJA-treated group remained at a significantly higher level within 144 h and peaked at 72 h after inoculation, which was 1.15 times and 1.43 times that of the control and SHAM groups, respectively (p < 0.05).
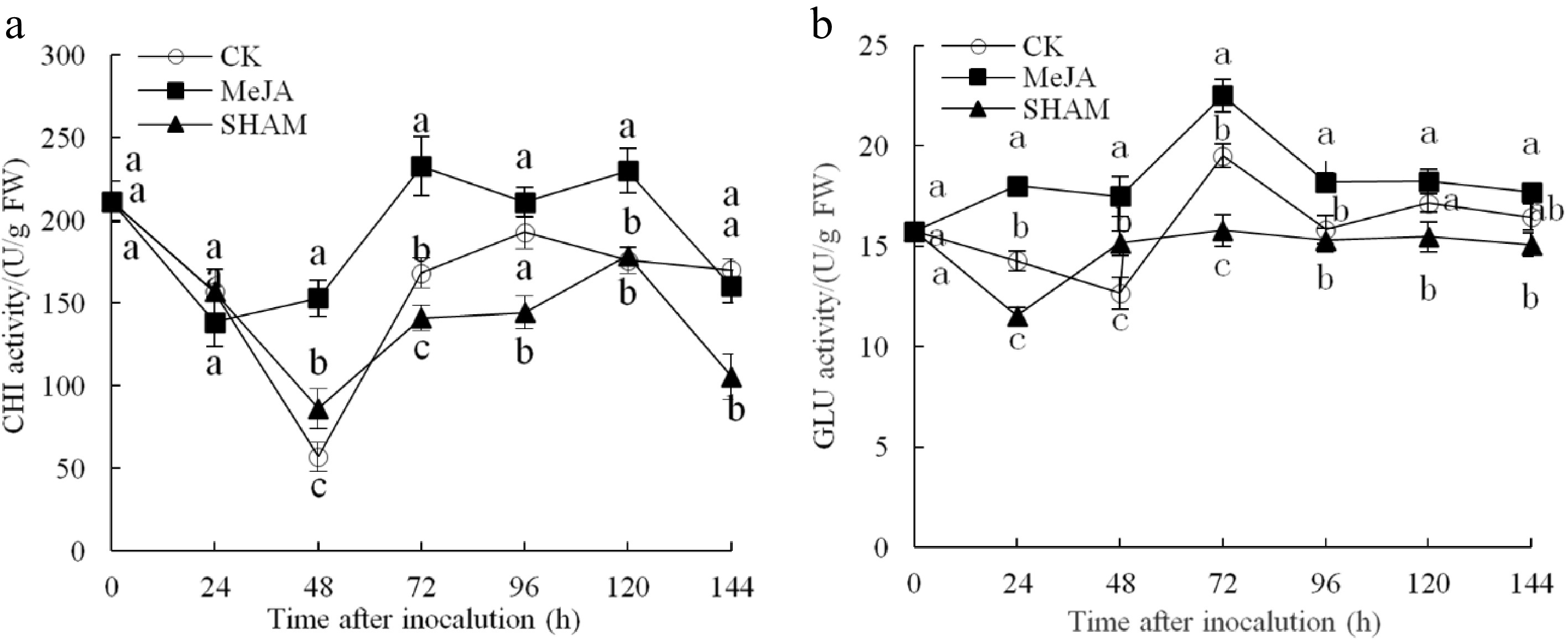
Figure 3.
Changes in the activities of CHI (a) and GLU (b) in pear fruit inoculated with Penicillium expansum after 100 μmol/L MeJA treatment for 36 h. Values are the means of three replicates, and vertical bars indicate the standard errors.
Effect of MeJA on the content of total phenol and MDA
-
The total phenol content continued to increase over time after inoculation in the three fruit groups, and the total phenol content in the MeJA group was higher than that in the other two groups from 72 to 120 h after inoculation (p < 0.05) (Fig. 4a). Figure 4b shows that the MDA content also continued to increase within 144 h after inoculation, which was significantly lower (by approximately 45.32% and 36.71%) in the MeJA-treated group compared with the control and SHAM groups, respectively (p < 0.05).
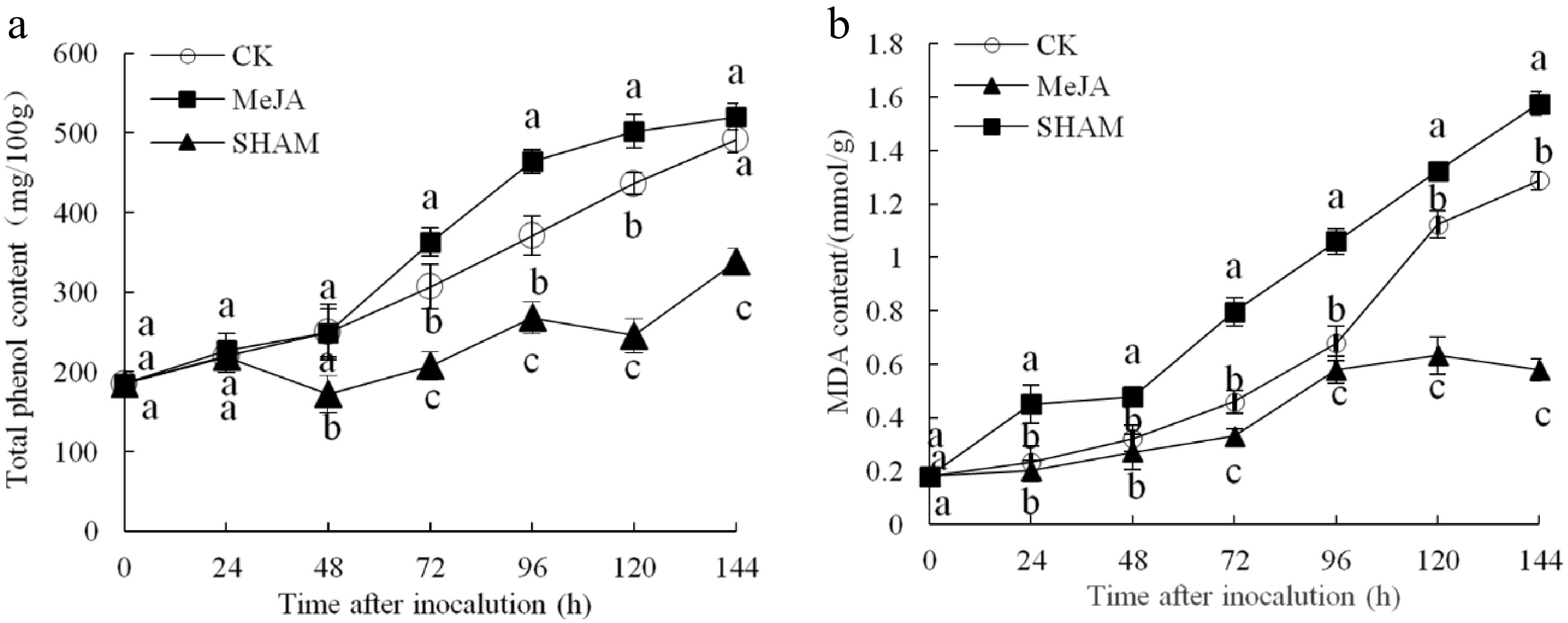
Figure 4.
Changes in the contents of total phenol (a) and MDA (b) content in pear fruit inoculated with of Penicillium expansum after 100 μmol/L MeJA treatment for 36 h. Values are the means of three replicates, and vertical bars indicate the standard errors.
Effect of MeJA on the expression of defence-related genes
-
After inoculation, the expression levels of PpPPO, PpPOD, PpCAT, Cu-ZnSOD, PpCHI and PpGLU in pear fruit tissue are shown in Fig. 5. The relative expression levels of these genes in the MeJA group were higher than those in the control and SHAM groups in most cases within 144 h.
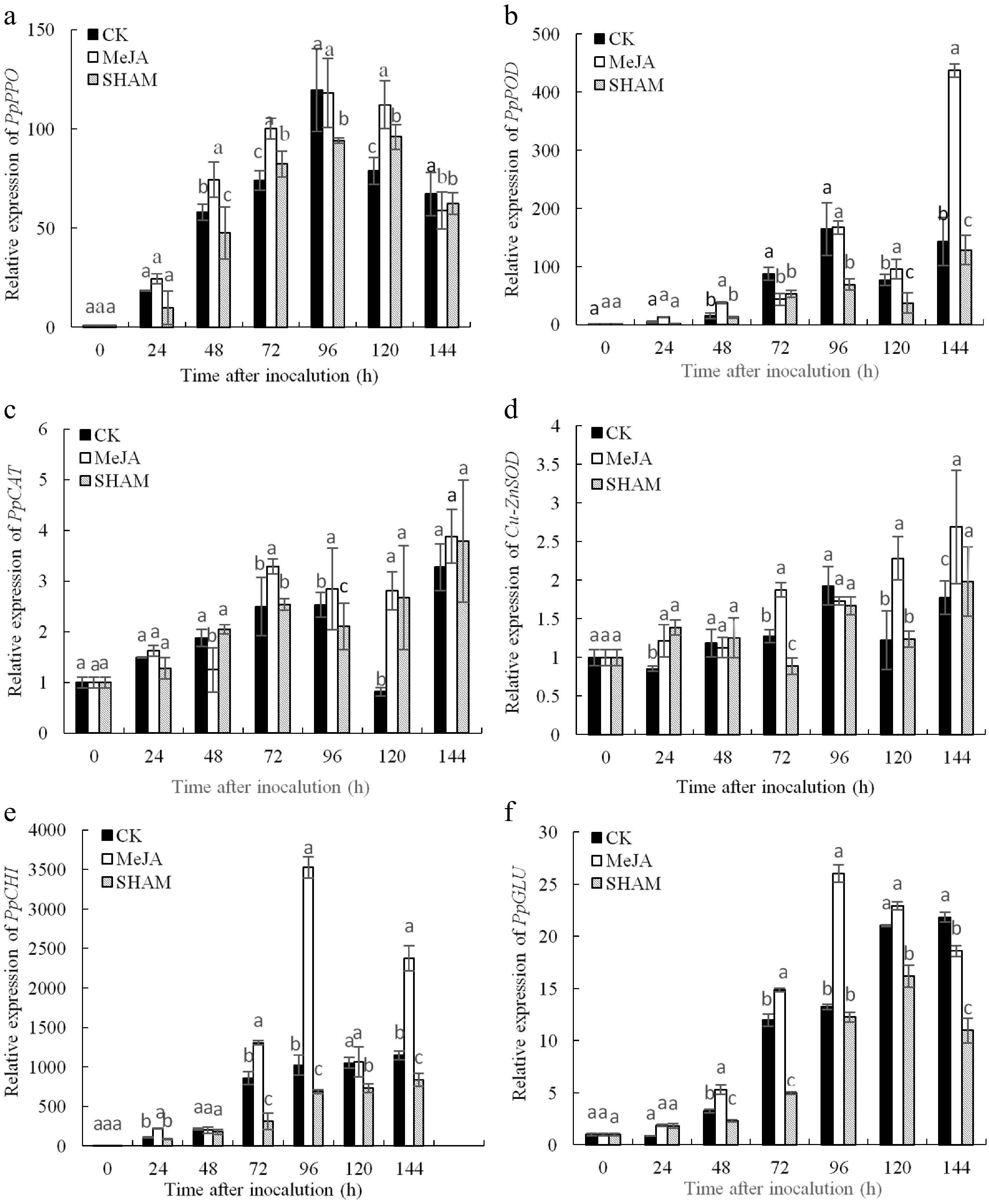
Figure 5.
Expression analysis of defence-related genes including PpPPO (a), PpPOD (b), PpCAT (c) Cu-ZnSOD (d), PpCHI (e) and PpGLU (f) in pear fruit inoculated with Penicillium expansum after 100 μmol/L MeJA treatment for 36 h. Values are the means of three replicates and vertical bars indicate the standard errors. Different letters indicate significant differences (p < 0.05) between the treatments determined using the Duncan’s multiple range test.
The expression level of PpPPO in the MeJA group was obviously higher than that in the control at 48 h, 72 h and 120 d, and the expression level of PpPPO in the MeJA treatment peaked at 96 h and was obviously higher than the SHAM treatment, except at 144 h (Fig. 5a) (p < 0.05). As shown in Fig. 5b, the expression level of PpPOD in MeJA group fruits was markedly higher than that of other groups at 48 h, 120 h and 144 h, whereas the expression of PpPOD in the SHAM group was lower than that in the control at 72−144 h. In addition, the highest value of PpPOD expression in the MeJA group appeared at 144 h, which was 3.06 and 3.40 times that of the control and SHAM treatment groups, respectively (Fig. 5b) (p < 0.05).
As shown in Fig. 5c, the PpCAT gene expression level in the MeJA group was markedly higher than that in the control and SHAM groups at 72−96 h; although it reached a peak at 144 h, there were no significant differences among the three treatments (p < 0.05). The expression level of Cu-ZnSOD in the MeJA group was markedly higher, which was 1.52 and 1.36 times that of the control and SHAM groups at 72, 120 and 144 h, respectively, and the highest value was observed at 144 h (Fig. 5d) (p < 0.05).
The change in PpCHI expression after inoculation in pear fruit is shown in Fig. 5e. The PpCHI expression level in the MeJA group was higher than that in the other two groups except at 48 h within 144 h after inoculation; the difference between MeJA treatment and control groups was significant at 24, 72, 96 and 144 h. PpCHI expression in the control was clearly higher than that in the SHAM treatment group at 72−144 h. The expression level of PpCHI peaked at 96 h in MeJA treatment fruits, which was 3.45 and 5.11 times that of the control and SHAM groups, respectively (p < 0.05). PpGLU gene expression in the MeJA group was obviously higher than that in the other two groups at 48−96 h, and its expression peaked at 96 h. The expression of PpGLU was lower in the SHAM group than in the control at 48−72 h and 120−144 h (Fig. 5f) (p < 0.05).
-
MeJA, a natural plant growth regulator, participates in the defence response and signalling of plants to external stress and induces the host immune response to reduce disease risk[6]. In this study, the results indicated that the induction effect of MeJA depended on treatment concentration and time before inoculation, and 'Cuiguan' pear fruits fumigated with 100 μmol/L MeJA for 36 h then inoculation were significantly improved the resistance to blue mould rot. Specifically, 10−100 μmol/L MeJA could effectively improve pear fruit resistance to blue mould rot, whereas 1,000 μmol/L MeJA treatment aggravated the occurrence of blue mould rot. Many previous studies showed that excessive MeJA treatment had opposite effects on fruit resistance to postharvest diseases. This conclusion was similar to the results obtained in kiwifruit[10], however, the optimum concentration of exogenous MeJA-induced resistance to Monilinia fructicola in sweet cherry fruit was 0.2 mmol/L[19], and 10 μmol/L MeJA treatment could significantly reduce the occurrence of loquat fruit decay caused by Colletotrichum acutatum[20]. Guo et al.[16] found that 100 μmol/L MeJA had the highest inhibitory effect on mandarin green mould disease. In addition, the suitable time between MeJA treatment and pathogen inoculation was 36 h in our test, whereas the optimum time for induction to Penicillium citrinum in Chinese bayberry[21], Botrytis cinerea in table grapes[17], and B. dothidea in kiwifruit[10] by MeJA treatment was 6, 12 and 24 h respectively. All of these results indicate that plant-induced resistance to the pathogen is maintained by plant and pathogen genetic factors that vary based on host-pathogen combinations.
Plant-induced resistance refers to the ability of plants to increase their immune defence enzymes and plant protection factors and thus form a self-protecting natural barrier to prevent infection by pathogens[22]. SOD, POD, PPO and CAT, which are key antioxidant enzymes in the reactive oxygen species (ROS) system because of the protection they provide to plant tissues against pathogen infection[23], are commonly involved in the plant defence response, which plays a pivotal role in the postharvest control area[24, 25]. In this study, the activities of SOD, POD, PPO and CAT were effectively induced by MeJA in pear fruit. Previous studies on defence responses have also shown that the activation of defence enzymes was largely related to the induced resistance mechanisms of table grape fruit against B. cinerea[17], tomato against Alternaria alternata[26] and kiwifruit against B. dothidea[10]. Various defence-related proteins in plants have been identified as PR proteins, including CHI and GLU, and chitin and β-1,3-linked glucans in fungal cell walls can be catalysed to inhibit pathogen growth[27]. Their accumulation in diseased plant cells and functions in different plant-microbe interactions have been intensively studied[28]. Experiments have shown that MeJA treatment can increase the activities of CHI and GLU in sweet cherry fruits and reduce the incidence of blue mould[29]; MeJA treatment can also increase resistance to anthracnose disease in avocado fruits[12]. Similarly, this study also showed that the CHI and GLU activities in MeJA-treated pear fruits were significantly higher than those in control fruits. Thus, the enhanced defence-related enzyme activities and proteins that were observed in this study may contribute to this resistance induction.
Total phenols, the most abundant secondary metabolite in plants, are antimicrobials and precursors of structural polymers, including lignin, or function as signal molecules, which have been shown to be involved in disease resistance in many plant–pathogen interactions[30]. Many studies have revealed that total phenols play a pivotal role in the plant's defence against diseases. For example, Min et al.[18] reported that MeJA treatment enhanced the accumulation of total phenolics and obviously inhibited the development of grey mould decay in tomato fruit. Moreover, total phenols were involved in the defence responses of peach fruit to P. expansum[31] and tomato fruit to B. cinerea[32]. MDA is one of the key factors of membrane lipid peroxidation and is an important indicator of disease resistance[33]. In our experiment, MeJA treatment significantly increased the total phenol content, but it inhibited MDA accumulation in pear fruit; similar results were observed in strawberry fruit against postharvest decay[34], pitaya fruit against wounding stress[35] and tomato fruit against grey mould treated with MeJA[18].
As an important elicitor in the field of plant-induced resistance research, MeJA can not only increase the activity of defence enzymes and the total phenol content but can also regulate defence enzyme genes at a higher level, which is strongly related to increased resistance to pathogens in plants. Jin et al.[15] found that MeJA could effectively increase the gene expression of POD, CAT and APX and decrease chilling injury during storage in peach fruit. Furthermore, MeJA treatment successfully increased the expression of PR and polygalacturonase inhibiting proteins (PGIP) genes and reduced the incidence of grey mould decay in Fragaria chiloensis fruit, then MeJA had a long-lasting effect on the reduction of Botrytis cinereal incidence[34]. In this study, RT-qPCR was carried out to detect the expression of defence enzyme genes, including PPO, POD, CAT, SOD, CHI and GLU, to explain their roles in pear fruit-induced resistance to P. expansum. All six of the aforementioned genes were significantly upregulated by MeJA treatment, which indicated that comprehensive defence reactions were activated by MeJA in pear fruit; this finding may reflect a transient rise in the activity levels of related enzymes. Our results are consistent with previous studies suggesting that higher expression levels of PR genes may stem from MeJA-induced defences in banana fruit[36] as well as defence-related genes, such as CHI, GLU, SOD and CAT, in table grape fruit[17]. Recently, our study showed that 0.1 mmol/L MeJA treatment activated kiwifruit defence resistance to B. dothidea by inducing higher expression levels of genes such as POD, SOD, CHI and GLU[10]. These results indicate that the induction of these defence-related genes may play an important role in the mechanism by which MeJA inhibits P. expansum infection in pear fruit.
-
In summary, the incidence of blue mould rot in pear fruits was inhibited in the MeJA group. These results highlight the fact that enhanced defence-related enzyme activities, higher content of total phenol and higher gene expression levels may all be associated with increases in the defence resistance of 'Cuiguan' fruit to P. expansum.
-
'Cuiguan' pear fruits were obtained from an orchard located in Xiajiang County, Jiangxi Province, China. The selected fruits were directly transferred to the postharvest laboratory at Jiangxi Agricultural University and sorted to obtain uniformly sized fruit without any mechanical wounds or disease symptoms for the following experimental procedures. The fruits were then stored in the laboratory to sweat for 24 h at 20 °C.
The test pathogen strain P. expansum isolated from diseased 'Cuiguan' fruit showing symptoms characteristic of blue mould rot was supplied by the plant pathology laboratory of Jiangxi Agricultural University[37]. P. expansum was cultured and kept on potato dextrose agar medium (PDA) at 25 °C for 5 d. The spore suspensions were then measured by a haemocytometer, and the concentration was adjusted to 1.0 × 106 spores/ml using sterile distilled water.
MeJA and SHAM
-
MeJA, SHAM and Tween 80 were supplied by Sigma Co. (Saint Louis, USA). The concentrations of MeJA used in the experiment were 10, 100 and 1,000 μmol/L. SHAM was first dissolved in 95% ethanol and then prepared with sterile water including 0.1% Tween 80 in 100 μmol/L SHAM reagent.
Chemical treatments
-
The pear fruits were randomly divided into five groups as follows: control, fruits exposed to air; 10 μmol/L MeJA, fruits exposed to 10 μmol/L; 100 μmol/L MeJA, fruits exposed to 100 μmol/L; 1,000 μmol/L MeJA, fruits exposed to 1,000 μmol/L; and SHAM, fruits exposed to 0.10 mmol/L (based on previous results). The filter paper was placed in a closed plastic box with liquid MeJA (6 L). The pear fruits were then treated for 36 h at 20 °C with a final vapour concentration (10, 100, 1,000 μmol/L), and 20 mL of 100 μmol/L SHAM was sprayed on the surface of pear fruits under the same conditions. The surfaces of pear fruit were inoculated with 10 μL of prepared spore suspension (1.0 × 106 spores/mL) after disinfesting in 75% (v/v) ethanol and wounding (1-mm diameter with 1-mm depth) with a sterilized needle. All fruits were incubated in plastic boxes at 25 °C (day/night, 12-h photoperiod, 90% relative humidity). Each treatment had three replicates, and 10 fruits were used per replicate. After inoculation, observations of the disease were made and recorded daily.
Pear fruits were fumigated with 100 μmol/L MeJA for 0, 12, 24, 36 and 48 h before inoculation. Other methods were the same as above.
Lesion size measurement on pear fruits
-
After inoculation, the lesion diameter of pear fruit was measured using the cross method with a Vernier gauge. The induction effect of MeJA on pear fruit resistance against P. expansum was calculated using the following formula: Inducing effect (%) = (lesion diameter of control fruits − lesion diameter of treatment fruits)/lesion diameter of control fruits × 100.
Measurement of defence-related enzyme activities
-
Pears were treated with MeJA at 100 μmol/L for 36 h before inoculation. The control was treated with sterile water, and the negative control pear fruits were sprayed with 100 μmol/L SHAM. Other methods were the same as the description in chemical treatments. Fruit flesh (approximately 0.5 g in fresh weight) around diseased sites was excised every 24 h after inoculation. The fruit flesh was quickly mixed and frozen with liquid nitrogen and kept at −80 °C for biochemical measurement and gene expression analysis.
To determine the activity of PPO and POD, crude enzyme solution was prepared as previously described[38]. Pear fruit tissue (1 g) was ground to powder using liquid nitrogen and homogenized in 10 mL of sodium phosphate buffer (0.1 mol/L, pH 6.8) containing 5% polyvinyl polypyrrolidone (PVPP). The homogenate was then centrifuged for 20 min at 4 °C. The supernatant liquid was used for analysis of PPO and POD activity following the method described by Pan et al.[10] and Dou et al.[38]. with slight modifications. The reaction mixture of PPO contained 4 mL of acetic acid-sodium acetate buffer (50 mmol/L, pH 5.5), 0.9 mL of pyrocatechol (50 mmol/L) and 0.1 mL of crude extract, and PPO activity was expressed as U/g FW based on fresh weight, where one unit (U) was determined as the absorbance increase 0.01 per min at 420 nm. The reaction mixture of POD contained 0.5 mL of supernatant (see above), 3.0 mL of guaiacol solution (25 mmol/L) and 200 μL of H2O2 solution (0.5 mol/L). POD activity was expressed as U/g FW based on fresh weight, where one unit (U) was expressed when the absorbance increased 0.01 per min at 470 nm.
To determine CAT activity, 1 g of pear fruit tissue was ground to powder with liquid nitrogen and homogenized in 5 mL of sodium phosphate buffer (50 mmol/L, pH 7.5) including 5% PVPP. The homogenate was then centrifuged for 30 min at 4 °C. The CAT reaction system contained 0.1 mL of supernatant and 2.9 mL of H2O2 solution (20 mmol/L). The absorbance was recorded every 30 s for more than 5 min at 240 nm. The CAT activity was expressed as U/g FW based on fresh weight, and one unit (U) was defined as the change of 0.01 per min in absorbance[10,38].
SOD activity was measured with a SOD assay kit (Jian Cheng Biotechnology Co., Ltd., Nanjing, China); CHI and GLU activity were determined using CHI and GLU assay kits, respectively (Solarbio, Beijing, China).
Determination of total phenol and malondialdehyde (MDA) content
-
The total phenol content of pear fruit was measured using the Folin-Ciocalteu method according to a previous study[39]. One gram of frozen pear fruit tissue was extracted with 8 mL of methanol and extracted by ultrasound at 50 °C for 30 min. The extracts were centrifuged for 20 min at 4 °C. The reaction mixture (0.5 mL supernatant, 0.5 mL Folin-Ciocalteu and 5 mL distilled water) was placed in the dark for 10 min and then mixed with 1 mL of 10% Na2CO3 for 60 min at room temperature, and the absorbance was determined at 765 nm. The total phenol content was calculated according to a standard curve made with gallic acid and defined as 100 grams of fresh gallic sample.
MDA content was determined via the method of Pan et al.[10]. One gram of pear fruit tissue was homogenized in 8 mL of trichloroacetic acid (TCA, 100 g/L), the homogenate was centrifuged for 20 min at 4 °C, 2 mL of supernatant liquid was mixed with 2 mL of 0.67% (w/v) thiobarbituric acid (TBA), and the mixture was boiled in 100 °C water for 30 min and centrifuged again after cooling. Finally, the supernatant was used to measure the absorbance at 450, 532 and 600 nm. The MDA content was calculated according to the following formula:
$\rm MDA (mol \;kg^{-1}) = [6.452\times(A_{532}-A_{600})-0.559\times A_{450}] \times\frac{Vt}{Vs\times W\times 100} $ where Vt = total volume of extract (mL); Vs = the extract volume (mL); W = fresh weight of sample (kg); and A532, A600 and A450 represent the absorbance values at 532 nm, 600 nm and 450 nm, respectively.
RNA extraction, cDNA synthesis and RT-qPCR
-
The total RNA of pear fruit tissue was isolated using an RNA extraction kit (Huayueyang Biotech Co., Ltd., Beijing, China). The total RNA concentration was measured by a spectrophotometer. First-strand cDNA was synthesized with a Prime Script RT reagent kit (TaKaRa, Dalian, China) following the manufacturer's protocols. The prepared cDNA was kept at −80 °C for RT-qPCR.
The expression levels of six defence-related genes were analysed using RT-qPCR with SYBR® Premix (Takara, Dalian, China). The specific primers of these genes for RT-qPCR were obtained from the literature (Table 1). The PCR program was set as follows: 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s, 60 °C for 30 s and 55 °C for 30 s. The 10-μL reactions included 3.4 μL of ddH2O, 1 μL of diluted cDNA strand, 5 μL of SYBR® Premix Ex Taq (TaKaRa, Dalian, China), and 0.3 μL of each primer. The expression levels of defence-related genes were normalized by actin and calculated using the 2−ΔΔCᴛ method[40, 41], and 3 replicates were measured for each sample. The RT-qPCR analysis was based on the primer sequences shown in Supplemental Table S1.
Statistical analysis
-
SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was used to process and analyse the data, and significant differences were determined by the Duncan multiple-range test at p = 0.05.
This work was funded by the National Natural Science Foundation of China (32160399) and Jiangxi Province Fruit and Vegetable Postharvest Processing Key Technology and Quality and Safety Collaborative Innovation Center Project (JXGS-03).
-
The authors declare that they have no conflict of interest.
- Supplemental Table S1 Sequences of primers used in quantitative real-time RT-qPCR.
- Copyright: 2022 by the author(s). Exclusive Licensee Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Chen M, Luo Z, Zhao X, Li S, Wu F, et al. 2022. Exogenously applied methyl jasmonate increased the resistance of postharvest pear fruit to blue mold. Fruit Research 2:11 doi: 10.48130/FruRes-2022-0011
Exogenously applied methyl jasmonate increased the resistance of postharvest pear fruit to blue mold
- Received: 14 April 2022
- Accepted: 07 July 2022
- Published online: 27 July 2022
Abstract: Methyl jasmonate (MeJA) is a plant-signalling molecule that plays significant roles in stress reactions and defence responses. The goal of this study was to characterize the effects of exogenous MeJA application on the resistance of postharvest pear fruit to blue mould rot caused by Penicillium expansum and investigate the mechanism underlying the observed effects of MeJA application. MeJA treatment effectively reduced the lesion diameter of blue mould rot in pear fruit. Furthermore, MeJA significantly enhanced the activities of antioxidant and defence-related enzymes, such as polyphenol oxidase (PPO), superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), β-1,3 glucanase (GLU) and chitinase (CHI); total phenol content also increased, and membrane lipid peroxidation decreased. MeJA treatment promoted the expression of PpPPO, Cu-ZnSOD, PpPOD, PpCAT, PpCHI and PpGLU. Overall, this experiment suggested that MeJA-induced pear fruit resistance against blue mould rot may be related to the enhanced activities of defence enzymes and gene expression.
-
Key words:
- Methyl jasmonate /
- Pear fruit /
- Penicillium expansum /
- Postharvest /
- Defence enzyme













