-
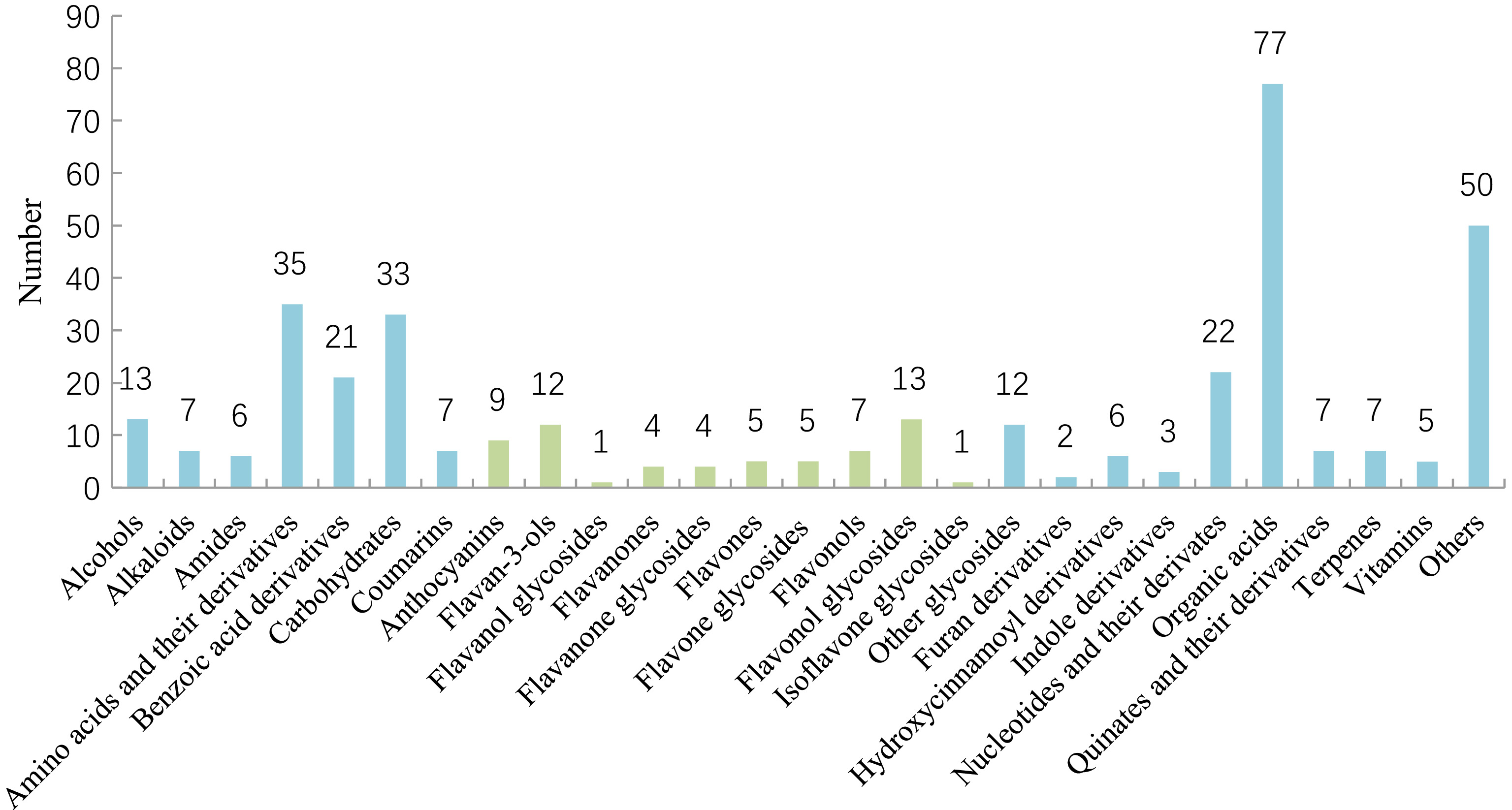
Figure 1.
Classification of metabolites for tea plant and its related species and varieties.
-
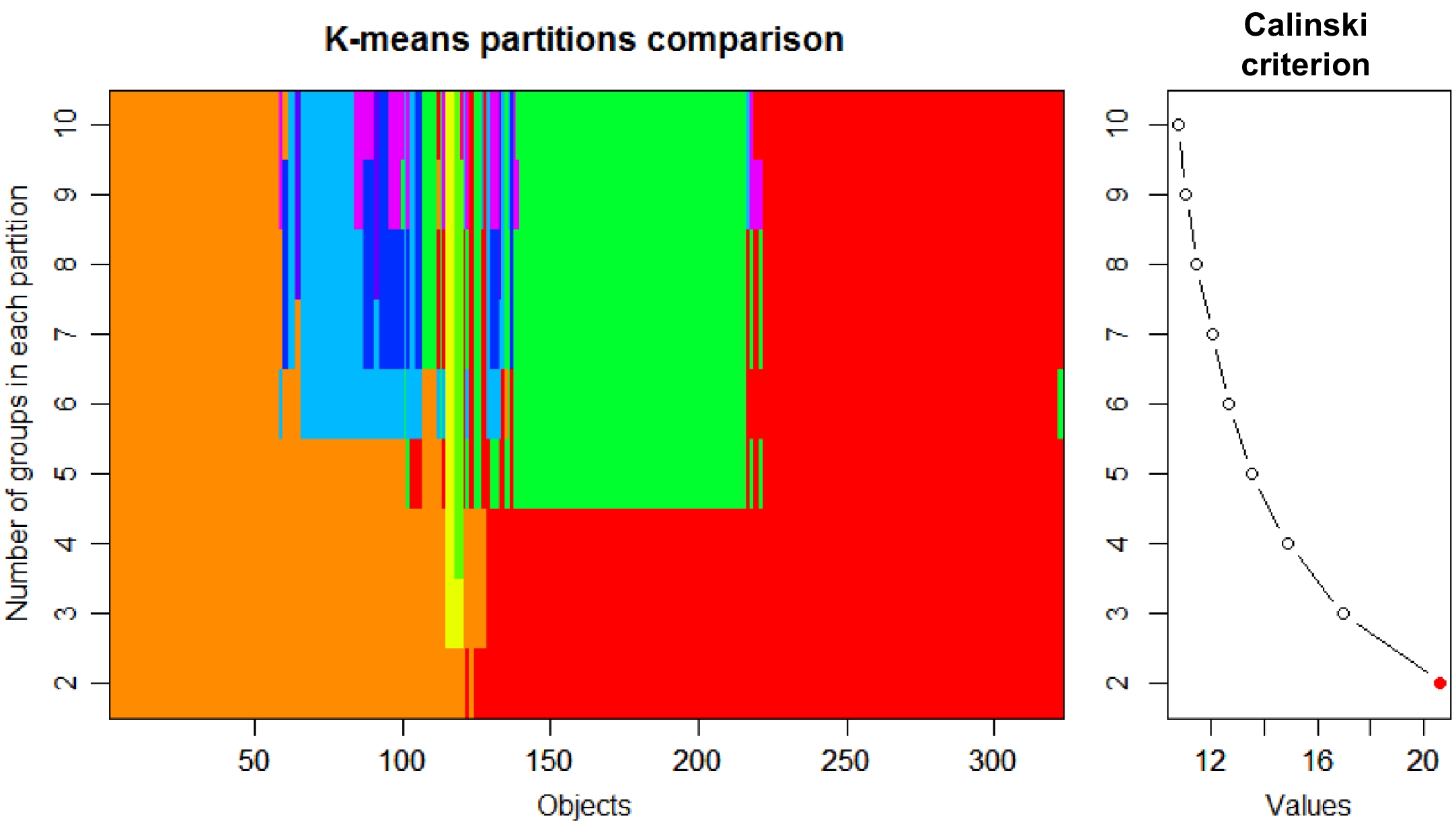
Figure 2.
Group number of tea plants determined based on Calinski criterion.
-
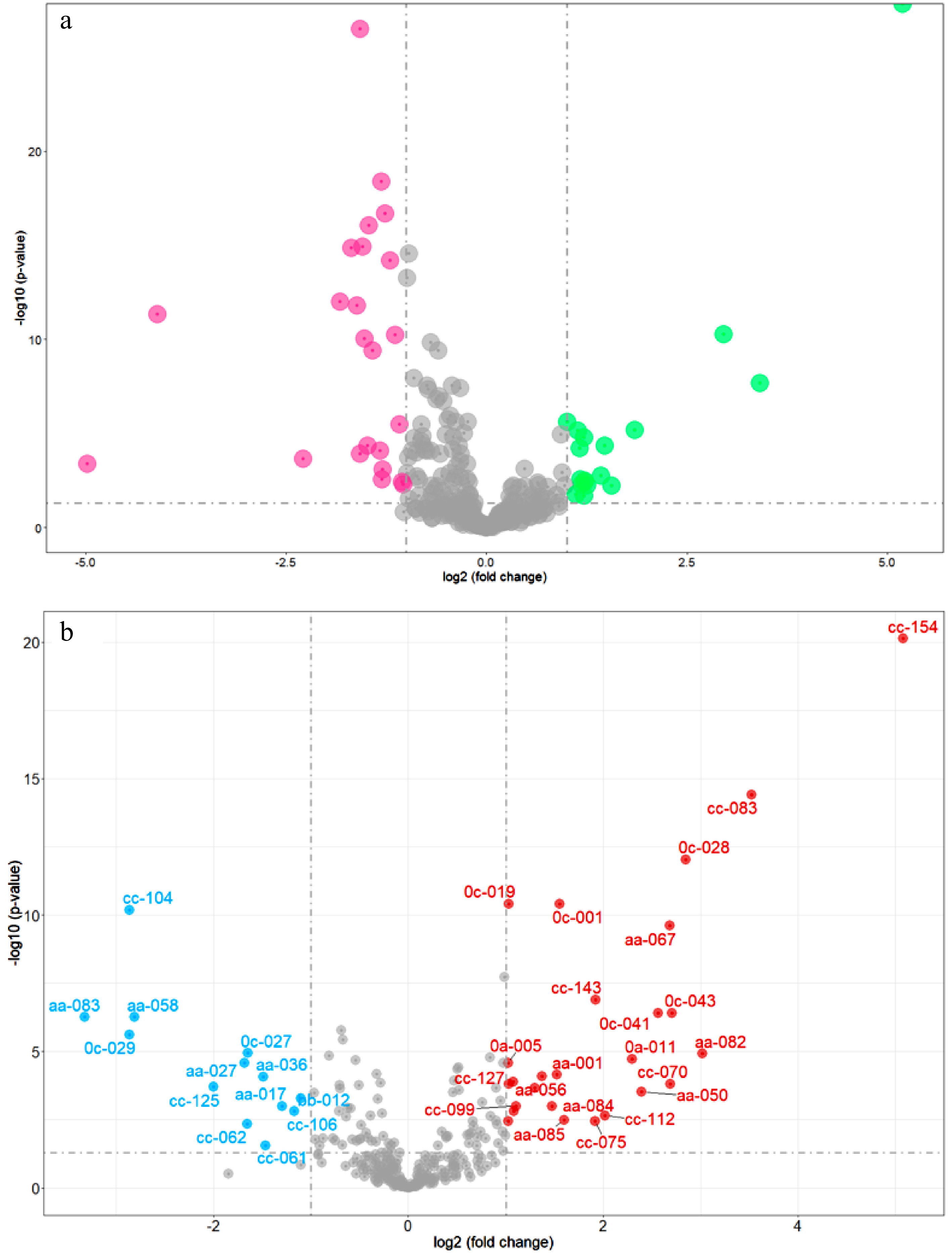
Figure 3.
Volcano plots of DAMs. (a) DAMs of natural population (red dots represent the content of DAMs that are higher in Group 2 compared to Group 1, green dots represent the content of DAMs that are higher in Group 1 compared to Group 2); (b) DAMs between Camellia sinensis and its relatives (red dots represent the content of DAMs that are higher in Camellia sinensis compared to relatives of Camellia sinensis, blue dots represent the content of DAMs that are higher in relatives of Camellia sinensis compared to Camellia sinensis).
-
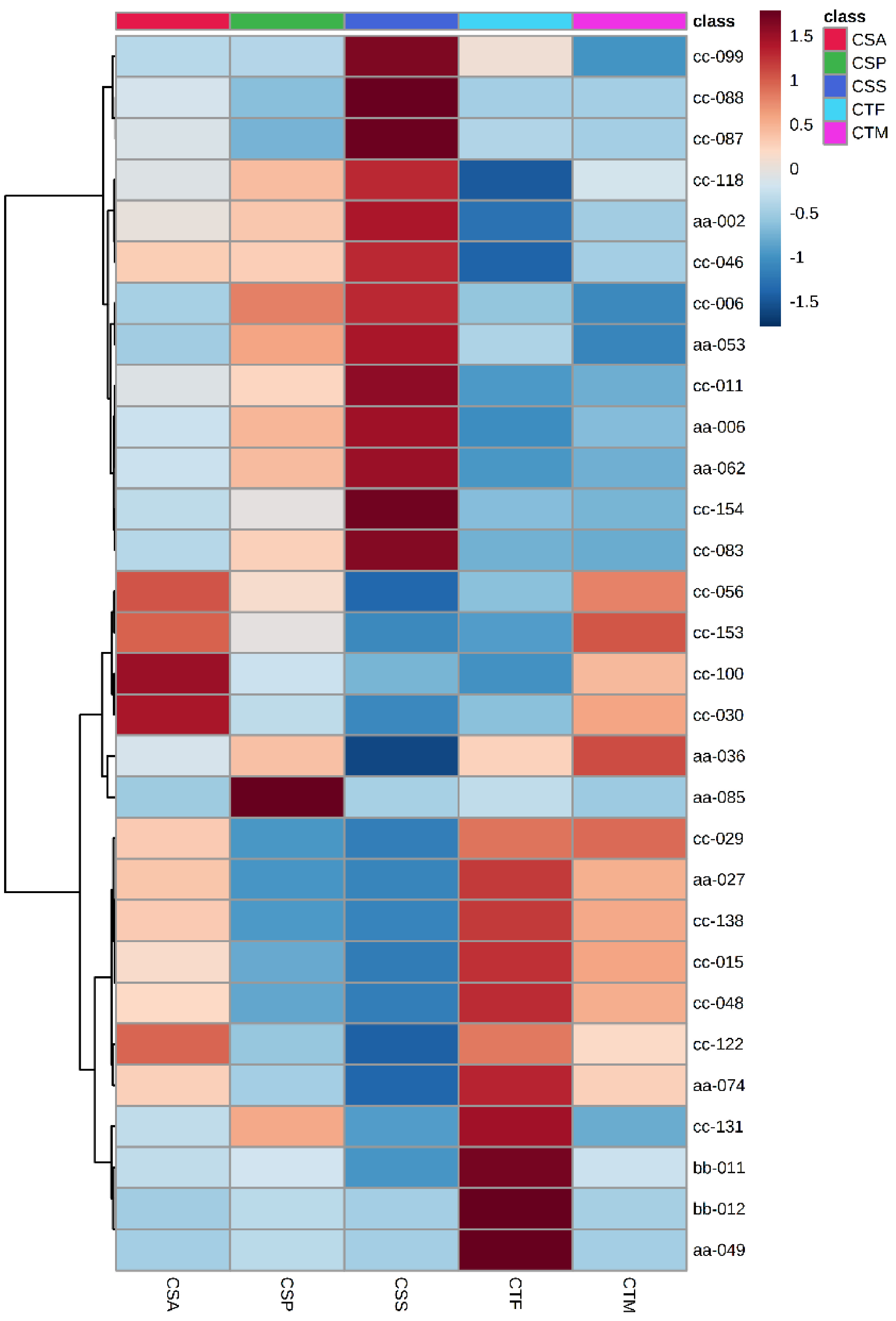
Figure 4.
Top 30 DAMs of five tea plant species and varieties.
-
Representative group* Metabolite Class Relative peak area CSA CSP CSS CTF CTM CSS L-Pyroglutamic acid Amino acids and their derivatives 5.E+07 7.E+07 8.E+07 4.E+07 4.E+07 CSS L-Serine Amino acids and their derivatives 1.E+06 1.E+06 2.E+06 1.E+06 1.E+06 CSS Sinapaldehyde glucoside Carbohydrates 3.E+05 2.E+05 4.E+05 2.E+05 2.E+05 CSS 7-Ethoxycoumarin Coumarins 1.E+05 8.E+04 2.E+05 1.E+05 8.E+04 CSS D-Quinic acid Quinates and their derivatives 3.E+06 3.E+06 4.E+06 2.E+06 2.E+06 CSA N-Acetyl-DL-tryptophan Amino acids and their derivatives 1.E+06 4.E+05 3.E+05 3.E+05 1.E+06 CSA Azelaic acid Organic acids 2.E+05 1.E+05 9.E+04 2.E+05 2.E+05 CTF 7-Hydroxycoumarine Coumarins 3.E+06 2.E+06 3.E+06 4.E+06 3.E+06 CTF diGC-GA Benzoic acid derivatives 2.E+07 2.E+07 1.E+07 3.E+07 2.E+07 CTF 2-Acetamido-2-deoxyglucose Carbohydrates 2.E+06 2.E+06 1.E+06 3.E+06 2.E+06 CTF Kaempferitrin Flavonol glycosides 2.E+04 5.E+05 2.E+04 6.E+06 3.E+04 CTF Nictoflorin Flavonol glycosides 2.E+05 7.E+05 2.E+05 7.E+06 2.E+05 CTF Traumatic acid Organic acids 5.E+05 3.E+05 3.E+05 6.E+05 5.E+05 CTF Neochlorogenic acid Quinates and their derivatives 7.E+06 2.E+06 2.E+06 1.E+07 9.E+06 CTM 5'-Xanthylic acid Quinates and their derivatives 5.E+05 3.E+05 3.E+05 5.E+05 6.E+05 CTM Chlorogenic acid Quinates and their derivatives 1.E+07 1.E+07 4.E+06 9.E+06 2.E+07 * CSS: C. sinensis var. sinensis (L.) O. Kuntze; CSA: C. sinensis var. assamica (Masters) Kitamura; CSP: C. sinensis var. pubilimba Chang; CTF: C. tachangensis F. C. Zhang; CTM: C. taliensis (W. W. Smith) Melchior. Table 1.
Signature metabolites of five tea plant species and varieties.
-
Name Classification Formula GC-GCG Anthocyanins C37 H30 O18 Procyanidin B1 Anthocyanins C30 H26 O12 Procyanidin B3 Anthocyanins C30 H26 O12 Procyanidin B4 Anthocyanins C30 H26 O12 Procyanidin C1 Anthocyanins C30 H26 O12 Tricatechins1 Anthocyanins C45 H38 O18 Tricatechins2 Anthocyanins C45 H38 O18 Tricatechins3 Anthocyanins C45 H38 O18 GC-diGA Benzoic acid derivatives C29 H22 O15 Eriocitrin Flavanol glycosides C27 H32 O15 Eriodictyol C-hexoside Flavanone glycosides C21 H22 O11 Naringin Flavanone glycosides C27 H32 O14 Naringin dihydrochalcone Flavanone glycosides C27 H34 O14 Prunin Flavanone glycosides C21 H22 O10 Baicalin Flavone glycosides C21 H18 O11 Cynaroside Flavone glycosides C21 H20 O11 Luteolin 7-O-(6-O-malonyl-β-D-glucoside) Flavone glycosides C24 H22 O14 Schaftoside Flavone glycosides C26 H28 O14 Astilbin Flavonol glycosides C21 H22 O11 Baimaside Flavonol glycosides C27 H30 O17 Isoquercetin Flavonol glycosides C21 H20 O12 Kaempferin Flavonol glycosides C21 H20 O10 Kaempferitrin Flavonol glycosides C27 H30 O14 Myricetin 3-O-galactoside Flavonol glycosides C21 H20 O13 Nictoflorin Flavonol glycosides C27 H30 O15 Quercetin 3-O-α-D-xylopyranoside Flavonol glycosides C20 H18 O11 Quercitrin Flavonol glycosides C21 H20 O11 Rutin Flavonol glycosides C27 H30 O16 Tiliroside Flavonol glycosides C30 H26 O13 Vitexin Flavonol glycosides C21 H20 O10 Vitexin-2''-O-rhamnoside Flavonol glycosides C27 H30 O14 Malonylgenistin Isoflavone glycosides C24 H22 O13 Table 2.
Polymers of flavonoids.
Figures
(4)
Tables
(2)