HTML
-
Horticultural crops, including fruit trees, vegetables and ornamental crops, have great significance in enriching human nutrition and beautifying human living environment. For most fruits and some vegetables, sugar and acid content is an important factor determining the fruit flavor quality. The main soluble sugars in fruits include sucrose, glucose and fructose, and the organic acids mainly contain malic acid, citric acid and tartaric acid[1]. As a unique organelle in mature plant cells, vacuoles can store metabolites such as sugar and acid, and this storage function mainly depends on tonoplast-localized transporters[2]. Therefore, the study of vacuolar sugar and acid transporters are very helpful in resolving the quality formation mechanisms of horticultural crops. In this paper, starting from the storage role of vacuoles, we summarized the tonoplast transporters that have been identified in main fruit crops, and discussed the methods for study on vacuoles, which will provide clues for subsequent research.
An overview of vacuole -
The term vacuole was originally named by famous French biologist Félix Dujardinand, referring to the blank space of protozoan contractile vesicles[3]. Due to the similar blank spaces were observed in the leaves and roots, this concept was later widely used in plant biology. For plants, vacuole is an important and specific organelle, as it does not exist in animal cells, but only exists in terrestrial plants or most fungi and algae[4]. The vacuoles can account for 80%−90% of the total volume of mature mesophyll cells and play diverse roles in plants[5]. The morphology, quantity, function and basic characteristics of plant vacuoles are also varied in different cell types and physiological conditions[6]. Just like in the young meristem, there are many small vacuoles in the cells, while there is a large central vacuole in the mature mesophyll cells. The substances in the vacuole determines what kind of function it has[7]. At present, there are two sources of cellular materials stored in vacuoles: endocytosis and intracellular biosynthesis[8]. According to the contents of these cells, vacuoles in cells can be divided into two different types. One is lytic vacuole (LV), which contains hydrolase that degrades unwanted impurities in cells and plays a role in storing ions to regulate cell balance, pressure buffering and pathogen resistance. The other is protein storage vacuole (PSV), which is transformed from the pre-existing vacuole[9]. There are many transporters in the membrane of protein storage vacuole to store various metabolites, such as carbohydrates, organic acids, secondary metabolites[10]. Therefore, the vacuolar membrane transporters are essential to accumulate more nutrient metabolites to promote the formation of horticultural crop yield and quality.
At the beginning, the study of vacuole membrane mainly from the yeast cells due to technical limitations. However, with the continuous development of technology, scientists can completely extract vacuoles from plants by improved approaches, and the functions of vacuoles have also been deeply studied[4]. They are mainly divided into the following four parts[11]. First, vacuoles act as lysosomes in plant cells, performing autophagy like animal cells, and decompose waste metabolites and toxic substances produced from inside and outside plant cells[12]. Second, when plants encounter biological stress (infected by pathogens or met with herbivores), the secondary compounds stored in vacuoles can convert into toxic compounds to the outside of cells, thus protecting plants from harm[13]. Third, vacuoles have higher osmotic pressure compared with cell sap, which can regulate swelling pressure and enhance the mechanical stability of plants[14]. Finally, and most importantly, vacuoles can be used as 'warehouses' to store nutrient metabolites, such as inorganic substances, organic acids, amino acids, carbohydrates, most of which exist in the cells of nutritional tissues such as seeds and fruits. For crops, vacuole is essential for the accumulation and metabolism of nutrients. Especially, the content of organic acids and carbohydrates in vacuole will affect horticultural fruit development and flavor, thus affecting its yield and quality.
Vacuolar transporters -
As an important storage space, most of the compounds found in vacuoles are hydrophilic. Even with charged molecules, they are not able to diffuse freely through the vacuolar membrane. Therefore, the mediation of tonoplast-localized proteins is required for the material exchange across the vacuolar membrane. The proteins located in the vacuolar membrane can be divided into various types, which can respectively correspond to the functions of the vacuolar membrane. For instance, the proton pump and proton antiporters located in the vacuolar membrane can control the pH of the vacuole, and maintain a slightly acidic environment in the vacuole[15]. Previously, the tonoplast Na+/ K+ proton antiporters (NHXs) were reported to affect the color change of morning glory during its opening by controlling vacuole pH value[16]. Besides, heavy metal ions are potentially toxic and are safely stored in vacuoles via the tonoplast-localized transporters[17]. ABC and MATE transporters were demonstrated to control the flow of endogenous secondary metabolites between vacuole and cytosol to protect the plant from stress conditions. It has been known that vacuoles store many substances conducive for the quality improvement of horticultural crops. In recent years, increasing research has been carried out to detect the function and specific regulation mechanism of vacuolar transports. With the discovery of transport pathway of metabolites in vacuoles, more and more vacuolar transporters involved in sugar and acid accumulation have been reported. These members can effectively regulate the sugar and acid content in mature fruit, so as to affect the quality of horticultural crops. The following section summarizes the vacuolar sugar and acid transporters and proton pumps that have been identified so far in horticultural crops, and briefly describes their functions and regulation mechanisms in fruit flavor quality formation.
Vacuolar sugar transporters -
Soluble sugars, as the main carbohydrate, not only provide energy for plant growth and development, but also affect the quality of fruits, as well as the yield of crops. The accumulation manner of soluble sugars has been well studied in plant, as sucrose equivalents are synthesized in the source leaves, and then transported to the storage tissues by symplast or apoplast pathway. During this process, sugar transporters are required for sugar loading or unloading. At present, the sugar transporters that have been identified in plants mainly include three categories: Monosaccharide Transporter-Like (MST) family, Sucrose Transporters (SUT/SUC), and Sugars Will Eventually Be Exported Transporters (SWEET). Monosaccharide transporters can be divided into 7 subfamilies, including Early Response to Dehydration-like 6 (ERDL6), Sugar Transport Protein or Hexose Transporter (STP/HT), Tonoplast Sugar Transporter (TST/TMT), Plastidic Glucose Transporter (pGlCT), Inositol Transporter (INT), Polyol/Monosaccharide Transporter (PMT), and Vacuolar Glucose Transporter (VGT)[18]. These sugar and acid transporters are located in different sub-cellular positions, as some of them are located in plasma membrane, and others are located in endomembrane system, like vacuolar membrane or other organelles’ membrane. According to the previous report, the vacuole consists of a group of transporters including SUTs/SUCs[19], SWEETs[20,21], VGTs[22], and TSTs[23], which of them work on sugar distribution between vacuole and cytosol space (Table 1, Fig. 1).
Table 1. The reported vacuolar membrane proteins involved in sugar and acid accumulation in fruit crops.
Proteins Species Functions Reference VvALMT9 Vitis vinifera L.(grape) Unidirectional transport of malic acid and tartaric acid to vacuoles. [56] MdALMT9 Malus domestica Borkh. (apple) WRKY31-ERF72-MA1 network regulates malic acid accumulation in apple fruit. [84] Ma1 Malus domestica (apple) ma1 reduces its malic acid transport function by removing the conservative C-terminal domain of MA1, resulting in low acidity of apple fruit. [59][54] ZjALMT4 Ziziphus jujuba Mill.(jujube) ZjWRKY7 activates the expression of ZjALMT4 to promote the accumulation of malate. [85] SlALMT5 Solanum lycopersicum (tomato) The SLALMT5 transported malate and inorganic anions such as nitrate and chloride, but not citrate. [56] SlALMT9 Solanum lycopersicum (tomato) SlALMT9 can determine the malic acid content and aluminum tolerance of tomato. [57] SlTDT Solanum lycopersicum (tomato) The overexpression of SlTDT significantly increased the content of malic acid in tomato fruit and decreased the content of citric acid. [50] MdTDT1 Malus domestica Borkh. (apple) MdMYB1 can affect the expression of acid transporters Ma1 and MdtDT to regulate fruit acidity. [86] CsCit1 Citrus sinensis. cv Washington CsCit1mediates CitH2- and CitH2--dependent H+ efflux from the vacuole and maintains vacuolar acidic pH and citric acid homeostasis. [62] DsSWEET17 Dianthus spiculifolius (caryophyllus) Influence on sugar metabolism and tolerance to stress in Arabidopsis thaliana. [87] MdSUT4 Malus domestica (apple) MdSUT4 may participate in the efflux of sucrose from the vacuolar membrane and may promote the synthesis of flavonoids. [28] CsSUT4 Citrus sinensis (citrus) In the natural state, CsSUT4 mainly mediates the output of sucrose in vacuoles. [29] BvSUT1 Beta vulgaris L.(sugar beet) BvSUT1 is a sucrose transport protein responsible for transporting sucrose to the main root of sugar beet. [26] VvTMT1 Vitis vinifera L.(grape) VvTMT1 participates in vacuolar monosaccharide transport and plays a major role in stress response. [88] VvTMT2 Vitis vinifera L.(grape) VvTMT2 gene is involved in the transport of hexose from cytoplasm to vacuole during berry ripening and over-ripening. [89] MdTMT1 and MdTMT2 Malus domestica Borkh. (apple) MdTMT1 and MdTMT2 are involved in the accumulation of fructose and sucrose during apple fruit ripening. [90] CmTST2 Cucumis melo L.(melon) CmTST2 plays an important role in sugar accumulation of melon fruit. [38] CmTST1 Cucumis melo L.(melon) CmTST1 transports sugar into vacuoles. [33] PbTMT4 Pyrus bretschneideri (pear) PbTMT4 participates in the sugar accumulation of vacuoles, thus affecting the growth and development of plants. [35] ClTST2 Citrullus lanatus (watermelon) ClTST2 encodes a vacuolar membrane protein, and its expression is related to the uptake and accumulation of vacuolar membrane sugar in watermelon pulp cells. [37] BvTST2.1 Beta vulgaris L.(sugar beet) The transporter BvTST2.1 is responsible for the uptake of vacuole sucrose in beet taproots. [40] PpTST1 Prunus persica L. (peach) Overexpression of PpTST1 led to a decrease in organic acid content and an increase in sugar content in peach and tomato fruits, indicating that it has dual functions in sugar accumulation and organic acid content. [39,91] StTST3.1 Solanum tuberosum L. (potato) StTST3.1-silenced leaves accumulated exceptionally high levels of maltose but low levels of sucrose and hexose. [92] StTST3.2 Solanum tuberosum L. (potato) Silencing of StTST3.2 in potato by stable transformation resulted in significantly lower RS content in tubers at harvest or after room temperature storage. [93] FvTST1 Fragaria vesca L. (strawberry) FvTST1 can mediate the uptake of different sugars, such as fructose, glucose, sucrose, and mannose. [94] MdVGT1 Malus domestica Borkh. (apple) MdVGT1 and MdTMT1 interact to transport glucose into vacuole. [95] MdERDL6-1 Malus domestica Borkh. (apple) MdERDL6-1 is a vacuole membrane H+/glucose co-transporter. [31] CsERD6L Citrus sinensis (citrus) The function of CsERD6L was verified to have glucose transport activity. [29] LeVHA-AP1 Lycopersicon esculentum L. (tomato) The concentration of sucrose in fruit of antisense transgenic tomato with V-ATPase A subunit was increased, but the concentration of glucose and fructose did not change. [71] MdVHA-A Malus domestica Borkh. (apple) MdVHA-A was involved in malate accumulation and vacuolar acidification in apple. [70] CitVHA-c4 C. reticulata Blanco cv. Ponkan CitVHA-c4 was reported to be involved in citric acid accumulation. [68] MdVHP1 Malus domestica Borkh. (apple) Overexpression or heterologous expression of V-PPase coding gene MdVHP1 significantly promoted the accumulation of malic acid in apple callus and tomato fruit. [96] MdPH1 and MdPH5 Malus domestica Borkh. (apple) MdPH1 and MdPH5 have been identified and shown to be involved in vacuolaracidification and malate accumulation [70] CitPH1 and CitPH5 Citrus sinensis. Cv Washington CitPH1 and CitPH5, are expressed in the fruits of sour lemon, orange, pummelo, and rangpur lime. [77] Ma10 Malus domestica (apple) Ma10 gene encodes P3A H+- ATPase of the vacuolar membrane that controls the vacuolar acidification of apple fruit. [55] 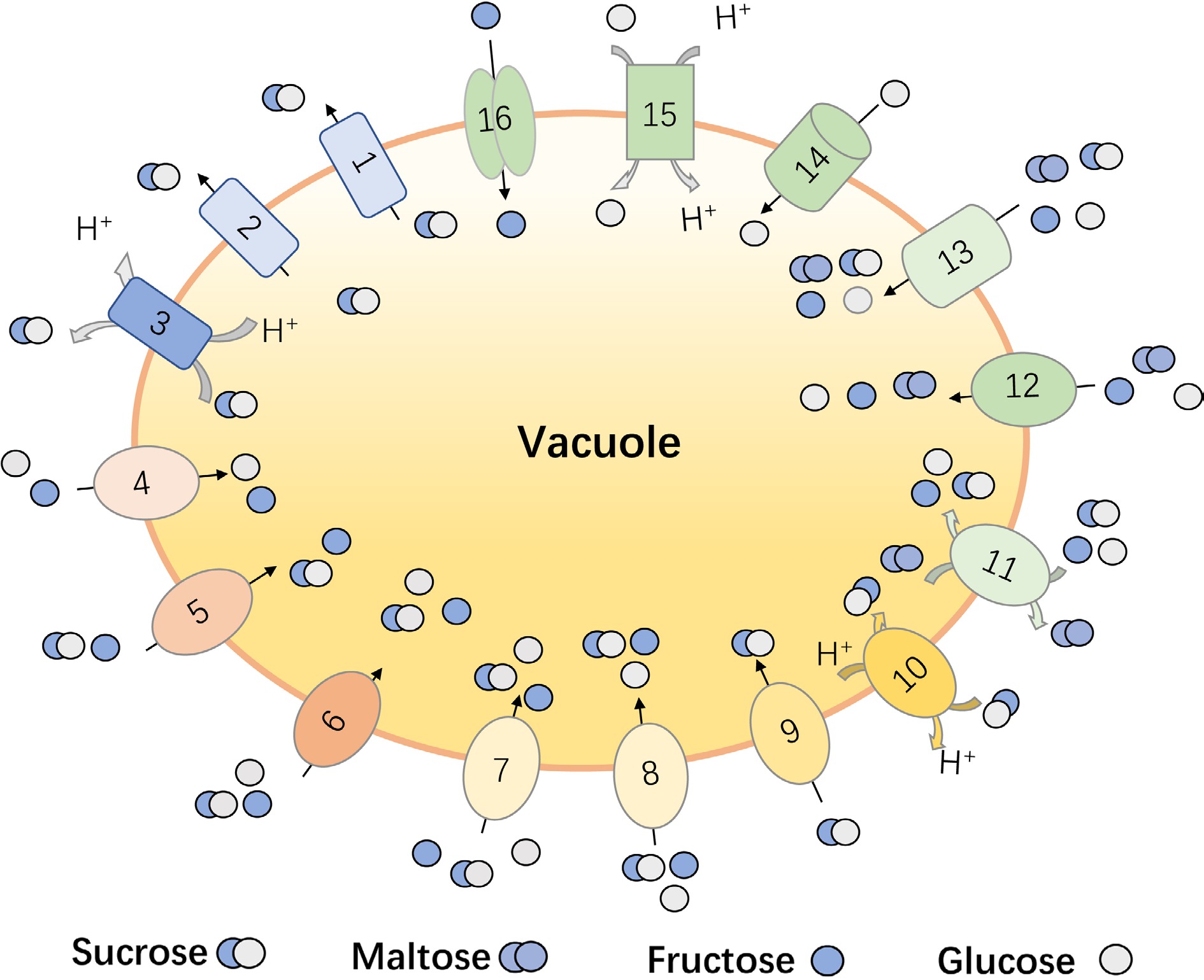
Figure 1.
The reported vacuolar sugar transporters in fruit crops. (1) Malus domestica sucrose transporters, MdSUT4; (2) Citrus sinensis sucrose transporters, CsSUT4; (3) Beta vulgaris sucrose transporters, BvSUT1; (4) Vitis vinifera tonoplast sugar transporter, VvTMT1 and VvTMT2; (5) Malus domestica tonoplast sugar transporter, MdTMT1 and MdTMT2; (6) Cucumis melo tonoplast sugar transporter, CmTST1 and CmTST2; (7) Pyrus bretschneideri tonoplast sugar transporter, PbTMT4; (8) Citrullus lanatus tonoplast sugar transporter, ClTST2; (9) Beta vulgaris tonoplast sugar transporter, BvTST2.1; (10) Solanum tuberosum tonoplast sugar transporter, StTST3.1; (11) Solanum tuberosum tonoplast sugar transporter, StTST3.2; (12) Prunus persica tonoplast sugar transporter, PpTST1; (13) Fragaria vesca tonoplast sugar transporter, FvTST1; (14) Malus domestica vacuolar glucose transporter, MdVGT1. (15) Malus domestica early response to dehydration-like 6, MdERDL6-1; (16) Citrus sinensis sugars will eventually be exported transporter, CsSWEET16.
With the deepening of research on vacuole, the function of some tonoplast sugar transporters has been verified. For the transportation of disaccharides in plants, SUT subfamily gene members have been isolated and identified from horticultural crops, such as Solanum lycopersicum[24], Solanum tuberosum[25], Beta vulgaris[26], Prunus persica[27], Cucumis melo[19], Malus domestica[28], Citrus sinensis[29], Fragaria[30]. Among them, SUT4 was proofed to be the only SUT member that localized in vacuole membrane for sucrose export from vacuole lumen. For example, CsSUT4 mainly mediates the efflux of sucrose from the vacuole, and its activity is significantly inhibited by the uncoupling agent CCCP and the cell membrane P-ATPase inhibitor, implying the co-transport with protons[29]. The apple MdSUT4.1 gene encodes a tonoplast localized protein and its expression level had a negative correlation with fruit sugar content. Overexpression of MdSUT4.1 in strawberry and apple callus inhibited the sugar accumulation, suggesting that it functions to export sugar out of the vacuole[28].
Besides, the increasing reports demonstrated that the tonoplast-localized TST/TMT members play vital roles for sugar accumulation during fruit development and ripening, which had been verified in Malus domestica[31], Citrullus lanatus[32], Cucumis melo[33], Prunus persica[34], Pyrus bretschneideri[35] etc. TST is responsible for sugars flowing from the cytoplasm into the vacuole, while the latter is responsible for the reverse outflow of hydrogen ions from the vacuole[36]. The heterologous overexpression of pear PbTMT4 in tomato significantly enhanced glucose and fructose content in tomato fruits[35], implying the hexose transport activity of PbTMT4. In sweet watermelon, the expression of ClTST2 is related to the uptake and accumulation of vacuole sugar in watermelon pulp cells[37]. The overexpression of the tonoplast sugar transporter CmTST2 in melon fruits also increased the sugar content[38]. In peach, the expression profile of PpTST1 was positively consistent with the sugar accumulation, and its silence could significantly inhibit the sugar content in peach fruits[39]. In addition to the monosaccharide transport ability, TST member has also been detected to have specific sucrose transport function in recent years. BvTST2.1 was reported to be a sucrose-specific transporter, and electrophysiological evidence suggested that it operated as a proton antiporter, coupling the import of sucrose into the vacuole to the export of protons in beet taproots[40]. Except for affecting the accumulation of sugar, these sugar transporters also take part in the development of organs. Lu et al. concluded that the overexpression of CmTST1 promoted the root growth under high sugar conditions[33]. The transgenic tomatoes with the overexpression of pear PbTMT4 had a higher glucose and fructose levels and an earlier flowering phenotype in comparison with wild-type plants[35].
In addition, other sugar transport protein subfamilies also have functional members that localized on the vacuole membrane[29]. In the genomes of many horticultural crops such as pineapple[1], tomato[41], grape[42] and sweet orange[29], the INT family members with high homology of Arabidopsis vacuolar membrane AtINT1 have been discovered, which is reported to be responsible for the efflux of inositol in vacuoles[43]. The tonoplast-localized ERD6L mainly mediate the simultaneous output of glucose/H+ in vacuoles and some of them can also mediate the diffusion of sugar in a non-specific way[44]. The apple MdERDL6 was proofed to mediate glucose efflux to the cytosol and then promote sugar accumulation in the vacuole through up-regulating TSTs in apple and tomato[31]. For the SWEET proteins, most of them are localized in the plasma membrane that participate in phloem loading in the process of plant sugar allocation and metabolism[45]. However, tea plant CsSWEET16 contributed to sugar compartmentation across the vacuole and function in modifying cold tolerance[46]. Together, these findings demonstrate that a various type of sugar transporters are localized in tonoplast for sugar accumulation in horticultural crop. Given the multiple roles of them in fruit quality formation, development and stress response, it is worthy to study the detail regulation mechanism of their transport activity in future.
Vacuolar acid transporters -
The flavor of the fruit is mainly determined by the sugar-acid ratio, so in addition to the sugar content, the acid content and types are also particularly important. The acid in the fruit is mainly organic acid, which can affect the acidity of the fleshy fruit, regulate the cell osmotic pressure, pH value, and take part in stress resistance and sensory quality of the fruit[47]. Although there are various types of organic acids in different fleshy fruits, malic acid and citric acid are the main organic acids in most horticultural crops. In fruits, malic acid and citric acid are transported and stored in the acidic environment of vacuole. In cells, malic acid and citric acid exist in the neutral or slightly alkaline environment of cytoplasm in the form of divalent anion and trivalent anion, respectively[2]. When it enters the acidic vacuole, it is protonated to maintain the transmembrane electrochemical potential difference, so that the organic acid enters the vacuole more continuously. Therefore, the transporters on the vacuole membrane play an important role in organic acid transport process[48]. For malic acid, proteins and pathways that mediate its transport and accumulation have been reported, which is catalyzed by at least one transporter and several channels[49] (Table 1, Fig. 2). The first type is tonoplast dicarboxylic transporter (tDT), and the corresponding tDT homologous genes have been cloned from horticultural crops such as tomato, apple, plum and citrus, respectively[47]. Among them, SlTDT1 can significantly increase the malic acid content of tomato fruit, but reduce the citric acid level simultaneously[50]. The other type is aluminum-activated malate channel protein family (ALMT) that has the malic acid transport activity. ALMTs are a small gene family of anion channels. They were originally identified as malate exporters conferring aluminum resistance[51]. This gene family was usually divided into four distinct phylogenetic clades, and most of clade II members, including ALMT3, ALMT4, ALMT5, ALMT6 and ALMT9 were proofed to be localized to the vacuole membrane[48,52]. ALMT9 is the earliest reported malate channel protein located in the tonoplast, which can mediate the inward current of malic acid and fumaric acid across the vacuole membrane, and its specificity for malic acid is higher than that of fumaric acid[53]. At present, the function of this transporter has been verified in horticultural crops such as grapes, tomatoes and apples[54,55]. VvALMT9 is an AtALMT9 homologue in grape, which mediates the accumulation of malic acid and tartaric acid in the vacuole of grape cells[56]. Two ALMT members, SlALMT4 and SlALMT5, are expressed during fruit development, and the overexpression of SlALMT5 alters malate content in seeds of tomato[57]. Besides, SlALMT9 was also found in the process of tomato domestication, which can determine the malic acid content and aluminum tolerance of tomato[58]. In apple, the ‘Ma’ protein, homologue of the ALMT9, has been implicated in fruit acidity via mediation of malate accumulation in vacuole. Moreover, the malate transport activity of Ma was proofed to be relied on the C-terminal conserved domain[59].
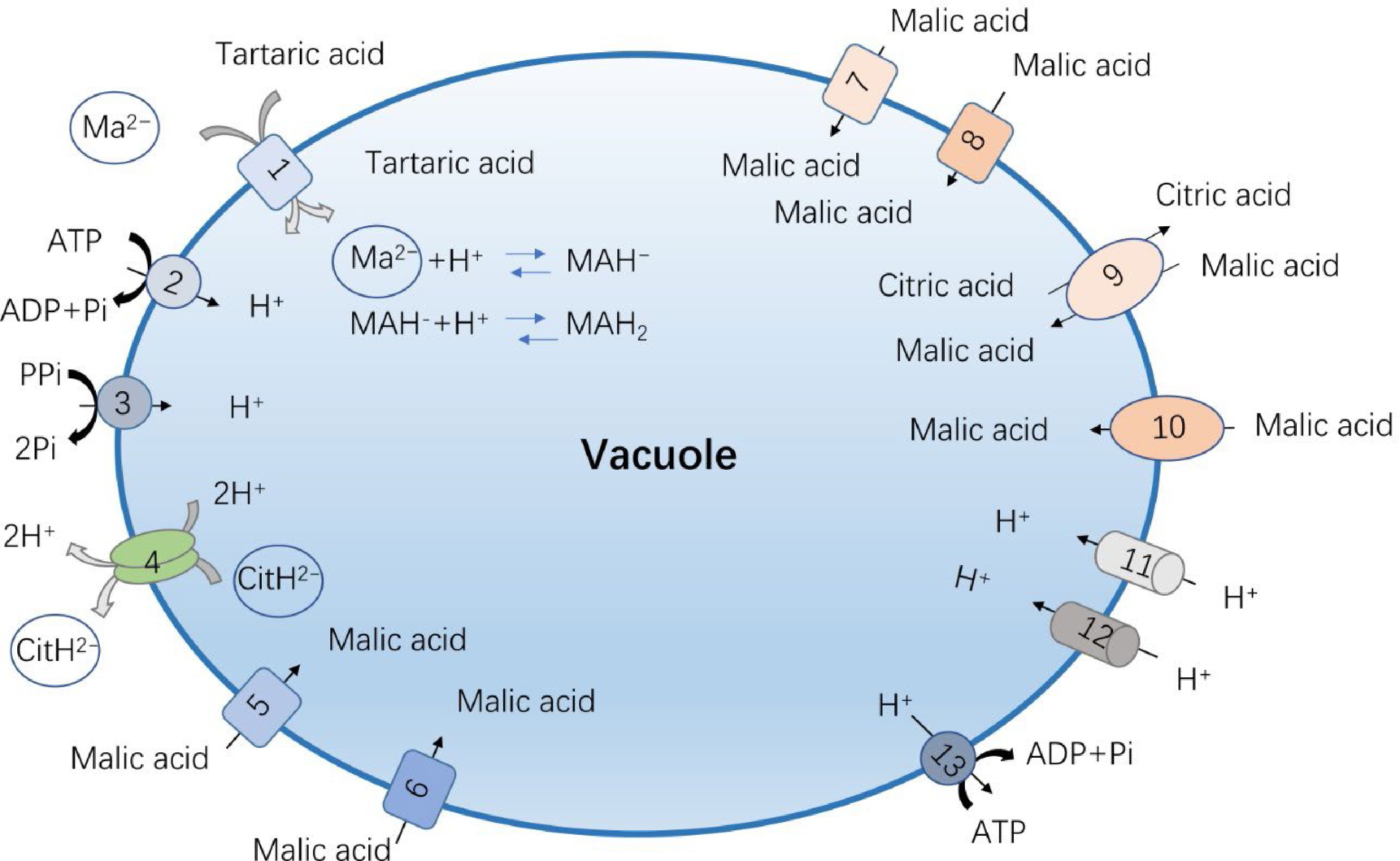
Figure 2.
The reported vacuolar acid transporters and proton pumps in fruit crops. (1) Vitis vinifera aluminum-activated malate channel protein, VvALMT9; (2) Malus domestica V-ATPase (vacuolar H+-ATPase, abbreviated VHA), MdVHA-A; (3) Malus domestica V-PPase (vacuolar H+-pyrophosphatase, abbreviated VHP), MdVHP1; (4) Citrus sinensis citrate transporter 1, CsCit1; (5) Malus domestica aluminum-activated malate channel protein, MdALMT9; (6) Ziziphus jujuba aluminum-activated malate channel protein, ZjALMT4;(7) Solanum lycopersicum aluminum-activated malate channel protein, SlALMT5;(8) Solanum lycopersicum aluminum-activated malate channel protein, SlALMT9; (9) Solanum lycopersicum tonoplast dicarboxylic transporter, SlTDT1; (10) Malus domestica tonoplast dicarboxylic transporter, MdTDT1; (11) Malus domestica P-type ATPases, MdPH1 and MdPH5; (12) Citrus sinensis P-type ATPases, CsPH1 and CsPH5; (13) Citrus sinensis V-ATPase (vacuolar H+-ATPase, abbreviated VHA), MdVHA-A.
The metabolic pathways of citric acid had been widely studied. However, the transport mechanism of citric acid in different species or even in the same species is different[60], and the transporter that mediates citric acid across vacuole membrane has not been found clearly. AttDT could play a role in the transport of citrate into the vacuole, but it is not the main tonoplast citrate carrier since the transport rate of citrate was higher in AttDT knockout plants[61]. Previous report shown that CsCit1, a homologue of tonoplast dicarboxylate transporter, can mediate the citric acid efflux from the vacuole and homoeostasis in citrus[62]. CitDIC and the cation/H+ exchanger CitCHX are also involved in citrate degradation during fruit development[63]. So, in comparison to the malate, the citrate transporter and accumulation mechanism in vacuole are still obscure. In citrus, more researchers proposed that an ATP-dependent citrate pump may operate in addition to the malate channel. The further study is needed to provide sufficient evidence that citrate transport is coupled to a specific transporter and not only through the tonoplastic pH gradient[2].
Vacuolar proton pumps -
At the early stage of fruit development, the content of organic compound such as sugar and acid are the similar in the cytoplasm and vacuole fluid. But with the fruit ripening, the organic compound is increased and transported into the vacuole under the mediation of tonoplast transporters, resulting the organic content in the vacuole is much greater than those in the cytoplasm[55,64]. An adequate electrochemical gradients and proton gradient (ΔpH) are required to facilitate this accumulation[65−67]. Proton pump is one of the key components that provide the driving force to metabolite accumulation[3,17]. There are three types of proton pumps currently being discovered in fruit vacuoles, V-ATPase (vacuolar H+-ATPase, VHA) , V-PPase (vacuolar H+-pyrophosphatase, VHP) and P-ATPase (P-type H+-ATPases)[68]. V-ATPase is structurally complex and is consisted with more than 10 different subunits, while V-PPase only has a single peptide. These two transporters have been identified from the fruit samples, and their functions in flavor quality formation have also been verified. Usually, the proton pump work synergistically with sugar transporters for sugars delivery. And for acid transport, protons are transported from the cytoplasm to the vacuole by means of energy released by the phosphoric acid bond in the hydrolysis of ATP or pyrophosphate mediated by proton pumps, thus reducing the pH value in the vacuole cavity and providing energy for acid transport. As reported previously, the transcription factor CitERF13 affected the accumulation of citric acid by protein interaction with the proton pump CitVHA-c4[69]. V-PPase is based on inorganic pyrophosphate as substrate that provides energy for metabolite transport across the vacuole membrane, and deeper studies on apples demonstrated that MdVHP1 favors the accumulation of malic acid and soluble sugars in apple fruit[70]. In addition, VHA or V-ATPase, plays multiple roles in fruit growth, seed formation and the sugar accumulation in tomato fruits[71]. P-ATPases are primary transporters that are energized by ATP hydrolysis and are characterized with a single catalytic subunit[72]. In plants, these ATPases are composed of five major subfamilies, P1-P5, which are classified according to the ions they transport[73]. Among them, P3 subfamily ATPases are responsible for energizing the electrochemical gradient used as the driving force of secondary transporters[74]. In petunia, PH5 encodes a tonoplast-localized P3A-ATPase proton pump that interacts with the P3B-ATPase PH1 complex to acidify the vacuolar lumen of petal cells, thereby affecting petal color[75,76]. In apple, MdPH1 and MdPH5 have been identified and shown to be involved in vacuolar acidification and malate accumulation[70]. As their homologs, CitPH1 and CitPH5 are significantly associated with the citrate content in citrus fruit[77]. Overall, the tonoplast proton pumps like to provide the 'acid trap environment' or kinetics for sugar and acids accumulation in vacuole[2].
Research strategies in vacuole and vacuolar transporters -
Due to the large size of vacuole in plant cells, it is more difficult to study plant vacuole in comparison to in yeast cells. After the 1970s, with the continuous advancement and improvement of the separation technology of intact cells, the research on plant vacuole transporters has become more and more in-depth[3]. Up to now, a complete system is applied for plant vacuole transporters research, but there are still some functional transporters that have not been identified, or their physiological and biochemical properties are unknown, or their regulatory mechanisms and interactions are poorly understood. Therefore, in order to have a more systematic and detailed understanding of the molecular pathways of sugar and acid metabolism in horticultural crops, methods and technologies need to be improved to better conduct research.
In recent years, a variety of bioinformatics approaches are developed to conduct multi-omics analysis of various horticultural crops, including genome, transcriptome, proteome and metabolome. The transcriptome is mostly used to study functional genomes, but it can not fully reflect the expression of functional proteins. Given the diverse functions of the vacuole, there should be a large array of proteins in vacuole to conduct all of these processes[49]. But the detailed knowledge of proteins targeted to the vacuole and their underlying molecular functions are still lacking in most of fruit crops. Therefore, the sequencing and analysis of vacuolar proteome is particularly important for the study of vacuole function and identification of new vacuolar proteins. The main challenge in vacuole proteomic assay is the generation of highly purified intact vacuoles. To date, vacuole proteome studies have been successfully performed on the plant vegetative tissues, including Arabidopsis thaliana leaf cells[49], barley mesophyll cells[78,79], cauliflower buds[80] and sugar beet taproots[40]. Most recently, Kuang et al. made a great progress on fruit vacuole proteome research, as they successfully isolated the intact vacuoles from grape and fig flesh tissues[81,82]. Based on the quantitative proteome analysis, 161 and 186 tonoplast transport proteins with differential abundance were respectively identified in different development stage of grape and fig, including proton pumps, aquaporins, sugar transporters, ATP-binding cassette transporters and ion transport proteins, which will be the important candidate functional proteins for fruit quality formation. Overall, the successful vacuole isolation and vacuolar proteome analysis should be an effective method to study vacuoles and vacuolar membrane proteins.
With the separation of vacuoles in different plants, radiolabeled and non-radiolabeled substrates can be used to effectively measure proton and membrane potential dependent transport[83]. Besides, the transport of sugar will cooperate with proton exchange, and the transport of acid depends on the voltage difference generated by proton pumps, both of which will generate weak current. Therefore, the transport activity can be directly measured by using electrophysiological related means, such as two-electrode voltage clamp (TEVC) and patch clamp technology. Patch clamp technology uses microelectrodes to obtain electronic information from a small film and measure the size of ionic current passing through the film. It can be used to analyze the ion channels on the membrane, so as to study the transport mechanism of proteins on the vacuolar membrane. Electrophysiology was first applied to the model plant Arabidopsis thaliana in plants. AtALMT6, a member of the aluminum-activated malate transporter family was function as a malate influx or efflux channel through patch-camp experiments[48]. AtINT1 is a tonoplast-localized H+/inositol symporter that mediates the efflux of inositol, which is generated during the degradation of inositol-containing compounds in the vacuolar lumen[43]. AtSUC4 resulted in proton/sucrose activity, TMT1/2 represents a proton-coupled antiporter capable of high-capacity loading of glucose and sucrose into the vacuole[36]. Subsequently, this method was widely used to study on vacuole membrane proteins in horticultural crops. Two tonoplast ALMT proteins in tomato was characterized by TEVC technique, which indicated that the SLALMT5 transported malate and inorganic anions such as nitrate and chloride, but not citrate[57]. In addition to the transport function, patch-clamp technology can reflect the strength of transport activity through the strength of current. BvTST2.1 in sugar beet taproots was reported to have the sucrose-specific transport ability, but not for the glucose through current value analysis under the patch clamp assay[40]. Vacuolar membrane protein VvALMT9 in grape was proved to have the function of transporting malic acid by patch-clamp technology via using isolated vacuole[56]. The malate transport activity of Ma1 in apple was proofed to depend on the C-terminal conserved domain through patch clamp and TEVC assays in vacuoles and Xenopus oocytes respectively[59]. In addition to the X. oocytes expression system, the researchers also used the human embryonic kidney cell-line 293T (HEK293T) to verify the transport function of ClTST2, which is a proton-coupled sugar antiporters in watermelon[37]. Overall, electrophysiological methods can be used to determine the substrate and dynamic of transporters and will provide more great support for the vacuole biology and tonoplast protein research.
Conclusions -
Taken together, eating fruit is like eating vacuole, as most of the metabolites and nutrients are transported and stored in the large central vacuole of ripening fruit cells. The tonoplast-localized transporters and pumps are contributed to the accumulation of metabolites and influence the fruit flavor quality and yield. Except for the above reported transporters and proton pumps, only a few vacuole membrane protein members and their functions have been verified in horticultural crops, and the study on molecular regulation mechanism is even less. With the deepening of research and the improvement of technology, the fruit vacuole and membrane transporters need to be fully explored, so as to better understand the molecular model of sugar, acid and other compounds’ accumulation in horticultural crops.
The authors acknowledge funding provided by the National Key Research and Development Program of China (2022YFF1003100) and the Huazhong Agricultural University (start-up funding to CL).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
Acknowledgments Conflict of interest Rights and permissions (2) Table(1) References(96) - About this article
Cite this articleLiu Z, Mao Z, Li M, Cai C, Wang Y, et al. 2023. Vacuole: a repository to control fruit flavor quality. Fruit Research 3:12 doi: 10.48130/FruRes-2023-0012 -












