-
When land plants first evolved on Earth in the early Paleozoic era (likely over 500 million years ago), they gained the capacity to grow, survive, and reproduce in comparatively drier environments than their algal ancestors, with initially little organic soil, limited nitrogenous nutrients, and not much protection against harsh UV-rich sunrays[1]. Early land plant relationships with fungal and bacterial endophytes helped plants to supplement their limited access to nitrogen from their substrate[2−7]. Plants interface with microbes in the soil through their roots, and plants become an integral part of the microbial community by using exudates to energize soil microbes and by absorbing nutrients from the microbial community[8]. Plants thus share in the nutritional bounty of the microbial community in the soil[8]. Early land plants like the fossil vascular plant Rhynia lacked true roots and instead used rhizoids to interface with the substrate[6]. Extant bryophytes (mosses, liverworts, and hornworts) lack roots and seeds, but possess non-photosynthetic filaments that have bacterial endophytes internally and extract nitrogen from them based on histochemical analyses (Fig. 1). Pteridophytes (ferns and relatives) have roots to host endophytic bacteria but lack seeds to readily pass microbes to offspring (ferns release spores, like bryophytes). Ferns in the sporophyte stage possess bacteria within root cells that have been shown to foster the rhizophagy cycle. The gametophytes of ferns produce filamentous rhizoids that contain clusters of bacteria internally and show production of ammonium around bacteria in plant filaments (Fig. 2).
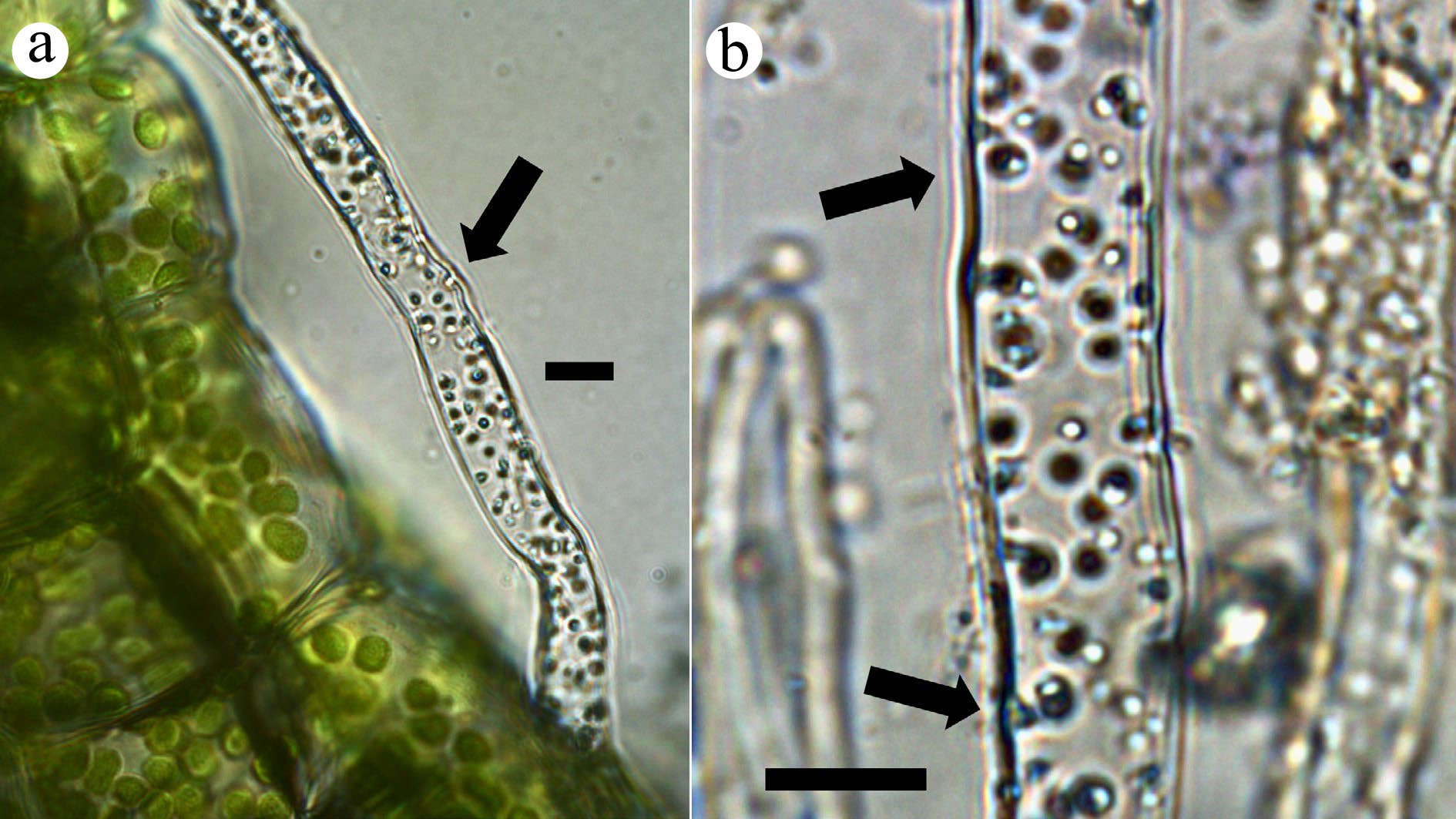
Figure 1.
Thallus surface and pegged rhizoid of a liverwort (Riccia fluitans). (a) Rhizoid stained with nitro blue tetrazolium (NBT) to visualize superoxide (blue color) around bacterial clusters within the filament (arrow; bar = 10 µ). (b) Rhizoid containing multiple clusters of bacteria, stained blue using NBT due to superoxide around bacteria (arrows; bar = 10 µ).
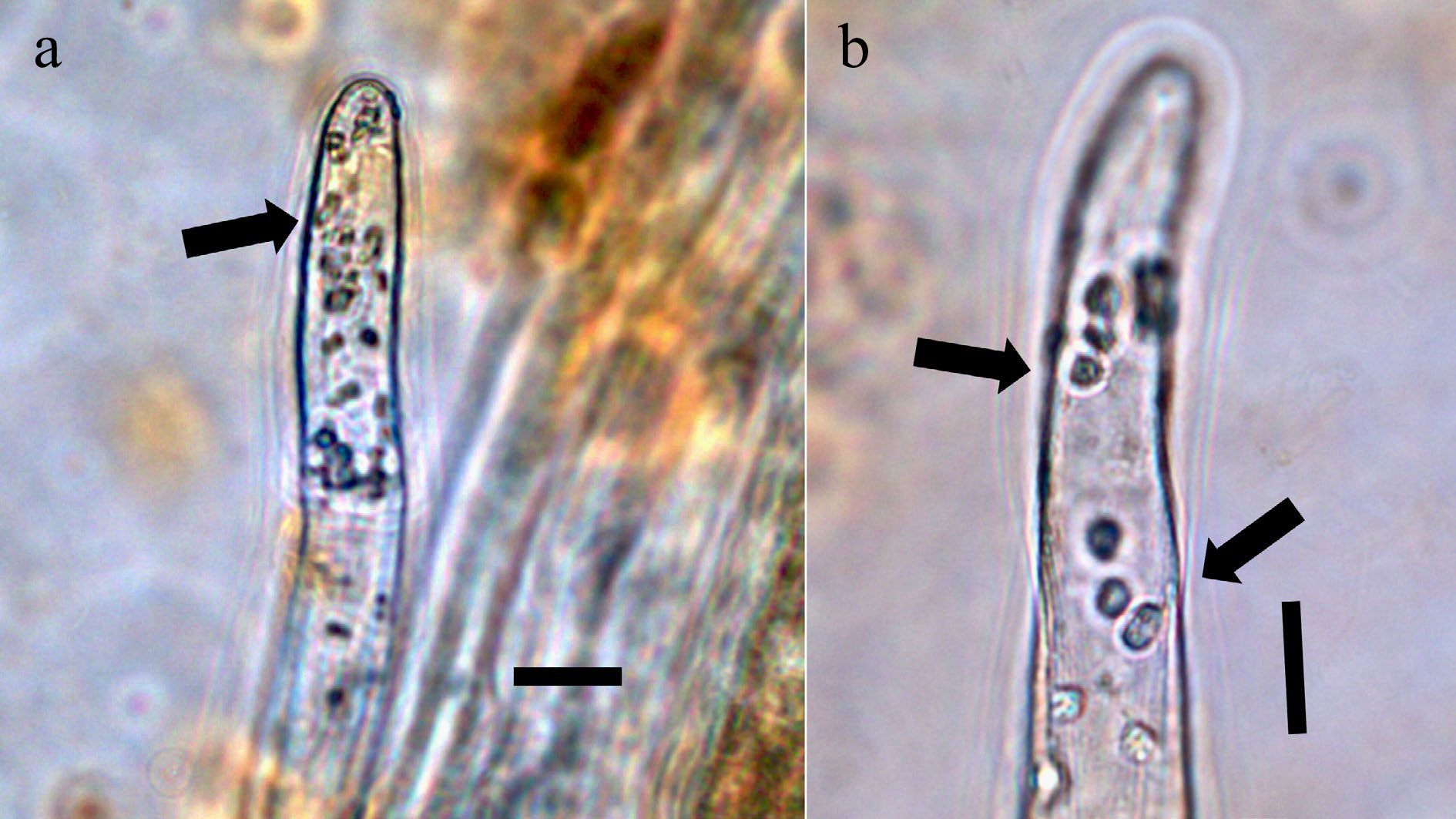
Figure 2.
Boston fern (Nephrolepis exaltata; Nephrolepidaceae) gametophyte rhizoids stained with a saturated solution of bromothymol blue to show ammonium (purple) around bacteria. (a) Rhizoid tip showing aggregation of bacteria in the tip (arrow; bar = 10 µ). (b) Rhizoid tip showing bacterial clusters in filament (arrow; bar = 10 µ).
-
The earliest land plants were small, poikilohydric, and non-vascular, most similar to modern bryophytes[2−7]. They lacked roots but instead had filaments such as rhizoids and other cells into which they likely absorbed and cultivated soil microbes similar to bryophytes[4−7]. In modern plants, and likely in early land plants, plant cells interact chemically with their internal bacteria to coax nitrogen and other nutrients from microbe cells[9]. This has been proposed to be a simple interaction whereby bacteria secrete ethylene that triggers plants to release sugars then used by the bacteria, while simultaneously, the plant cell produces superoxide (formed from oxygen in the air) and covers the bacterial cells with it[9,10]. Exposure to superoxide causes the bacteria to secrete reduced forms of nitrogen (from bacterial nitrogen fixation) as antioxidants to protect themselves from oxidative damage stemming from the superoxide exposure[9, 10]. This simple but likely ancient interaction with microbes has permitted plants to obtain nutrients within their own cells from bacteria, especially nitrogen. Most plants today engage in this symbiotic interaction with bacteria absorbed into their cells and tissues[8−16]. We refer to the microbes that were internalized into plant tissues and cells as endophytes [endo = internal; phyte = plant][8]. Endophytes are defined as mostly nonpathogenic microbes that inhabit plant tissues and often provide beneficial outcomes for the plant[8]. Plants in the wild absorb soil microbes to the extent that some of their cells may become filled with microbes[8−16]. Land plants are also associated with fungi in a symbiotic interaction called mycorrhizae[17]. Mycorrhizal fungi attach to roots and extend into the soil, improving nutrient absorption from soils[17]. Mycorrhizal fungi also internalize soil microbes in their hyphae[17]. Plants associate with and contain communities of microbes (bacteria and fungi) to such a large extent that they may be considered 'farmers of soil microbes'[8].
-
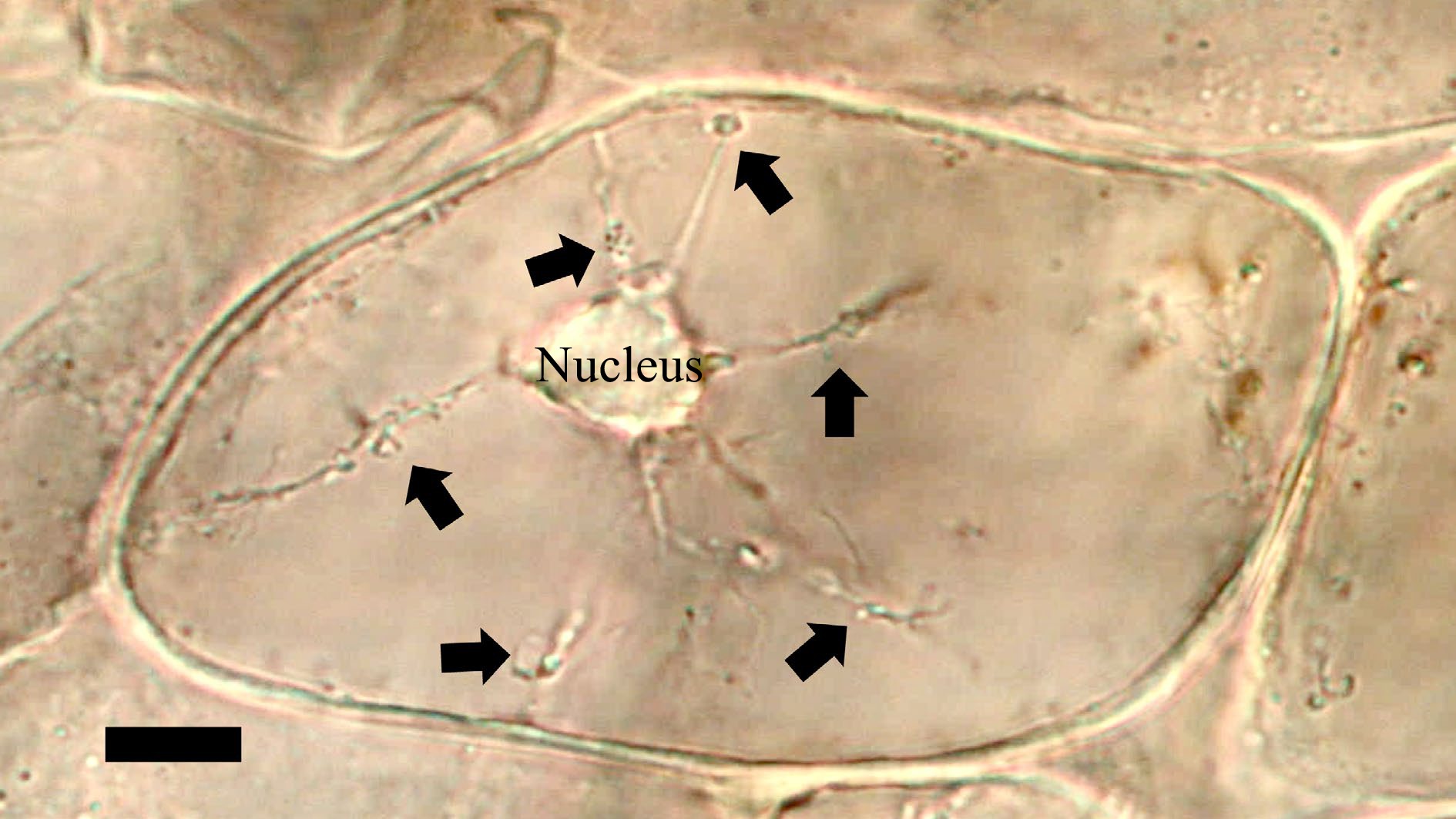
Modern plants have evolved sophisticated systems for attracting, internalizing, and managing soil microbes. They have developed structures, including root hairs and leaf hairs, to foster, host, and process soil microbes. One of the processes these plants use to acquire microbes from the soil is called the rhizophagy cycle [rhizo = root; phagy = eating] (see Fig. 3)[8−16]. In the rhizophagy cycle, plants attract soil microbes to root tips by secretion of root exudates[8]. These exudates are composed of sugars produced during photosynthesis, organic acids, and sometimes other nutrients that signal to microbes that nutrients are available at the plant root tips[8]. Microbes from the soil are then attracted to the root tips.
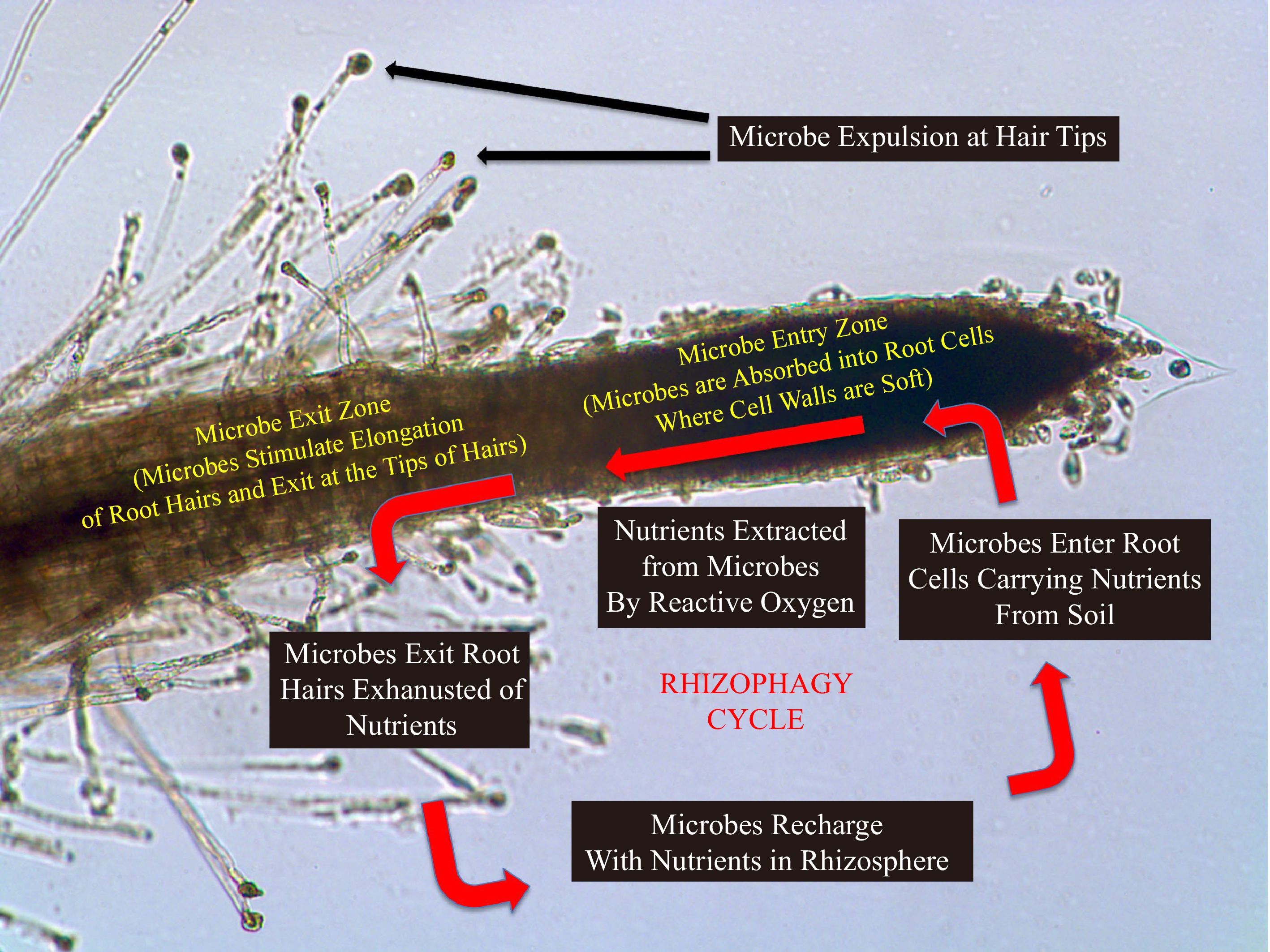
Figure 3.
Diagram showing the rhizophagy cycle, with a schematic of a root, with root cap to the right and developing root hairs to the left. In the rhizophagy cycle microbes alternate between a free-living phase in soil and an intracellular phase in root cells. The microbes obtain nutrients in the soil phase, and nutrients are extracted by the plant from microbes oxidatively in the endophytic phase inside the root. Microbes are expelled from root hairs at tips where they reform cell walls and reenter soil to acquire additional nutrients[12].
Scientists are still trying to decipher how plants are signaling soil microbes for absorption and influencing the environment in the soil to favor solubilization of soil nutrients[18]. These close interactions between plants and soil microbes are areas of active study by plant biologists and agronomists. It has been hypothesized that plants can obtain particular nutrients by absorbing microbes that specifically carry nutrients by changing their exudate composition[18]. For example, if a plant requires additional nitrogen in its tissues, it will secrete exudates that lack nitrogen but are rich in sugars[18,19]. Absence of nitrogen in plant root exudates then selects for soil bacteria that produce nitrogen through nitrogen fixation from the air[18]. Once the nitrogen fixing bacteria are absorbed, then their host plant may obtain nitrogen from these bacteria[8,9]. Plant cells produce superoxide in response to microbe-produced ethylene within the plant cell[9]. Superoxide is a powerful oxidant that oxidizes the cell walls off the microbe cells—resulting in formation of bacterial protoplasts without cell walls[8]. The microbe cell walls that are oxidized thereby provide nutrients directly to the plant within its cells[9−12]. Soil microbe cell walls contain many nutrients within them, such as metals, phosphorus, nitrogen and other compounds and elements[12]. The plant continues to subject the endophytic microbes, in this case as wall-less protoplasts, to superoxide, and some of these protoplasts eventually become fully degraded releasing all their nutrients[8−12]. Other bacteria will not be fully degraded; some bacteria are not capable of nitrogen fixation and some produce antioxidants that can counteract superoxide and similar oxidants[8,12].
-
One of the important functions of root hairs (in addition to uptake of water and solubilized nutrients) is the processing and extraction of nutrients from soil microbes—this includes stimulating nitrogen fixation in bacteria and coaxing them to secrete their nitrogen—or rather reduced forms of nitrogen that serve to neutralize plant-produced superoxide[9,10]. The function of root hairs is tied intimately to the microbiome of the plant and the soil. Bacteria that may produce and secrete nitrogen are hosted within the root hairs, and in tandem with hormones, this stimulates development of longer root hairs. These endophytic bacteria accumulate in the root hair initial, which appears as a bump on root epidermal cells[9]. The bacteria in the root hair initials have been found to secrete ethylene and nitric oxide[9]. Both of these compounds are growth hormones for plants that then result in a growth spurt resulting in the development of a long root hair[9].
During the growing of a root hair, bacterial protoplasts accumulated in the tips of the root hairs are ejected from the root tip through pores in the plant cell wall into the soil[12]. The plant also releases small amounts of exudate through the tip of the root hair to enable the bacteria that were recently ejected to reform their cell walls and their soil-capable mode of growth[8]. The remaining bacteria inside the root hairs reproduce by division or budding of the protoplast, and continue to fix nitrogen[9] (Fig. 4). Root hairs move endophytic bacteria around the periphery of hairs at a speed of approximately 9-11 micrometers/second in a process that is similar to cyclosis[8], although precisely how this movement happens is still a mystery. This movement of bacteria around the root hair stimulates replication of the microbes[9]. It is hypothesized that plants facilitate reproduction of microbes in root cells that help it acquire nutrients through the rhizophagy process[10]. A few bacteria enter a root cell at the root tip, but later many more cells of those bacteria are ejected back into the soil from the root hair tip[8]. Thus, the plant facilitates endophytic replication and increases the numbers of bacteria within its cells and in the soil for optimal nutrient uptake. Root hairs are one of the hypothesized sites in plants that accumulate microbes that sustain nitrogen fixation and transfer to plant cells.
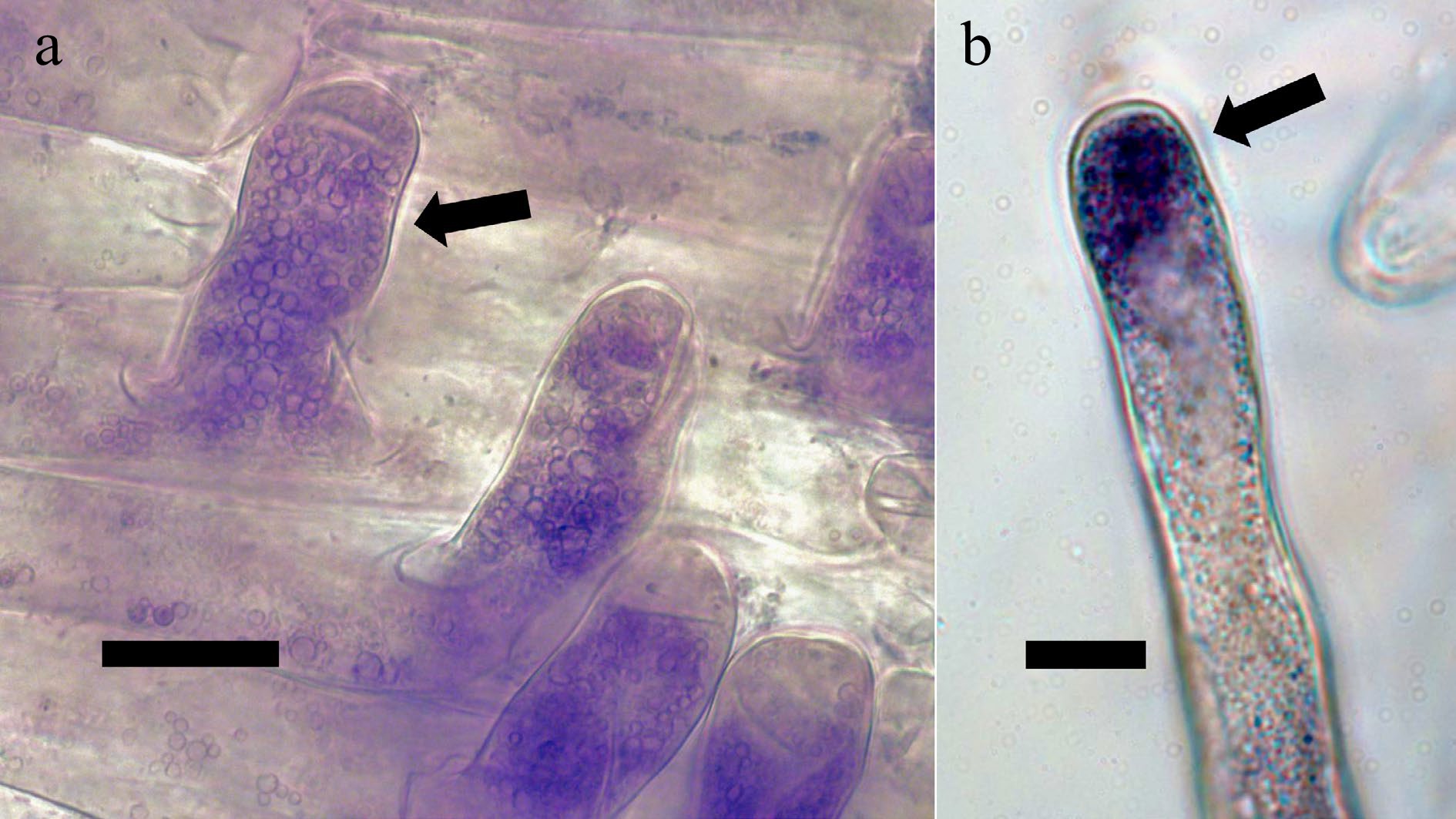
Figure 4.
Root hairs containing bacteria internally[9]. (a) Love-lies-bleeding (Amaranthus caudatus; Amaranthaceae) root hairs (arrow) stained with diaminobenzidine tetrahydrochloride to stain hydrogen peroxide (purple-brown color) around bacteria internally (bar = 10 µ). (b) Root hair of tall fescue (Festuca arundinacea; Poaceae) stained with nitro blue tetrazolium to visualize superoxide (blue color) around the bacteria in the tip of the hair (arrow; bar = 10 µ).
-
Above ground plant hairs also function to replicate microbes and foster plant growth.
Trichomes (epidermal hairs) are also symbiotic nitrogen fixing structures on plants that function like tiny root nodules[10]. Trichomes (Fig. 5) are commonly found on leaves but may also be present on stems (including rhizomes), flowers, fruits, and seeds. Most extant plants have some type of hairs on them, and their morphology varies considerably. The simplest hair forms are unbranched, filamentous (sometimes with pores along their sides) and often possess microbes inside them[10]. Microbes tend to accumulate at the tips of these hairs, similar to root hairs. Filamentous trichomes with pits along their sides are also quite common in plants[10]. Microbes appear in the pits in the pores in the trichome cell. In the pitted trichome the pores appear to somehow relate to the function of those trichomes. Microbes appear at the pits and there are multiple interactions between plant trichome cell and groups of microbes distributed beneath the lateral pits. Thus, a single trichome with lateral pits interacts with many clusters of bacteria, while non-pitted filamentous trichomes interact with a single cluster of bacteria at the hair tip. This suggests greater efficiency in extraction of nutrients from microbes inside pitted trichomes than in non-pitted trichomes where clusters of bacteria accumulate only inside the tips[10]. A third kind of trichome, the glandular trichome type, has been shown to be very efficient at absorbing nitrogen from the air. Glandular trichomes are present in tomato, hemp, hops, and many other plants. Their leaves and flowering structures bear glandular trichomes that are filled with various chemicals including antioxidants[10]. We speculate here that the antioxidants scavenge oxygen around bacteria to increase the efficiency of nitrogen fixation by bacteria inside the glandular trichome cells. Scavenging oxygen around the bacteria is needed because oxygen inhibits nitrogen fixation in bacteria[19].
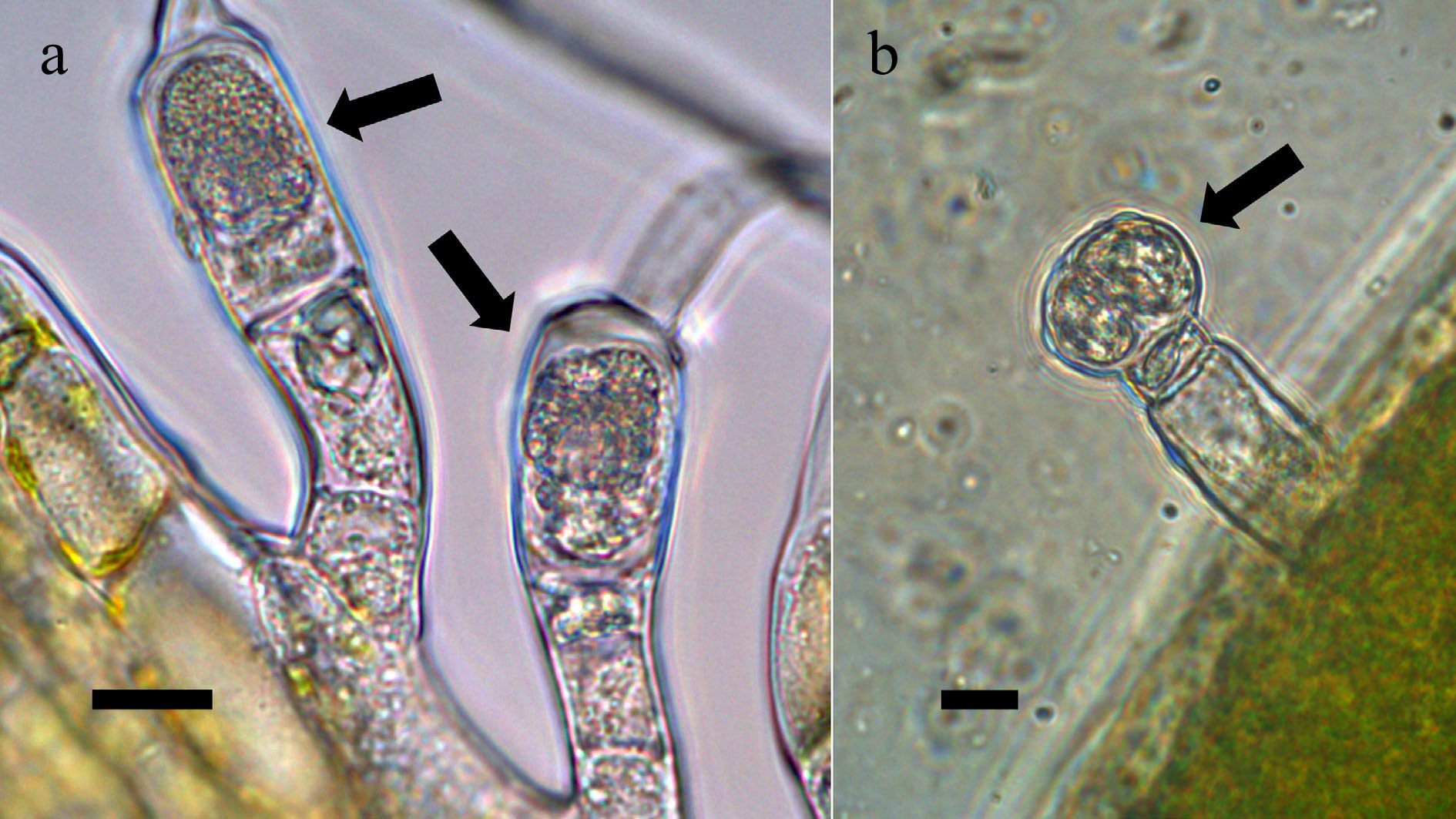
Figure 5.
Trichomes on seed plants stained using acidified diphenylamine stain to visualize nitrate (purple; arrows) around clusters of bacteria in hairs[10]. (a) Two trichomes (at arrows) on petals of Canadian goldenrod (Solidago canadensis; Asteraceae) (bar = 10 µ). (b) Trichome on young leaf of common mullein (Verbascum thapsus; Scrophulariaceae) showing accumulation of nitrate around bacterial mass in head of trichome (bar = 10 µ).
-
It's widely known that legumes (Fabaceae) and some other plant families may form root nodules that contain nitrogen fixing bacteria. People frequently cultivate legumes to increase nitrogen content in soil. It is less known that plants in many families may produce structures in leaves that contain nitrogen fixing bacteria. These structures are called leaf nodules[10] (Fig. 6). Our experiments with isotopic nitrogen showed that leaf nodules are efficient at acquiring nitrogen from the air[10]. Many plants that do not produce root nodules but possess endophytes in leaf nodules, hairs or other structures, are known to be effective at increasing nitrogen content in their tissues and grow under nutrient-poor soil conditions[10, 20, 21]. Leaf nodules are likely therefore another way to obtain the nutrients that plants require[10].
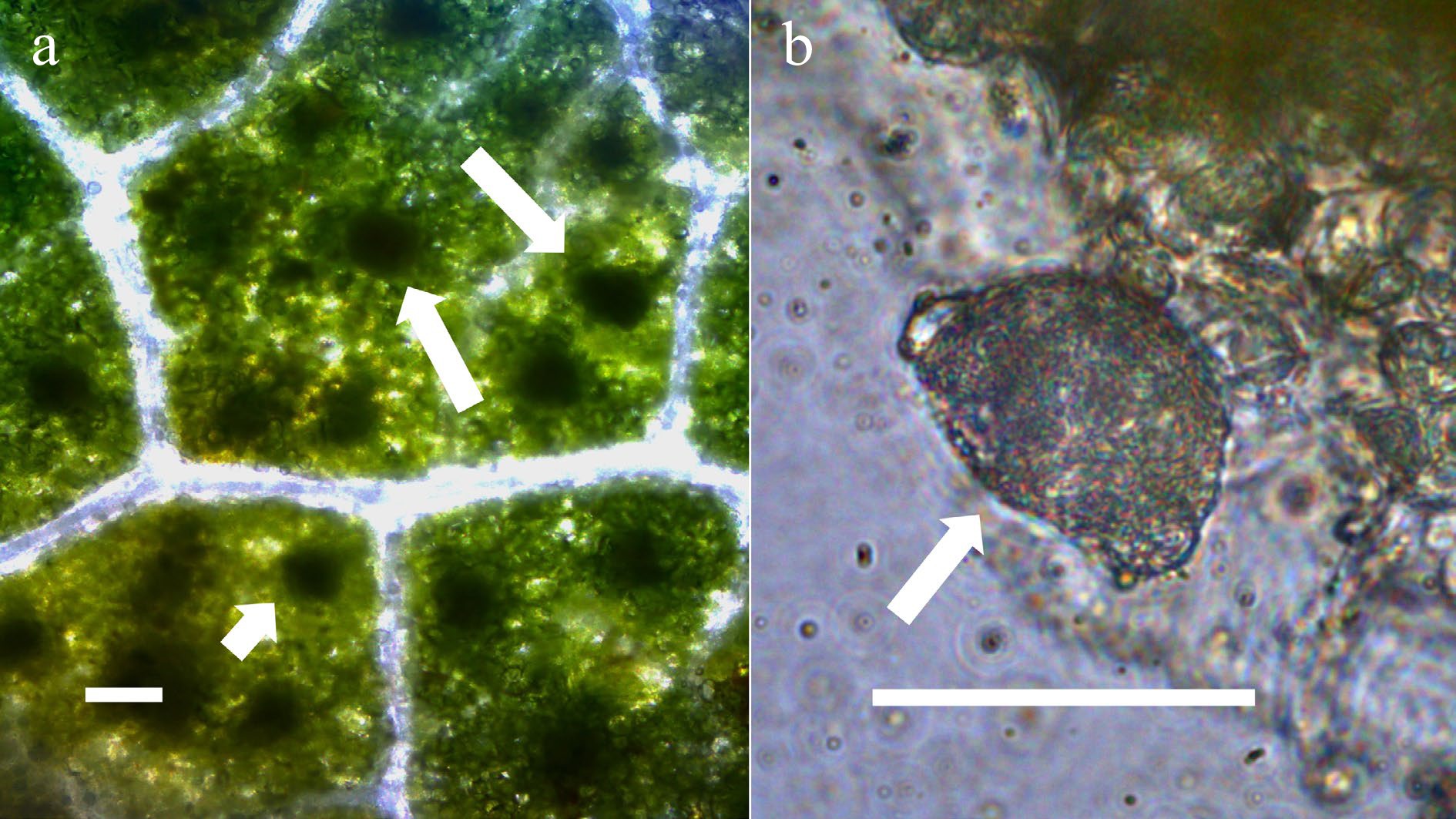
Figure 6.
Leaves of portia tree (Thespesia populnea; Malvaceae) with bacterial leaf nodules[10]. (a) Leaf stained with acidified diphenylamine to show nitrate (blue color) in leaves around bacterial masses (arrows point to leaf nodules) (bar = 50 µm). (b) Arrow points to mass of bacteria from a leaf nodule stained with acidified diphenylamine to show nitrate (blue color) around bacteria (bar = 50 µm).
-
In non-photosynthetic cells of leaves there is a different kind of symbiosis, called nuclear envelope symbiosis, that appears to result in nitrogen formation and nitrogen transfer to the developing plant cell (Figs 7 & 8). This is when bacteria colonize into the nuclear envelope and replicate there[10, 22, 23]. Generally, microbes entering the nuclear envelope produce proteins called nucleomodulins that affect plant gene expression[22,23]. Nuclear colonization could therefore play a role in control of cell development, however, very little is understood about the nuclear envelope colonization phenomenon. A previous study suggested that the nitrogen fixation phase may be happening in the nuclear envelope when the bacterial cells are replicating using plant cell provided sugars[10]. As the plant cell develops, the bacteria emerge from the nuclear envelope into the cytoplasm where they are exposed to superoxide produced by the plant, which results in nitrogen being released from the bacteria[10]. These nuclear envelope symbioses are often located in epidermal cells of meristems of roots, stems and leaves where chloroplasts are lacking. We hypothesize that the bacteria enter, or may already be present, in meristematic cells during chromosomal replication while the nuclear envelope is not present, then after cell division, they become trapped between the two membranes of the nuclear envelope[10]. The bacteria replicate in the nuclear envelope then escape from the nuclear envelope, perhaps through pores in the outer membrane of the nuclear envelope, and then move through the cytoplasm to the periplasmic space just underneath the cell wall where nitrogen extraction continues[10]. Use of copper sulfate to visualize reducing sugars suggests that the plant cell supplies sugars to the bacteria within the nuclear envelope[10]. Nuclear envelope colonization is a means whereby bacteria may be readily transmitted reliably from parent cell to daughter cells in the plant meristem and can thereby colonize the entire plant if present early in development.
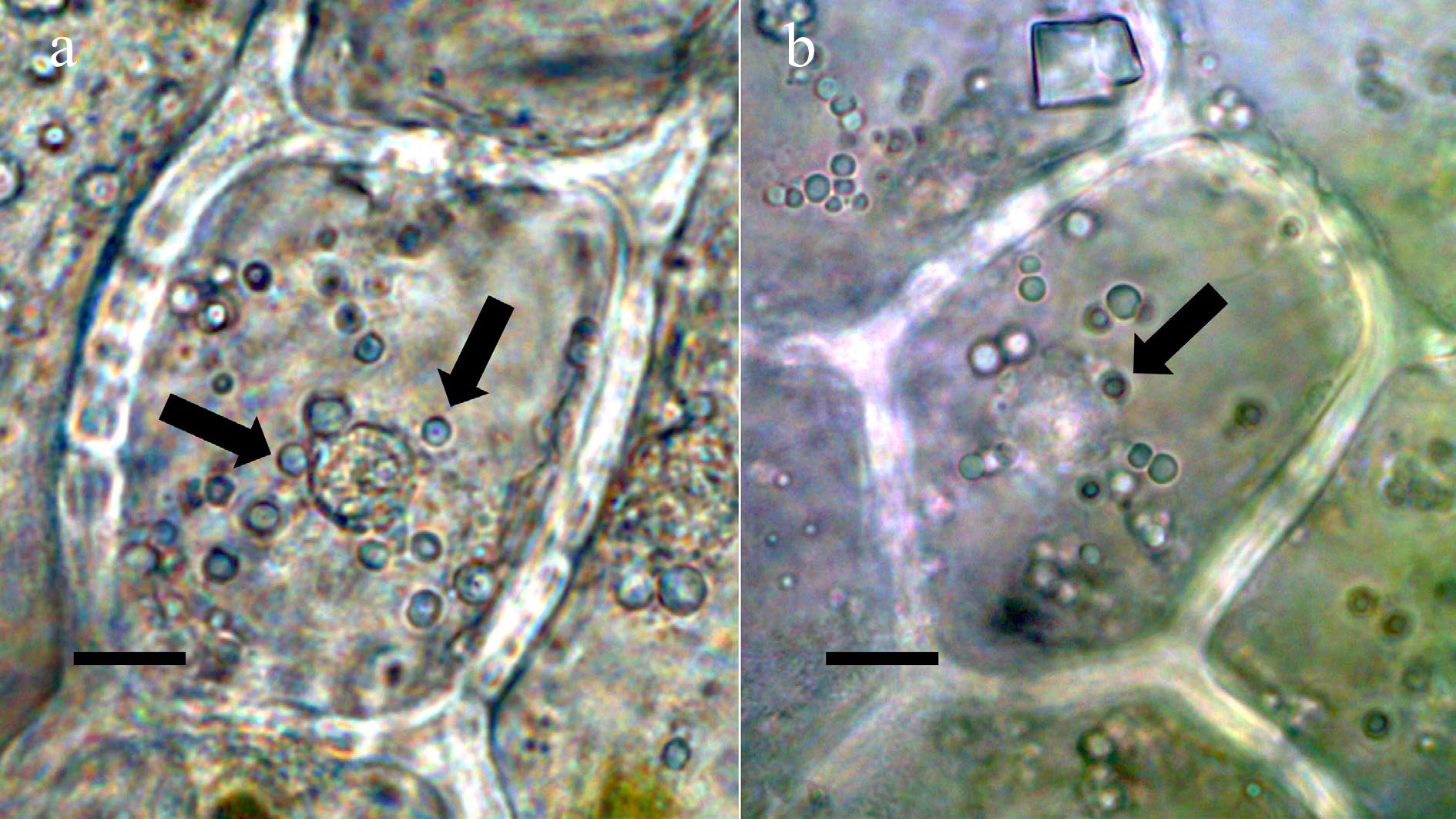
Figure 7.
Oblong-leaved vanilla (Vanilla phaeantha; Orchidaceae) showing bacteria within nucleus and emergence from nuclei[10]. (a) Nucleus stained with acidified diphenylamine to show nitrate (blue-purple color) (bar = 10 µm). (b) Nucleus stained with nitro blue tetrazolium to show superoxide (blue color) around bacteria (arrows) in cytoplasm (bar = 10 µm).
-
Plants often have multiple types of structures and processes in which they cultivate microbes and extract nutrients from them. We refer to the presence of multiple types of nutrient transfer symbioses in a plant as 'symbiosis stacking'[10]. For example, a plant may possess rhizophagy cycle activity in roots where plants acquire a broad set of soil nutrients through oxidative extraction in root cells just behind the apical meristem, nuclear envelope symbiosis in epidermal cells, and the same plant may also possess other nitrogen transfer symbiosis in trichomes and leaf nodules[10, 15, 24, 25]. Plants that engage in nitrogen transfer symbiosis stacking are more efficient in terms of nitrogen use; they require less added nitrogen than plants that do not have these symbioses. High nitrogen use efficiency in crops is indicative of crops that have greater access to soil microbes and have acquired or contain more endophytic microbes active in nitrogen fixation[10, 12, 13, 15, 24, 26, 27].
-
There has been a discussion extending back decades regarding declining food nutritional content that is thought to correlate with the advent of industrial (conventional) agriculture and abundant use of inorganic (synthetic) fertilizers[28−30]. This association of reduced food nutrient quality with the use of agrochemicals, particularly fertilizers, has been difficult to confirm. Wang et al.[29] showed that antioxidant capacity and flavonoid content of organically grown blueberries were notably higher than those of conventionally grown blueberries. Often experiments do not include sufficient control data sets or sample size to get statistically significant results on nutrient density. However, in experiments that we have conducted, we have found that many plants with microbial endophytes accumulate higher amounts of nutrients (particularly including, manganese, iron, magnesium, zinc, nitrogen, etc.) than plants without symbiotic microbes or with reduced amounts of microbes[8]. From a perspective of nutrient density, it is clear that more symbiotic microbes result in higher rhizophagy cycle activity, and more nutrients available to plants[8, 12]. In addition, the oxidative interactions between plant cells and endophytic microbes result in formation of more phenolics, carotenoids, and other kinds of antioxidant compounds that accumulate in plants[8, 24, 25, 31]. Thus, it is reasonable to expect that plants with greater symbiosis with microbes have greater accumulation of nutrients than plants without microbes or with reduced amounts of microbes.
-
Seed plants transport symbiotic microbes from soils throughout the plant and onto seed surfaces and into seeds[32−35]. Microbes are moved into the developing ovary of a plant and the fertilized ovule inside the ovary develops into a seed[33, 34]. The microbes are often located within the interior of seeds in the nucellus tissues and perhaps within the embryo itself[32, 33]. Plants also carry microbes on the surfaces of seeds or their associated tissues, like dry leaf or husk material[33, 35]. It is thought that some of the microbes that a plant has acquired are located in the seeds, and when those seeds germinate, they already have a small community of microbes nearby to use for rhizophagy cycle activity, nitrogen fixation, and mineral solubilization in soils[8,32−35]. These 'early' microbes are extremely important for plant seedling health. Many experiments have shown that seedlings with microbes perform better than seedlings from seeds that have lost or have reduced numbers of symbiotic microbes[8, 25, 31]. A healthy seed microbiome requires time to develop on maturing seeds. To obtain a healthy seed microbiome, seeds should have sufficient time to mature in their natural setting[35]. In the wild, seeds may remain on the plant for weeks, fall to soil, or may be vectored by animals internally or in some other manner, while the microbiome community develops on and within the seeds[35]. Failure to permit such seed microbiome maturation results in seeds that may have fewer endophytic microbes, and are of reduced hardiness[35]. This period of development of the microbiome on seeds may be referred to as the 'microbiome ripening process'[8, 35]. Failure for microbiome ripening results in seeds that have incompletely developed microbiomes[35].
-
Healthy plant seeds come with their microbiomes, and this microbiome is part of the immune system of plants[8, 34−38]. Many of the microbes that vector on and within seeds and colonize plants also have the capacity to grow out away from the seedling or the mature plant, into the soil[8, 37]. Such symbiotic microbes may colonize potentially pathogenic fungi, for example Fusarium species, which may cause a damping off disease[37]. Instead, once the fungus is colonized by the bacteria the behavior of the fungus changes[37]. The fungus may show reduced virulence, be less destructive to plants, or be non-destructive, causing no disease at all[8, 37]. In some cases the fungus may then actually grow inside the plants as an endophyte[8]. When seeds have fully established endophytic microbiomes, they are at least partially resistant to fungal pathogen attacks[8, 37]. However, when the microbiome on seeds is absent, seedlings become vulnerable to attacks by many species of fungi[36].
Endophytic microbes also stimulate plants to upregulate their oxidative stress traits[8, 39−42]. Plants produce antioxidants and other stress-reducing traits that help them cope with reactive oxygen generated during stress periods[39, 40]. Through this process, plants become more resistant to oxidative stress and to superoxide and other oxidants that the plant is producing to manage microbes in the rhizophagy cycle or to coax nitrogen from microbes throughout the plant[8, 12, 13, 16]. This increased stress tolerance makes plants with endophytic microbes more resistant to many kinds of environmental stresses and diseases[40−42].
-
Most plants in nature have symbiotic nutrient acquisition systems that help them to acquire nutrients that they need to grow and reproduce. Plants in nature don't require our fertilizers or other agrochemicals[8−12]. The process of domestication and modern cultural practices negatively impact symbiotic systems within plants, and as a consequence crop plants have become reliant on chemical inputs that further degrade activities of plants with regard to endophytic microbes[16, 43−49]. Some of the modern practices that degrade symbiotic systems in crop plants are discussed below.
-
Many of our food crops have come through a process of domestication and cultivation that has resulted in loss of microbiomes from seeds or fruits[43−46]. An example here is corn. The wild corn ancestor teosinte possessed a thick and hard husk (outer layer)[44]. The microbiome of teosinte is in part carried on the husk of the kernels[44]. In the development of modern corn, one of the changes that happened was that the husk became soft and thin on the kernels[44]. This means that the natural way of carrying some of the microbes to the next generation changed. The indigenous Americans discovered ways to cultivate corn while promoting endophytic microbes[14]. The Iroquois used what they referred to as 'corn medicine' to reestablish the microbiome on depleted corn seedlings[47]. They would gather wild grasses, including grasses like Canada wild rye (Elymus canadensis), Virginia wild rye (Elymus virginiana), and common reed (Phragmites australis), then they took the wild grass roots and ground them up in water. In some cases they would heat up the water, which had the effect of reducing the amount of some microbes but triggered some spore-forming bacteria to increase in number since heat activates their endospores to germinate and grow[47]. They would take corn seeds from the previous harvest and place them in this water and let the seed germinate slightly so that the root emerged, thereby permitting colonization of the roots by bacteria from wild grasses[47]. The Iroquois farmers then planted the endophyte-colonized seedlings in their fields. These historical agricultural practices are instances of 'microbiome transference', moving microbes from wild plants to corn to improve the growth and health of their corn crops.
An experiment conducted a few years ago involved cultivating the annual coyote tobacco (Nicotiana attenuata, Solanaceae)[37]. Investigators gathered wild seeds of the species and brought it into cultivation, planting it each spring, and then harvesting seeds in the fall for storage in a cool dry place until the following spring. After approximately 7 years of continuous cultivation, investigators found that a wilt disease developed in the cultivated crop. They went back to the wild population and isolated microbes from the wild plants. They found that continuous cultivation resulted in loss of endophytic microbes from the plants. Reinfection of the endophytic microbes back into the cultivated plants from the wild population resulted in elimination of the wilt disease from the cultivated crop. Thus, simply the process of repeated cultivation may result in loss of components of the microbiome[37].
-
Tissue-culturing of plants has a negative impact on the plant microbiome and on any endophytes that engage in fixation and transfer of nitrogen to plants and in rhizophagy cycle activity[8, 16]. When plants are tissue-cultured, they are frequently treated with antibiotics to control bacteria or fungi. The plant tissues that form in these tissue cultures are often free of microbes or show reduced microbe populations[16], and therefore may lack a healthy microbiome.
Seed treatments with acids to remove surface tissues as in delinting in cotton, or with fungicides to protect plants from diseases such as damping off diseases, also negatively affects seed microbiomes. In cotton, seeds are often treated with powerful acids to digest away cotton fibers so that seeds flow better in mechanical field planters, however, the beneficial microbes are transmitted within and on the seed fibers[32, 41]. Loss of those microbes results in plants that are vulnerable to fungal diseases[36−38]. Repeated use of antifungals on crop seeds has the long-term effect of degrading the seed microbiome. Many plants contain fungal endophytes that are eliminated when antifungal agrichemicals are applied to seeds and seedlings. Thus, antifungal applications to the surfaces of seeds results in more damage to the seed microbiome. The preferred solution to the problem of reduced population numbers of beneficial microbes on seeds is to add such microbes to seeds. It seems counterintuitive that the solution to a fungal disease problem is to add microbes to seeds, however, that is what has been found to protect seeds and seedlings[37, 38].
Tillage that results in deep turning of soils may interfere with the soil microbiome, burying microbial communities deep in the soil column. Deep turning is sometimes carried out to kill insects and weeds that may accumulate in the surface layers of soils; however, it is in those surface inches that most of the microbes live and grow. Displacement of the microbial layer at lower layers may result in reductions in the soil microbiome. Under these conditions when plants begin to grow, their roots enter the soil, but because there are few microbes there, they extract fewer nutrients from that soil[8, 12].
Many wild plants utilize bacteria that fix nitrogen as one source of nitrogen[8−14, 20, 21]. Overuse of synthetic nitrogen fertilizer suppresses nitrogen fixation in endophytic and soil microbes[48, 49]. This result in plants not getting their nitrogen from symbiotic microbes even if such bacteria are present in plant tissues or the soil[49]. Humans commonly cultivate plants that thrive due to fertilizer inputs and therefore do not need to invest in endophytic systems, i.e., our commercial agriculture is largely based on plants that are dependent on synthetic fertilizer. There are many things we still do not know about endophyte-plant relationships. We do not know how much nitrogen prevents or reduces endophytic nutrient transfer. What is clear is that synthetic/commercial fertilizers negatively affect plant use of endophytic and soil microbes.
-
Plants have evolved with microbes since they conquered the terrestrial environment. In the harsh new terrestrial environment, plants relied on symbiotic microbes to get the nutrients they needed to survive. We still understand little about how plants manage their soil and internal microbiomes, but it is clear that plants generally internalize or absorb beneficial microbes into their cells and tissues. Wild plants grow by using their internal microbiomes as a source of nutrients. Many of the commercial agricultural practices currently in use have a large potential to disrupt the microbial endophytes of plants and thereby increase plants' dependency on agrochemicals, especially nitrogen fertilizers. We need to gain a better understanding of how these endophytic and symbiotic associations have evolved in plants, how they function, and how they are regulated. It is imperative to develop data on how we can manage and manipulate endophytic associations in crop plants to maximize their benefit to crops (resulting in better plant health and higher crop yields) and minimize agronomic practices that negatively impact the environment and plant health.
-
All authors contributed equally to research and writing of this review article.
-
All data will be provided upon request to the corresponding authors.
The authors are grateful for support from USDA Multi-state project 4147 (Protecting Plants from Diseases and Stress Using Endophytic Microbes) and USDA NIFA (Corn/Endophyte Partnerships for Organic Farmers).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Chang X, Young B, Vaccaro N, Strickland R, Goldstein W, et al. 2023. Endophyte symbiosis: evolutionary development, and impacts of plant agriculture. Grass Research 3:18 doi: 10.48130/GR-2023-0018
Endophyte symbiosis: evolutionary development, and impacts of plant agriculture
- Received: 27 June 2023
- Accepted: 17 August 2023
- Published online: 11 September 2023
Abstract: Land plants can absorb soil microbes (bacterial, fungal and algal) into their cells and tissues. Plant endophytes enhance plant growth, stimulate elongation of root hairs, increase branching of roots, allow plants access to more nutrients, and stimulate oxidative stress tolerance. In the rhizophagy cycle, microbes are absorbed from soil directly into plant root cells where nutrients are extracted oxidatively, which provides nutrients to support plant growth. Early land plants lacked true roots, but possessed non-photosynthetic filaments (e.g., caulonemata, rhizoids) that may have cultivated diazotrophic bacteria within their cells as a source of nitrogen, just as bryophyte and pteridophyte rhizoids do today. Extant land plant lineages, such as bryophytes, pteridophytes, gymnosperms, and flowering plants, often produce epidermal structures (e.g., trichomes, papillae, paraphyllia, scales) on their roots, leaves, stems, or thalli; these often contain symbiotic nitrogen-fixing bacteria. Little is understood about how plants interact with soil and plant microbiomes. In this article we present novel endophytic phenomena in diverse lineages of land plants (liverworts, ferns, monocots, and eudicots) and explain how such symbiotic systems might have evolved over hundreds of millions of years. Due to these endophytic and symbiotic systems, land plants have the capability to obtain nutrients from the environment. Cultivation practices used in commercial agriculture can detract from the innate capabilities of plants to use microbes as a source of nutrients and might be harmful to plant health.
-
Key words:
- Bacteria /
- Endophytes /
- Evolution /
- Nutrient use efficiency /
- Rhizophagy cycle /
- Stress tolerance.