-

Figure 1.
Xinhui Chenpi production process flow chart.
-
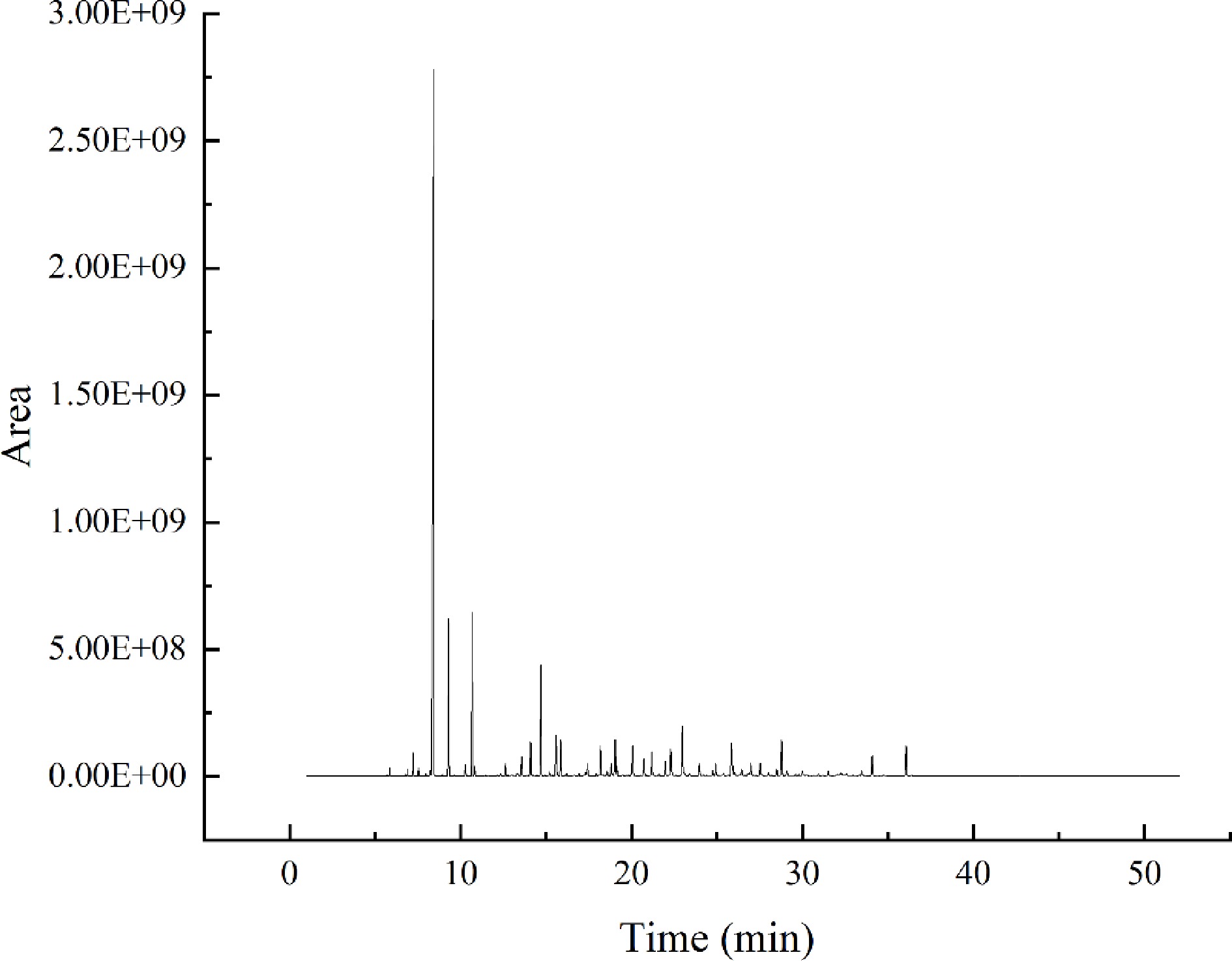
Figure 2.
Total ion chromatogram (TIC) of volatile compounds of Chenpi.
-
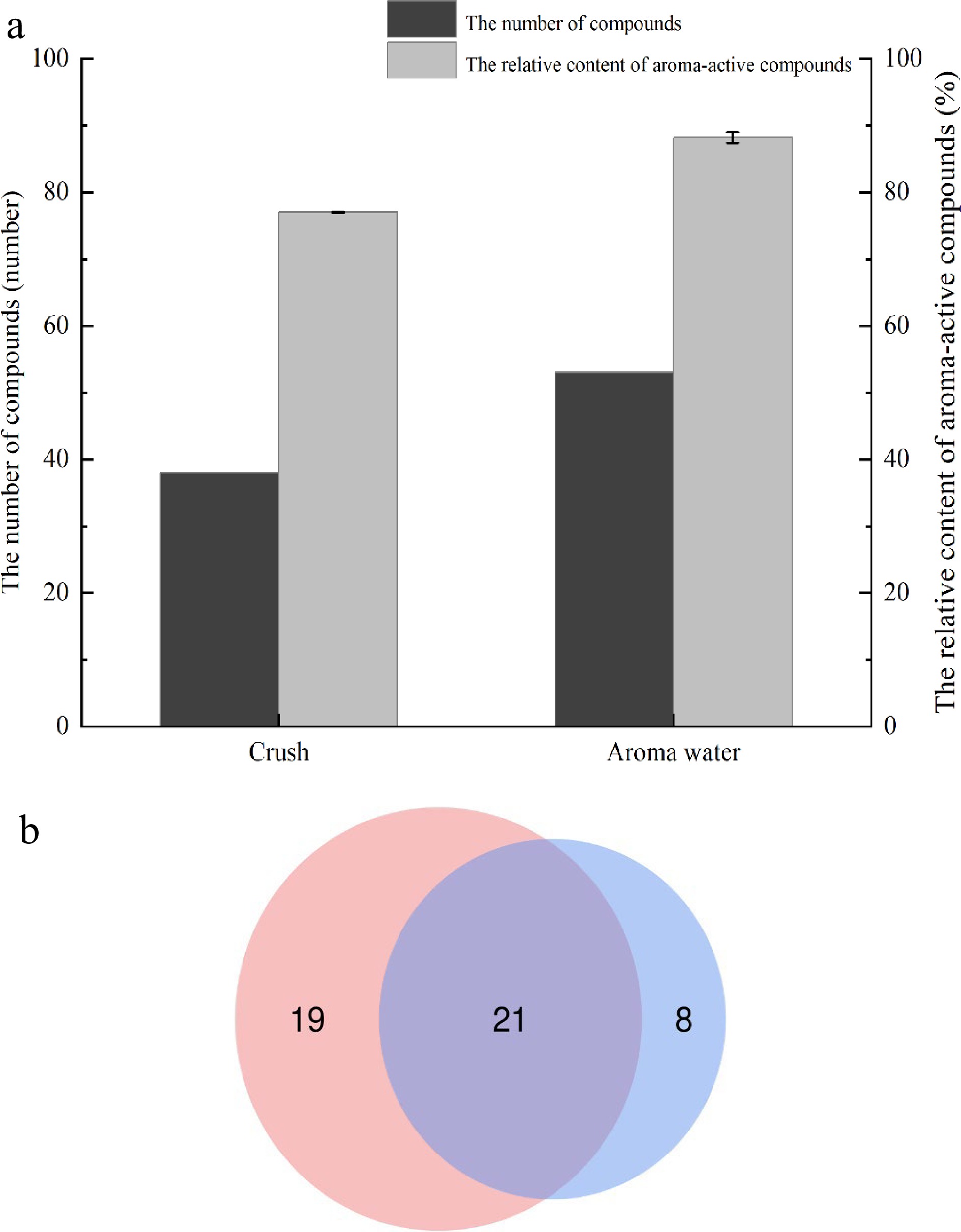
Figure 3.
Comparison of aroma-active compounds in two forms, (a) concentration and number; (b) the number of compounds.
-
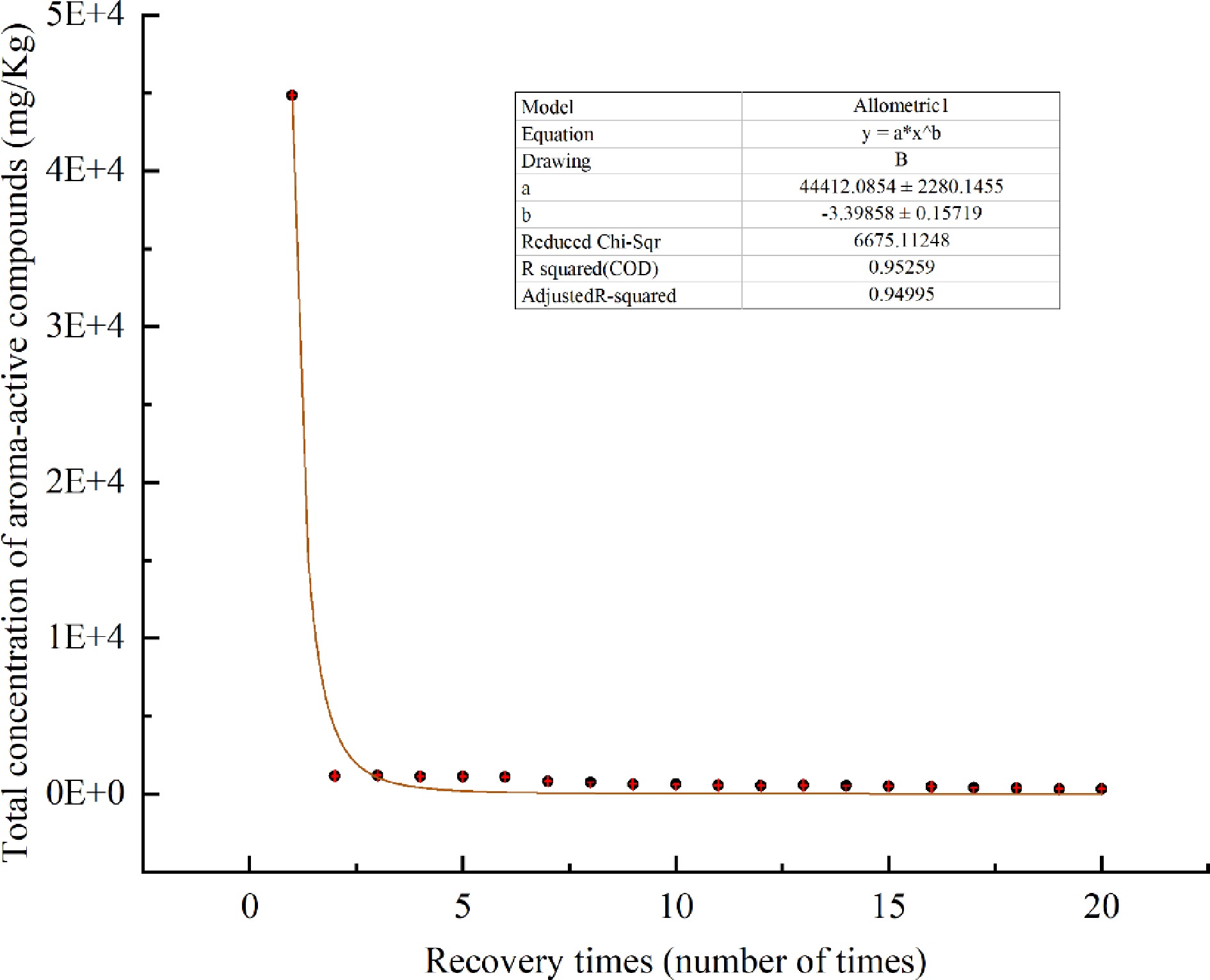
Figure 4.
Changes of total concentration of aroma-active components in Chenpi with heating time.
-
No Time
(min)RI Compound Molecular formula Calibration curves R2 Linear range
(mg/kg)Content
(mg/kg)aRSD (%)b 1 4.12 840 Furfural C5H4O2 y = 2.08E+09x + 1.76E+06 R2 = 0.9965 92.34-4.62 274.80 17.64 2 7.42 983 Myrcene C10H16 y = 3.55E+11x – 1.25E+07 R2 = 0.9939 1.37-0.07 21.19 24.52 3 7.74 1002 Octanal C8H16O y = 3.05E+10x – 4.37E+06 R2 = 0.992 2.34-0.12 78.71 31.41 4 8.19 1009 α-Terpinene C10H16 y = 2.73E+11x – 6.85E+06 R2 = 0.9919 0.51-0.03 3.47 27.55 5 8.44 1017 p-Cymene C10H14 y = 4.27E+11x – 5.02+05 R2 = 0.9959 0.41-0.02 12.85 13.06 6 8.60 1023 d-Limonene C10H16 y = 1.06E+11x + 2.55E+08 R2 = 0.9916 127.98-6.40 3291.64 28.02 7 9.53 1054 γ-Terpinene C10H16 y = 1.50E+11x – 3.95E+07 R2 = 0.9941 12.92-0.65 354.97 26.94 8 9.87 1071 1-Octanol C8H18O y = 1.62E+11x + 6.65E+06 R2 = 0.9923 0.40-0.04 3.32 20.64 9 10.91 1101 Linalool C10H18O y = 1.37E+11x + 9.32E+08 R2 = 0.9902 33.41-3.34 561.39 17.00 10 11.08 1105 Nonanal C9H18O y = 1.61E+11x + 4.96E+06 R2 = 0.9915 0.38-0.04 6.45 18.43 11 13.58 1168 1-Nonanol C9H20O y = 1.05E+11x + 3.83E+07 R2 = 0.9942 1.38-0.14 10.10 21.04 12 13.90 1192 4-Terpineol C10H18O y = 9.17E+10x + 3.02E+08 R2 = 0.9909 13.14-0.66 370.81 25.28 13 14.40 1202 α-Terpineol C10H18O y = 1.08E+11x + 1.66E+08 R2 = 0.9908 19.11-1.91 250.54 30.68 14 14.70 1219 Octanoic acid C8H16O2 y = 2.00E+09x + 1.41E+07 R2 = 0.9946 - - 15 16.52 1291 (-)-Carvone C10H14O y = 2.07E+11x + 4.62E+07 R2 = 0.9909 092-0.09 6.18 24.79 16 16.92 1304 Geraniol C10H18O y = 2.00E+11x + 4.34E+07 R2 = 0.9901 1.39-0.14 16.99 28.71 17 18.51 1358 Thymol C10H14O y = 6.54E+11x + 6.06E+08 R2 = 0.9922 7.88-0.79 38.18 26.40 18 18.89 1371 Cymenol C10H14O y = 6.14E+11x + 7.10E+07 R2 = 0.9916 0.77-0.08 29.62 21.95 19 19.40 1340 2-Methoxy-4-vinylphenol C9H10O2 y = 3.41E+10x + 2.15E+08 R2 = 0.9911 31.33-3.92 253.67 30.06 20 21.00 1360 Citronellyl acetate C12H22O2 y = 8.53E+11x + 1.20E+07 R2 = 0.9907 0.14-0.01 1.68 26.58 21 21.56 1380 n-Decanoic acid C10H20O2 y = 3.55E+11x – 1.42E+07 R2 = 0.9924 0.48-0.05 7.17 31.25 22 22.22 1385 Geranyl acetate C12H20O2 y = 1.01E+12x + 6.69E+06 R2 = 0.9906 0.08-0.008 0.54 30.38 23 23.22 1453 Methyl methanthranilate C9H11NO2 y = 1.16E+11x + 7.55E+06 R2 = 0.9914 0.53-0.05 15.20 10.21 24 26.40 1486 β-Ionone C13H20O y = 5.98E+11x + 3.47E+06 R2 = 0.9994 0.06-0.007 1.94 23.68 a: The data of concentration is mean (n = 3 for aroma water sample). b: The RSD is standard deviation (n = 3 for aroma water sample). −: Indicates no detection results. Table 1.
The concentration of volatile compounds detected in aroma water samples.
-
No Compound Odor description* FD** 1 Furfural Nut 8 2 Myrcene Pungent 32 3 Octanal Orange flavor 512 4 α-Terpinene Wax, orange 16 5 p-Cymene Refreshing 8 6 d-Limonene Citrus 32 7 γ-Terpinene Woody 8 8 1-Octanol Oily, fruity 128 9 Linalool Flowers, sweet 8192 10 Nonanal Oily, sweet, orange 8 11 1-Nonanol Orange scent 2 12 4-Terpineol Woody, loamy incense 2048 13 α-Terpineol Flowers, woody 2048 14 Octanoic acid Fruity 32 15 (−)-Carvone Mint, spicy 2048 16 Geraniol Rose 512 17 Thymol Medicine 1024 18 Cymenol Pungent, refreshing 8192 19 2-Methoxy-4-vinylphenol Pungent, flowers 8192 20 Citronellyl acetate Flowers 16 21 n-Decanoic acid Flowers 2048 22 Geranyl acetate Medicine 2048 23 Methyl methanthranilate Orange, flowers 1024 24 β-Ionone Woody 4096 * Description of the sniffing results by the sensory evaluator (n = 3 for sensory evaluator).
** Maximum dilution of the aroma-active compound.Table 2.
The FD factor of aroma-active compounds.
-
No Compound Concentration
(mg/kg)Odor threshold in
water (mg/kg)OAV 6 d-Limonene 3291.64 0.14b 24026.58 9 Linalool 561.39 0.03a 20049.61 19 2-Methoxy-4-vinylphenol 253.67 0.02b 13351.10 16 Geraniol 16.99 0.01a 1699.27 17 Thymol 38.18 0.10b 381.80 3 Octanal 78.71 0.23b 342.23 13 α-Terpineol 250.54 0.86b 291.32 24 β-Ionone 1.94 0.01c 231.49 18 Cymenol 29.62 0.18b 164.56 8 1-Octanol 3.32 0.02b 144.30 15 (-)-Carvone 6.18 0.07a 92.26 1 Furfural 274.80 3.00c 91.60 10 Nonanal 6.45 0.10a 64.50 12 4-Terpineol 370.81 6.40a 57.94 21 n-Decanoic acid 7.17 0.13b 55.19 23 Methyl methanthranilate 15.20 0.35b 43.54 2 Myrcene 21.19 0.67a 31.63 11 1-Nonanol 10.10 1.00a 10.10 7 γ-Terpinene 354.97 55.00c 6.45 22 Geranyl acetate 0.54 0.15a 3.60 5 p-Cymene 12.85 7.20b 1.78 20 Citronellyl acetate 1.68 1.00b 1.68 4 α-Terpinene 3.47 2.40b 1.45 14 Octanoic acid − 0.86b − Odor thresholds in water found in the literature. a: Indicates reference[18]. b: Indicates reference[35]. c: Indicates reference[21]. −: Indicates no detection. Table 3.
The results of OAVs calculation of aroma-active compounds.
-
Time (min) Compound Concentration (mg/Kg) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 4.12 Furfural 4.07 2.46 2.49 2.48 2.59 2.69 2.71 2.70 2.53 2.41 2.24 2.32 2.63 2.58 2.54 2.66 2.70 2.61 2.51 2.21 7.42 Myrcene 1053.51 0.37 0.25 0.22 0.31 0.40 0.19 0.51 0.20 0.37 0.35 0.39 1.09 0.44 0.85 0.69 1.20 0.77 0.77 0.98 7.74 Octanal 69.18 4.19 6.48 5.87 6.80 5.30 6.97 5.64 5.25 5.56 5.53 5.08 6.29 4.98 5.10 4.96 5.89 7.61 5.99 4.75 8.19 α-Terpinene 114.06 0.56 0.59 0.36 0.52 0.51 0.44 0.56 0.50 0.52 0.56 0.42 0.75 0.59 0.78 0.72 1.10 0.97 0.88 1.11 8.44 p-Cymene 53.16 1.18 1.18 0.89 1.24 1.18 0.88 1.38 0.82 1.16 1.17 1.17 1.34 1.34 0.72 1.71 1.58 1.41 1.33 1.97 8.60 d-Limonene 36762.66 20.30 20.33 21.24 20.29 21.32 18.23 44.92 20.00 32.18 30.15 33.80 45.82 36.91 42.08 30.50 20.79 21.79 29.84 26.29 9.53 γ-Terpinene 3591.33 1.63 1.52 1.72 1.81 1.61 1.59 3.73 1.78 2.80 2.76 3.12 4.00 3.05 3.77 4.13 3.67 4.07 3.65 3.33 9.87 1-Octanol 20.45 2.86 2.86 1.72 1.87 1.81 1.42 1.08 1.17 1.02 0.88 1.11 0.75 0.58 0.47 0.39 0.45 0.55 0.42 - 10.91 Linalool 1080.19 396.41 408.52 350.55 346.02 352.47 270.33 240.68 232.30 211.12 194.25 173.38 180.08 161.11 140.28 138.62 121.05 112.08 61.12 59.41 11.08 Nonanal 58.69 3.08 3.30 3.00 3.72 3.61 3.49 2.96 2.91 3.43 3.59 3.29 3.56 3.28 3.50 3.56 3.54 3.34 3.04 3.39 13.58 1-Nonanol 16.30 7.72 7.62 7.31 6.96 6.80 6.62 5.51 4.71 3.90 3.23 3.29 3.28 3.23 3.29 3.36 2.98 3.00 2.97 2.79 13.90 4-Terpineol 498.61 147.36 144.59 136.17 133.69 123.46 117.43 108.56 112.45 110.63 104.22 104.54 103.89 103.29 102.11 106.10 89.27 84.65 50.56 79.04 14.40 α-Terpineol 394.39 134.60 127.01 114.39 113.81 100.99 79.61 68.79 70.19 67.84 61.17 56.70 56.03 56.12 55.62 55.00 43.90 41.62 41.01 40.69 14.70 Octanoic acid − 4.28 4.11 − − − − − − − − − − − − − − − − − 16.52 (−)-Carvone 30.11 15.90 14.41 13.32 11.47 9.65 6.81 5.31 4.92 4.14 3.34 2.86 2.87 2.10 1.84 1.55 1.04 0.98 0.91 0.78 16.92 Geraniol 24.16 18.26 17.67 15.09 14.85 13.24 10.37 7.84 8.16 7.78 6.63 5.70 5.60 5.28 4.82 4.95 3.85 3.43 2.84 2.66 18.51 Thymol 207.17 187.75 184.75 180.91 168.43 134.25 103.75 86.04 83.95 74.10 57.64 54.99 51.60 47.08 40.06 38.67 28.19 23.13 18.93 17.40 18.89 Cymenol 72.98 152.32 175.42 189.58 198.40 198.78 116.71 82.08 20.87 20.15 20.01 20.93 21.03 27.24 30.94 24.91 8.89 7.49 14.32 13.62 19.40 2-Methoxy-4-vinylphenol 22.97 23.21 31.70 62.03 68.22 71.49 54.06 53.21 51.45 50.46 50.34 50.10 49.72 48.85 48.53 49.54 49.70 44.43 44.72 39.54 21.00 Citronellyl acetate 21.93 5.32 5.30 5.20 5.55 5.26 3.65 3.40 1.73 1.52 1.48 1.41 1.73 1.67 1.48 1.44 1.56 1.46 1.86 2.09 21.56 n-Decanoic acid − 12.05 14.52 14.52 15.45 16.58 12.05 10.00 8.03 7.42 7.31 7.73 7.30 7.33 5.27 5.73 5.61 5.11 5.72 5.50 22.22 Geranyl acetate 7.13 1.12 1.16 1.29 1.16 1.29 1.20 1.18 1.18 1.16 1.16 1.15 1.16 1.04 1.16 1.17 1.09 1.09 1.18 1.13 23.22 Methyl methanthranilate − 5.12 6.93 5.65 5.02 4.89 4.84 4.82 3.10 2.82 2.52 2.16 1.98 1.68 1.54 1.49 1.43 1.05 0.89 0.73 26.40 β-Ionone 7.72 6.95 5.23 4.88 4.88 3.82 3.21 3.02 2.85 2.62 2.36 2.23 2.06 1.84 1.72 1.66 1.46 1.09 0.99 0.99 Total concentration 44839.78 1155.54 1187.93 1138.38 1132.86 1081.40 826.57 743.90 641.10 615.12 562.88 537.87 554.55 521.93 498.49 483.52 400.94 373.74 326.45 310.42 - indicates no detection. Table 4.
Changes in the concentration of aroma-active compounds of Chenpi with extraction time.
Figures
(4)
Tables
(4)