-
Dear Editor, it has been understood that the expression rates of programmed death ligand 1 (PD-L1) in solid cancer is directly related to immunotherapy treatment in different platforms, different clones, and different cutoff values[1−3]. However, the need for scoring methods to correctly and accurately measure expression rates in the tumor microenvironment cannot be separated from the relationship between treatment and expression[1−3]. Currently, PD-L1 immunohistochemistry assays/algorithms, scoring methods, clones, and platforms have been approved by the United States Food and Drug Administration (FDA) in many tumors[1−4]. For these reasons, scoring methods should be correct and certain, facilitating the workflow for pathologists and preventing doubts about immunotherapy options.
Tumor-associated macrophages (TAM) with heterogeneous phenotypes can undergo polarization changes during tumor development and polarize from the tumor-fighting M1 phenotype to the tumor-depleted or inactive M2 phenotype[5−7].
In the study by Dong et al.[8], it was understood that TAMs increase the expression of PD-L1, an inhibitory molecule, in tumor cells and other immunosuppressive cells by secreting cytokines and metabolites. It is also known as the main cause of resistance to immune checkpoint inhibitors[9]. In the study by Arlauckas et al.[10], it was stated that TAMs negatively manage anti-PD-1/PD-L1 treatment through Fcg molecules. It was understood that they can directly express inhibitory molecules, PD-L1[5,9,11]. Apart from gastrointestinal tumors, it is known that macrophages in breast cancer have an M2 phenotype expressing many M2 genes such as CD163, MS4A6A, and TGFBI and also exhibit immunosuppressive results with the effect of genes known to promote tumor progression and angiogenesis such as PLAUR13 and IL-8[12].
The presence of TAMs has generally been associated with a poor prognosis in solid tumors[5,9,13]. The formation of this negative relationship has provided an opportunity for the development of targeted treatment methods. Although immunotherapy agents targeting molecules in the macrophage-tumor axis have toxic effects, it is known that they have been tried in different tumors. The use of combination therapeutic moleculesis another handicap, as it increases the patient's medical costs[9]. As in these treatments, in cases where an inhibitory molecule blockade is required in colon adenocarcinoma, one of the analyses is the use of scoring methods that have provided prognostic consensus on the cutoff values of activity on PD-L1. The Combined Positive Score (CPS) is one of these scoring methods and is an FDA-approved method that combines tumor and immune cells for gastrointestinal system tumors[3−5]. However, considering the tumor heterogeneity and the evolutionary change process of the tumor, it is understood that the units in the CPS score analysis (immune cells such as lymphocytes, macrophages) for each tumor still need to be analyzed individually due to the ongoing discussion of different platforms, different cutoff values and clones, and the detailed presentation of the functions of these units with new studies. This letter was written in the light of this information to draw attention to the discussion of the existence of a hypothesis with the results of our new study[5].
CPS is an approach based on cell counting, where the number of PD-L1 stained cells (tumor cells, immune cells) is divided by the total number of tumor cells and multiplied by 100[3,5]. At this point, I would like to state that a new scoring method, which was first published and shows the PD-L1 expression status in tumor-associated macrophages in the colon adenocarcinoma microenvironment, can be developed. In a recent study, a PD-L1 macrophage score (MS) for TAMs was established by examining the Dako 22C3 clone in 122 resected colon adenocarcinoma specimens, and the score rate and prognostic efficacy were analyzed[5]. The aim of the study was to provide a simple visual scoring method that could lead to treatments focused on TAMs in colon adenocarcinoma. TAMs are found in the tumor center, tumor gland lumen, cancer gland rupture periphery, and tumor-associated reactive stroma[5]. To determine the PD-L1 MS score, the stroma containing macrophages in the cancer gland and gland rupture microenvironment in colon adenocarcinoma was first determined in hematoxylin and eosin-stained samples. Specifically, the expression of pancytokeratin, CD68, CD163, and PD-L1 in similar tumor areas was examined at 20× and 40× high field power (HFP). PD-L1 MS was found out at 20× HFP by dividing the number of PD-L1 positive macrophages by the total clear seen tumor field and multiplying the ratio by 100 (PD-L1 macrophage score = Number of PD-L1 positive macrophages / Number of tumor cells in the tumor area × 100)[5]. Membranous, cytoplasmic and punctate staining of TAMs of any intensity was considered as PD-L1 positive staining (Fig. 1). PD-L1 expression intensity was determined as negative (0) if there was no staining, weak expression (1+) if it was much weaker than the positive control, moderate expression (2+) if there was light staining, and strong expression (3+) if there was an expression equivalent to or stronger than the positive control.
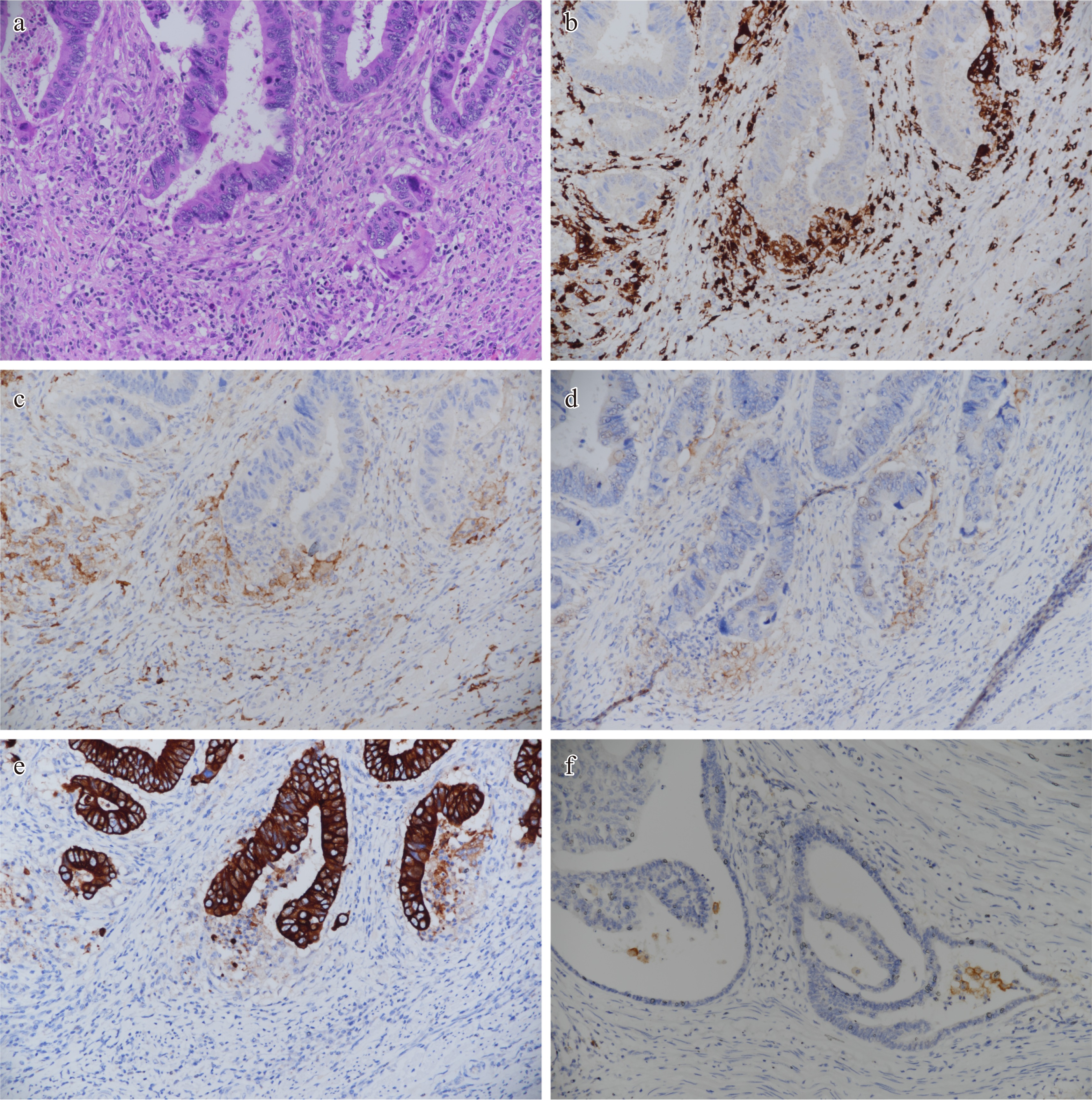
Figure 1.
Sample mapping of areas where PD-L1 macrophage score can be generated in the tumor gland microenvironment of colon adenocarcinoma. Immunohistochemical study findings in the same tumor microenvironment are shown. Similar findings can be replicated. (a) Especially tumor gland and gland rupture areas, HE 20×. (b) CD68 positive macrophages around the tumor gland, 20×. (c) CD163 positive macrophages in the tumor gland microenvironment, 20×. PD-L1 weak and moderately positive macrophages in the tumor gland microenvironment (PD-L1 macrophage score = PD-L1 positive macrophage number / Tumor cell number × 100). (d) The image that other immune cells are negative and it is possible to generate the PD-L1 macrophage score, 20×. (e) It is seen that cells expressing CD68, CD163 and PD-L1 in the same area do not stain with pancytokeratin, 20×. (f) Another distinct tumor area and PD-L1 positive macrophages around the tumor gland stained strongly positive, 20×.
PD-L1 MS score was statistically significant in tumors with ≥ 1% and the risk of death was four times higher in patients with PD-L1 expression rate ≥ 1% in TAMs[5]. In the last few decades, different scoring systems for immune checkpoint inhibitors have been rapidly implemented[1−3]. In the literature, we first analyzed the modified PD-L1 MS, a simple and visual-based method for scoring tumor cells and macrophages together. The introduction of another PD-L1 scoring method (PD-L1 MS) in a population confused about the gastrointestinal system may be perceived as a limitation.
It may not be possible to analyze the treatment results or prognostic effect in a hypothesis. What is important is that the foundations on which this hypothesis is based are on the agenda and are still open to further investigation. TAMs are also one of the CPS units. The recommendation of PD-L1 MS score in colon adenocarcinoma was considered and presented to take into consideration and not to overlook the fact that the macrophage tumor axis is a treatment target in addition to CPS in a colon adenocarcinoma. This new visual method may be a way to add appropriate adjuvant treatments to immunotherapies targeting the colon tumor cell-macrophage axis without compromising the accuracy of patient selection. The major drawback is that the original study[5] lacked a comparison with the existing assessment method, the CPS. Our primary expectation is that this new PD-L1 MS method will be considered, investigated, and standardized as a method in new studies.
HTML
-
As the study only involved the analysis of anonymized pathological specimens and did not require any direct patient intervention, no new informed consent was obtained. The use of these samples was in compliance with ethical guidelines, and patient confidentiality was strictly maintained.
-
The author confirms the solo responsibility to all aspects to this publication, i.e., study conception and design; data collection; analysis and interpretation of results; draft manuscript preparation and revision; resources, supervision and project administration.
-
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
-
I would like to express my gratitude to our patients, and to the Pathology department technicians who provided laboratory support during the course of this study.
-
The author declares that there is no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Baş Y. 2024. Is the "PD-L1 macrophage score" possible as an immunotherapy target in colon adenocarcinoma? Gastrointestinal Tumors 11: e003 doi: 10.48130/git-0024-0004 |