-
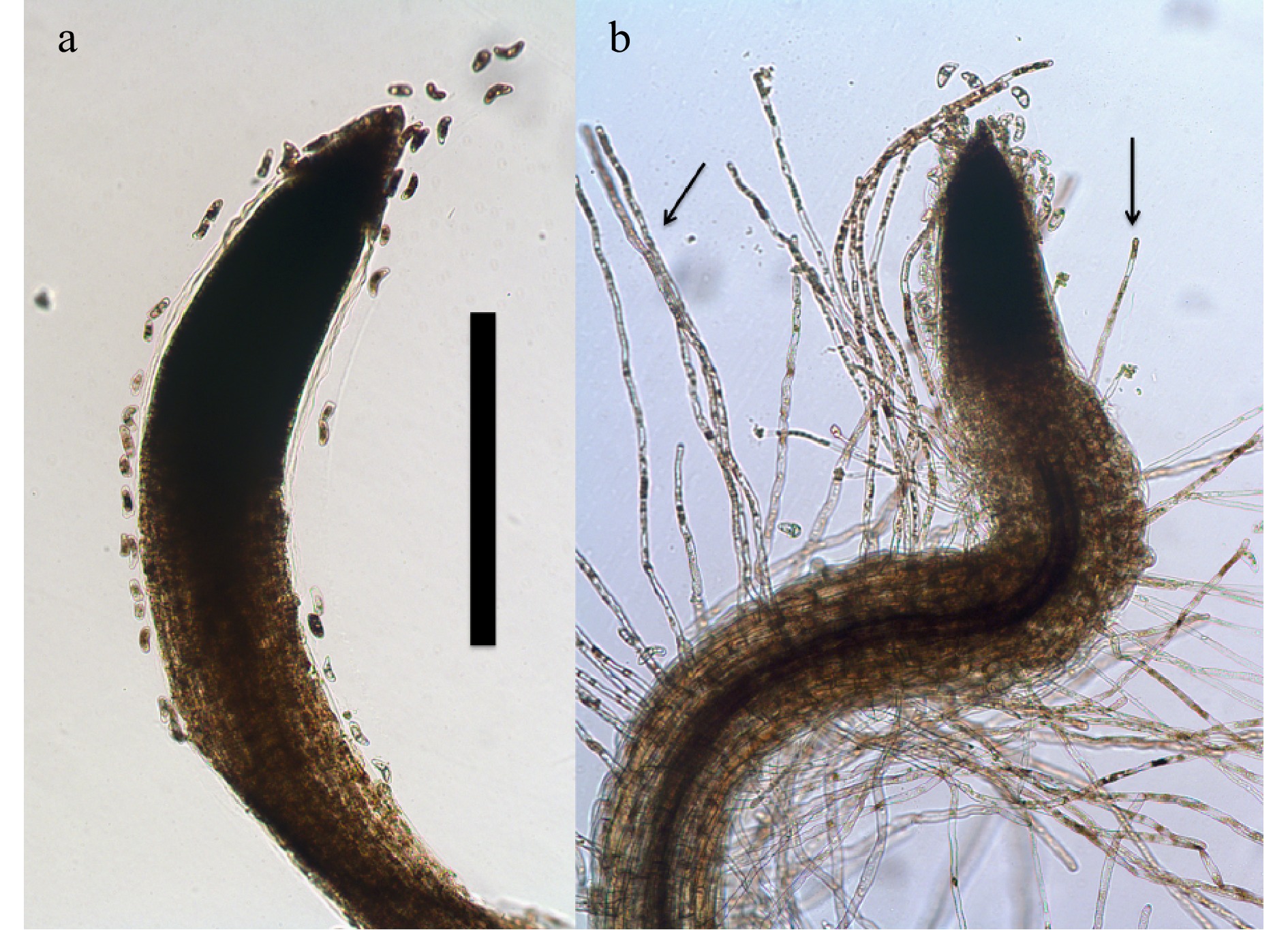
Figure 1. Root tips of 7-day-old Bermuda grass (Cynodon dactylon) seedlings growing on 0.7% agarose (stained with diaminobenzidine tetrahydrochloride; bar = 1 mm). (a) Root tip lacking root hairs due to absence of bacteria. (b) Root tip of seedling inoculated with a commercial product strain of Bacillus showing abundant presence of root hairs (arrows).
-

Figure 2. Root epidermal cells of wheat (Tritichum aestivum) seedling showing bacteria (arrows) within the root cell (stained with diaminobenzidine tetrahydrochloride; bar = 10 µm).
-
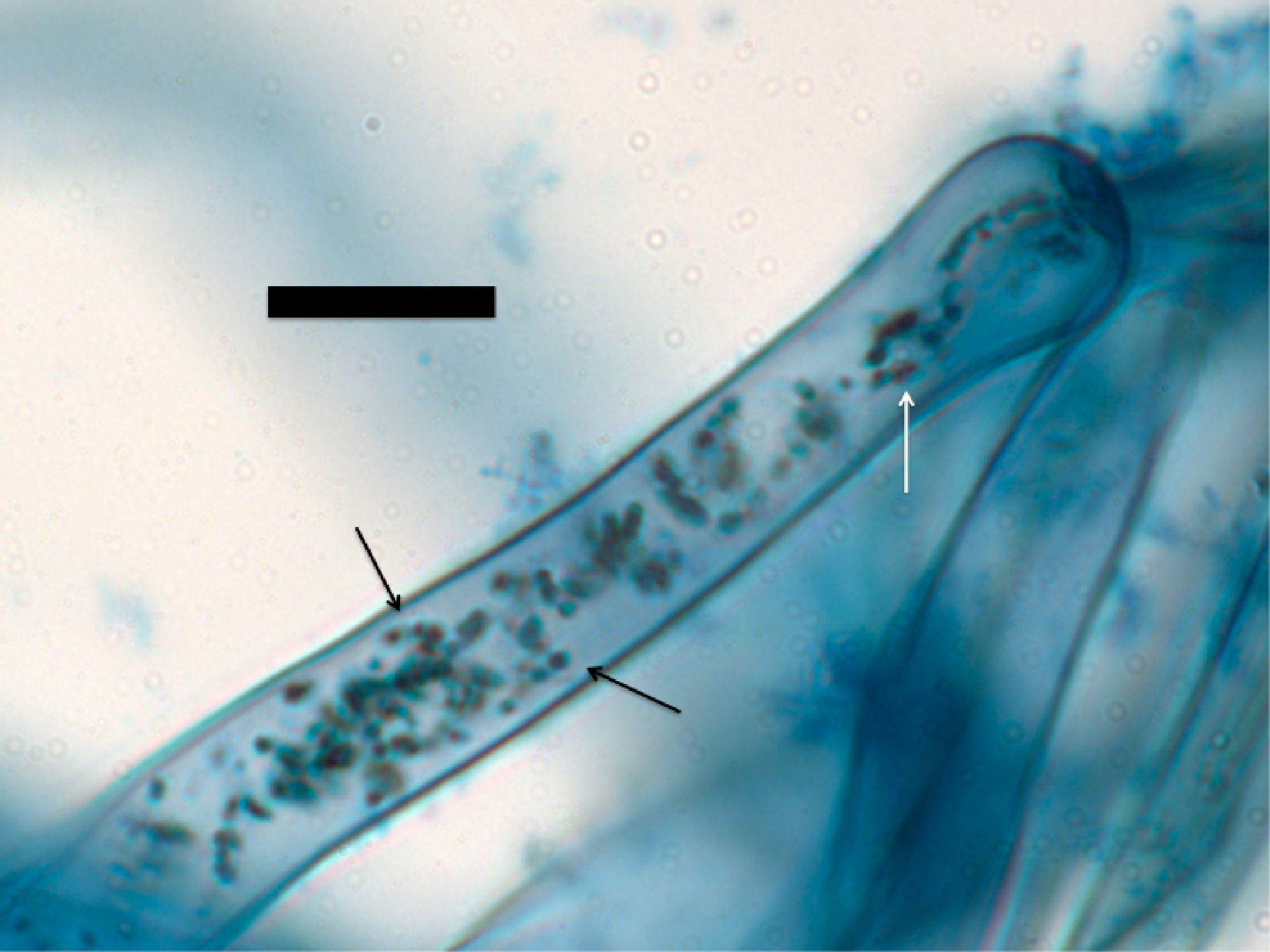
Figure 3. Root hair tip of Bermuda grass (Cynodon dactylon) seedling inoculated with commercial product strain of Bacillus sp. showing abundant presence of bacteria (arrows) within root hairs (stained with diaminobenzidine tetrahydrochloride; bar = 10 µm).
-
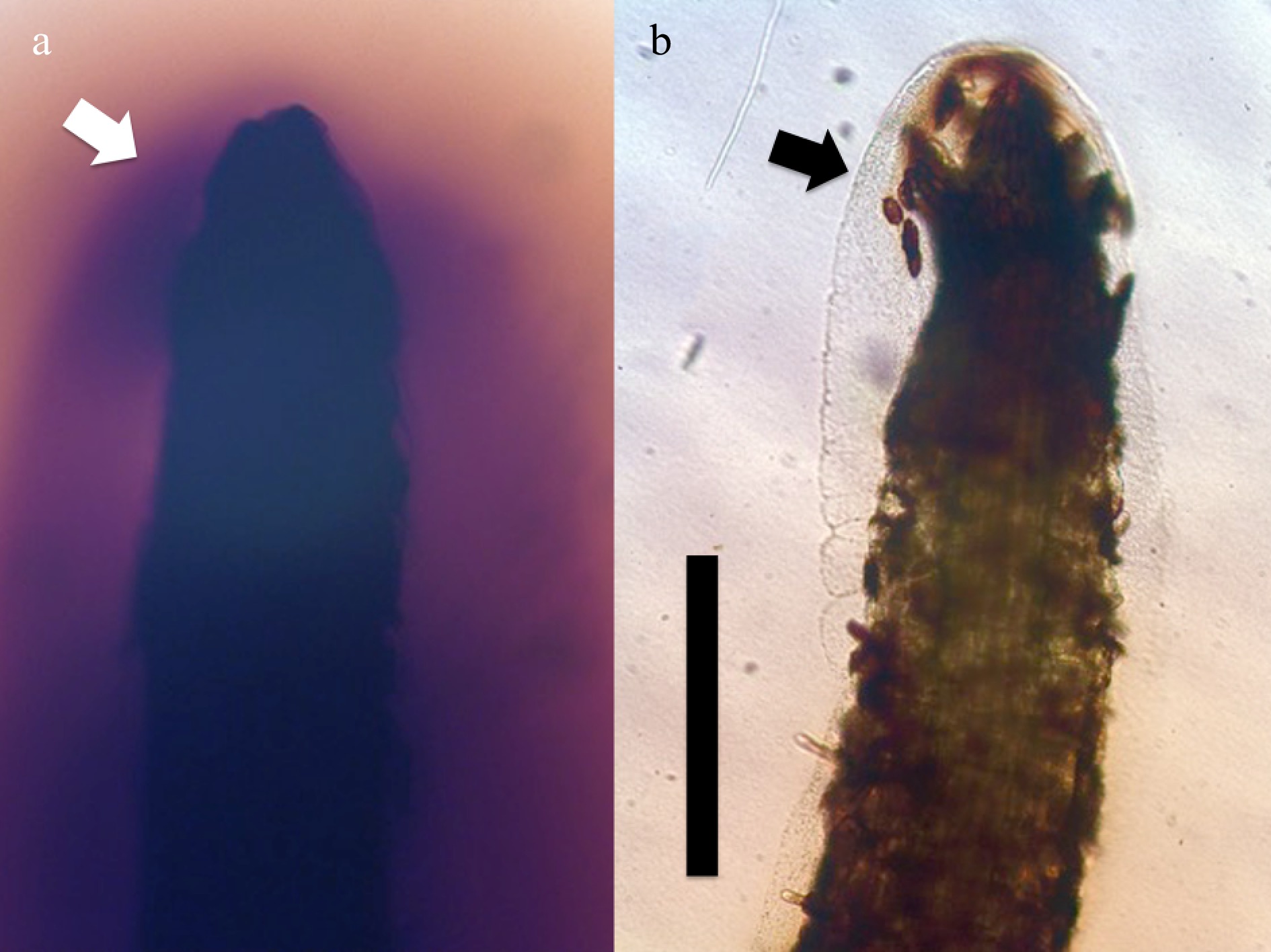
Figure 4. Root tips of grass Poa annua seedlings stained for ten hours at 20 oC with hydrogen peroxide stain diaminobenzidine tetrahydrochloride (bar = 1 mm). (a) Root tip (arrow) in agarose without humic substances, showing secretion of hydrogen peroxide (brown color) from root tip and absence of bacterial masses. (b) Root tip of Poa annua from the same experiment showing lack of reactive oxygen around root tip where humic substance (0.01% wt./vol.) is present in medium; bacteria (arrow) are also evident around the root tip.
-

Figure 5. Germination of Bermuda grass in 125 µM arsenate. (a) Control (no microbes). (b) Inoculated with Pantoea eucalypti MC-12. (c) Inoculated with Pantoea conspicua MC-K1. (d) Inoculated with the bacterial mixture isolated from the seeds and seedlings.
-
Humic acid concentration
(wt./vol.)Gravitropic response2 Root hair length (µm)3
(n = 18)Root length
(n = 12)0% 41% (n = 177) 197 ± 10.4 9 ± 1.3 mm 0.01% 88.5% (n = 113) 537 ± 50 19 ± 2.1 mm 0.1% 89.5% (n = 143) N/A 39 ± 3.1 mm 1 Humic acid (mined and powdered Leonardite from EarthWorks Health, LLC (Norfolk, Nebraska, USA)) was incorporated into 0.7% agarose media. Seeds were then germinated and grown on the agarose for 7 days in lab ambient conditions, then seedling parameters were assessed.
2 Gravitropic response % = number of seedling roots that grow down into agarose ÷ total number of seedling roots × 100.
3 Data are presented as mean ± SD.Table 1. Humic acid effects on grass seedling (Poa annua) root gravitropic response, root hair and root length growth1.
-
Strain Species As MIC2 Phosphate solubilization3 Auxin
(µg/mL)% Inhibition Alternaria sp. (LB/PDA4) MC-12 Pantoea eucalypti 200 mM ++ 18.96 ± 8.79 0/0 MC-10 Bacillus siamensis 1 mM + 1.1 ± 0.12 100/82.9 MC-13 Pantoea sp. 200 mM + 2.3 ± 1.56 0/0 MC-14 Acinetobacter radioresistens 200 mM − 0.83 ± 0.03 25/0 MC-15 Unidentified 200 mM + 4.97 ± 0.36 20/43 MC-19 Unidentified 200 mM + 2.45 ± 0.29 0/0 MC-K1 Pantoea conspicua 450 mM + 1.96 ± 0.07 40/0 Bacterial mix N/A 200 mM ? 4.46 ± 0.01 20/65 1 Methodology and bacterial identifications in Molina et al.[30].
2 As MIC = As minimal inhibition concentration.
3 Bacteria were screened for phosphate solubilization by a plate assay method using Pikovskaya agar media: ++ Halo greater than a centimeter in diameter; + Halo less than a centimeter in diameter; − No halo[30].
4 Percent inhibition of radial growth = R1−R2/R1 × 100 where R1 is the farthest radial distance grown by the pathogen in the opposite direction of the antagonist and R2 is the distance grown on a line between the inoculation of the pathogen and the antagonist on media Luria Bertani agar (LB) and potato dextrose agar (PDA)[30].Table 2. Plant growth promotional features of Jasione Montana bacteria1.
Figures
(5)
Tables
(2)