-
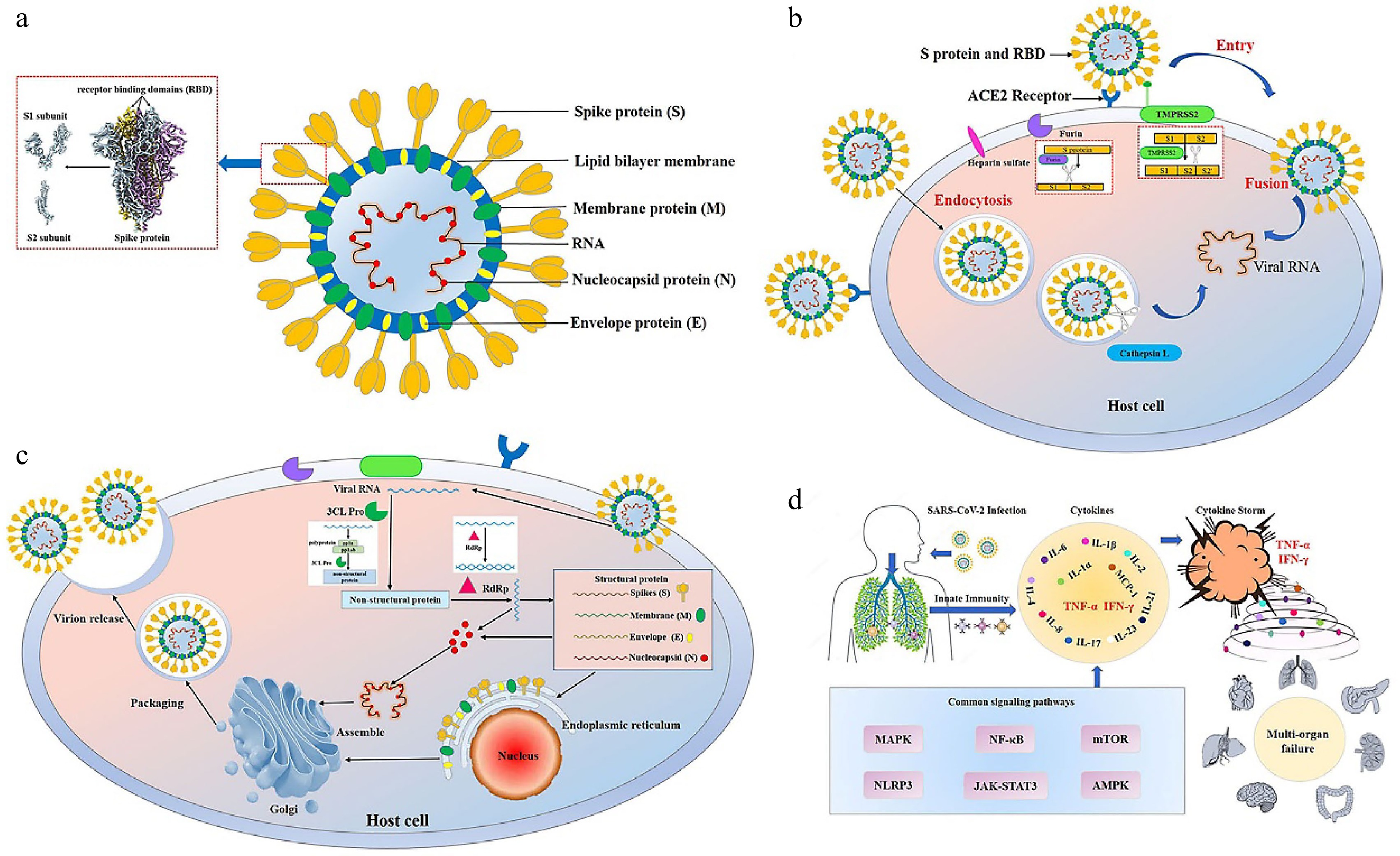
Figure 1.
Structure, infection and pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). (a) The structure of SARS-CoV-2. SARS-CoV-2 has four main structural proteins, namely spike (S), envelope (E), membrane (M) and nucleocapsid (N). The S protein of SARS-CoV-2 includes S1 and S2 subunits. The receptor binding domain (RBD) is located on the S1 subunit[111]. (b) Entry of SARS-CoV-2 into the host cell. SARS-CoV-2 entry into the host cell depends mainly on the action of host proteases, such as angiotensin-converting enzyme 2 (ACE2) (responsible for recognizing the virus S protein), transmembrane serine protease 2 (TMPRSS2) (responsible for endocytosis-independent entry and spike protein cleavage at the S2 site), cathepsin L (responsible for endocytosis-dependent entry) and furin (responsible for spike protein cleavage at the S1/S2 site). The S1 protein binds to and recognizes the host receptor ACE2, and then the host TMPRSS2 targets the S2 site for cleavage, releasing the spike fusion peptide and promoting virus entry into the target cell. (c) Replication of SARS-CoV-2 in the host cell. After entering and unhulling, genomic RNA acts as a transcript and is translated to produce multi-protein pp1a and pp1ab. Under the action of 3C-like protease (3CL Pro), pp1a and pp1ab are cleaved to produce 15-16 non-structural proteins with various functions. Under the action of RNA-dependent RNA polymerases (RdRp), genomic RNA generates double strands and then unwinds to generate new plus strands, helping the genome to replicate. Transmembrane structural proteins (S, M, and E) and some membrane-related helper proteins are translated in the endoplasmic reticulum, while N proteins are translated by cytoplasmic free ribosomes. The virion is then assembled in the golgi apparatus, transported in smooth-walled vesicles, and transported by the secretory pathway for release into the extracellular domain by exocytosis. (d) Cytokine storm. The infection of SARS-CoV-2 leads to the release of a large number of cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), interferon γ (IFN-γ) and monocyte chemotactic protein-1 (MCP-1) by immune cells, resulting in acute respiratory distress syndrome and multiple organ failure.
-

Figure 2.
The effect of functional components from edible plants on COVID-19. (a) Bioactive components inhibit virus entry into host cells. Various functional ingredients act on angiotensin-converting enzyme 2 (ACE2), transmembrane serine protease 2 (TMPRSS2) and furin or bind to the viral spike (S) protein, thereby preventing the interaction between the receptor binding domain (RBD) of the virus S protein and the host ACE2 and the subsequent membrane fusion, ultimately preventing the virus from entering the host cell. (b) Bioactive components inhibit virus replication in host cells. Bioactive components inhibit virus replication and spread of the virus by inhibiting the liquid phase condensation of the nucleocapsid (N) structure protein and the activity of replication-related enzymes, mainly 3C-like protease (3CL Pro) and RNA-dependent RNA polymerases (RdRp). (c) Bioactive components inhibit cytokine storm. Bioactive components can inhibit the infection of SARS-CoV-2, regulate immunity and inhibit the increase of inflammatory factors, so as to prevent cytokine storm.
-
Functional components Common source Experimental methods Observations Mechanisms References Polyphenols Quercetin, kaempferol and puerarin Fruits, vegetables, spices and medicinal food homologous plants Network pharmacology, molecular docking simulation and SPR The binding free energies of quercetin, puerarin and kaempferol to ACE2 were −7.92 kcal/mol, −7.46 kcal/mol and −7.21 kcal/mol, respectively. Quercetin showed high binding affinity for SARS-CoV-2 RBD with KD value of 2.21x10-6 M. The interaction between S protein RBD and ACE2 was almost completely eliminated by quercetin. It binds ACE2 and RBD to eliminate the interaction between S protein RBD and ACE2 and prevent the virus from recognizing the host and entering. [17] Luteolin and quercetin Same as above Enzyme kinetics and fluorescence experiment Luteolin showed the highest inhibitory effect on ACE2 activity with IC 50 value of 23 μM, followed by quercetin with IC 50 value of 43 μM. By inhibiting ACE2 [18] Hesperidin In the peel of citrus fruits Molecular docking simulation Hesperidin binds to S protein and its receptor ACE2 with a binding energy of -8.99 kcal/mol. Binding ACE2 and S protein makes the binding structure of ACE2 and S protein unstable and prevents SARS-CoV-2 from entering host cells through ACE2. [19] Resveratrol Grapes, berries, peanuts, etc Molecular docking simulation Good affinity (> −7 kcal/mol) The highly stable binding conformation with the viral protein-ACE2 complex affects the role of S protein and inhibits viral entry. [20] EGCG and TF Green tea and black tea Molecular docking simulation The binding fractions with RBD were −9.7 kcal/mol and −11.6 kcal/mol, respectively. The binding fractions with ACE2 were −8.5 kcal/mol and −8.0 kcal/mol, respectively. Virus recognition is inhibited by binding to virus S protein and ACE2. [21] EGCG, TSA and TFDG Tea Cell assay (VeroE6/TMPRSS2 cells) Three compounds strongly block the binding between ACE2 and RBD. It interacts with RBD and prevents the interaction between RBD and ACE2. [22] Epigallocatechin and epicatechin Tea Molecular docking simulation The binding energies of epigallocatechin and epicatechin with furin were
−7.7 kcal/mol and −7.1 kcal/mol, respectivelyFurin activity is inhibited by binding to the active site of furin. [23] GCG Tea Cell assay (A549, A549-hACE2-Flag, H1299, HEK293T) Viral titers were dramatically inhibited, IC50 = 44.4 uM; the selective index (ratio of CC50 to IC50) was 3.5. It inhibits SARS-CoV-2 replication by disrupting the LLPS of N. [24] EGCG Tea Network pharmacology, molecular docking simulation, SPR and FRET KD = 6.17 μM, IC50 = 0.847 μM. Binding to 3CL Pro and inhibiting 3CL Pro activity, thereby affecting viral replication. [26] EGCG Tea Cell assay (RD cells were infected with HCoV-OC43 and HCoV-229E) EGCG treatment decreased the 3CL Pro activity of HCoV-OC43 and HCoV-229E in a dose-dependent manner, with IC50 of 14.6 μM and 11.7 μM, respectively. EGCG treatment aslo reduced viral cytotoxicity, plaque formation, viral RNA and protein expression. It interferes with coronavirus replication by inhibiting 3CL Pro activity and reducing viral RNA and viral protein production. [27] EGCG, curcumin, resveratrol, quercetin
and ellagic acid— Molecular docking simulation, SPR and enzyme kinetics IC50 = 13.9, 11.9, 16.9, 23.4 and 11.8 µM;
KD = 311 ± 69 μM (ellagic acid).Binding to 3CL Pro and inhibiting 3CL Pro activity, thereby affecting viral replication [33] Naringenin In the peel of citrus fruits Molecular docking simulation and cell assay (Vero E6) Naringin showed moderate activity against SARS-CoV-2 at non-cytotoxic concentrations in vitro, with a significant selectivity index(CC50/IC50 = 178.748/28.347 = 6.3) By inhibiting the activity of M Pro and SARS-CoV-2. [29] Rutin and hesperidin Rue leaves, dates,
apricots, tomatoes,
buckwheat flowers and citrus fruitsMolecular docking simulation The binding free energy of rutin was −9.55 kcal/mol and that of hesperidin was -9.02 kcal/mol. By tightly combining 3CL Pro. [31] Luteolin Pepper, celery and other vegetables; honeysuckle, perilla and other natural Chinese herbal medicine Molecular docking simulation The binding energy of luteolin with 3CL Pro was −5.37 kcal/mol, forming five hydrogen bonds with GLN-189, LEU-4, ASN-142 and THR-26. Virus replication is inhibited by tightly combining 3CL Pro. [30] Silybin Silybum marianum Molecular docking simulation and enzyme kinetics The binding energy of silybin with 3CL Pro was −8.9 kcal/ mol , IC50 = 47.11 mg /L. Virus replication is inhibited by good binding of 3CL Pro and inhibiting enzyme activity. [28] Resveratrol Grapes, berries, peanuts, etc Cell assay (Vero E6) Inhibiting the replication of SARS-CoV-2,
EC50 = 4.48 μM.Viral infection is inhibited by inhibiting the replication of SARS-CoV-2 and activating SIRT1 signal in cells. [35] Resveratrol Same as above Cell assay (MRC5 and
Vero E6)Inhibiting the replication of HCoV-229E,
EC50 = 4.6 uM, CC50 = 210 µM, SI = 45.65.
Inhibiting SARS-CoV-2 replication,
EC90 = 11.42 µM, EC50 = 10.66 µM.The virus titer was reduced by direct inhibition of SARS-CoV-2 replication. [36] Polysaccharides Fucoidans Edible seaweed Molecular docking simulation, SPR and cell assay (Vero cells) EC50 = 8.3 ± 4.6 μg/mL (equivalent to 83 nM) Sulfated polysaccharides bind tightly to S protein of SARS-CoV-2 in vitro, thus interfering with the binding of S protein to heparin sulfate co-receptors in host tissues and inhibiting virus entry. [45] SJ-DSH and GN — SPR, NMR, cell assay (HEK293T) SJ-DSH binding to heparin competing virus pseudotype particles (A and B), IC50 = 27 nM; GN binding to heparin competing virus pseudotype particles (C and D), IC50 = 231 nM By inhibiting the interaction between S protein and heparin, S protein contact ACE2 was reduced. [46] κ- carrageenan Seaweed Molecular docking simulation The binding free energy of caffeine was
−14.37 kcal/mol; Ki = 29.35 pM.Virus replication is inhibited by good binding of 3CL Pro. [47] Sulfated polysaccharide Caulerpa lentillifera Cell assay (HeLa cell) IC50 = 48.48 μg/mL SARS-COV-2 activity is inhibited. [48] Lectin FRIL Hyacinth beans (Lablab purpureus) Glycan array analysis,
cell assay (MDCK and
Vero E6), animal experiment (BALB/c mice)EC50 = 0.80 μg/mL (7.15 nM); FRIL closely bound to the recombinant S protein at the concentration of 10 ng/mL. By effectively neutralizing SARS-COV-2 and binding to S protein with complex N-glycan (natural glycosylation), the virus can be influenced to recognize hosts. [63] MASL Maackia amurensis seed Cell assay (HSC-2 cell) ACE2 mRNA, ACE2 protein expression, glycosylation expression, fruin and ADAM17 mRNA were significantly decreased. Virus recognition and fusion are affected by inhibition of ACE2 and furin activity. [65] H84T-BanLec Musa acuminata Cell assay; animal experiment and human lung tissue in vitro SARS-CoV-2 infection significantly decreased. By targeting viral entry, binding to the mannose site of S protein and competitive binding to ACE2 [64] Alkaloids Bis-benzylisoquinoline alkaloids Poppies Cell assay (293T-ACE2, Calu-3, A549 and
Vero E6)Effectively protect different cells from coronavirus infection; The virus-induced cytotoxicity and viral RNA levels were partially inhibited. To some extent, SARS-CoV-2 can be directly inhibited or Ca2+ -mediated fusion and virus entry can be inhibited by blocking host calcium channel. [71] Caffeine and
theophyllineCocoa beans, kola nuts, tea and coffee beans, etc Molecular docking simulation The binding free energy of caffeine was −7.95 kcal/mol and that of theophylline was −8.91 kcal/mol. Virus replication is inhibited by good binding of 3CL Pro. [72] Berberine Coptis and berberis Cell assay (Vero E6 and Vero FM) It was effective against SARS-CoV-2 in Vero E6 cells at low micromolar concentrations, EC50 = 9.1 µM. The level of SARS-CoV-2 RNA in supernatant of the nasal epithelial cell model was inhibited, EC50 = 10.7 µM. By acting on the later stage of the virus life cycle, it slightly affected viral RNA synthesis and reduced the infectious virus titer, thus inhibiting SARS-CoV-2 replication in primary target cells. [73] Berbamine
hydrochlorideBerberis Cell assay (Vero E6 and Caco2 cell) In Vero E6 cells: EC50 = 1.732 μM, CC50 = 66.88 μM; In Caco2 cells: EC50 = 1.887 μM,
CC50 = 31.86 μMBy inhibiting S-mediated cell-cell fusion and targeting the stage of viral entry. [75] Terpenoids and saponins Ginseng saponin Ginseng Molecular docking simulation and cell assay (HEK293, MASMC and 16HBE) KD(Ra2) = 9.44 μM, KD( Rb1) = 0.47 uM, KD(Rb3) = 1.00 μM. By acting on the S1 subunit, it disrupts the S-RBD/ACE2 interaction of SARS-COV-2, thereby inhibiting virus entry. [89] limonin and limonin glycosides In the peel of citrus fruits Molecular docking simulation The binding energies with fruin were
−8.7 kcal/mol and −7.8 kcal/mol, respectively.
The binding energies with TMPRSS2 were
−8.3 kcal/mol and −8.1 kcal/mol, respectively.Binding to the active site of furin and TMPRSS2, thus inhibiting the activity of furin and TMPRSS2 and affecting virus fusion and entry. [23] Platycodon D Platycodon grandiflorum Cell assay (H1299, HEK293T, A549, MRC-5, Caco2 Vero and Calu-3) Inhibition of pSARS-CoV-2 into
ACE2/TMPRSS2 cells, IC50 = 0.72 μM;
Inhibition of sIPSC frequency;
SARS-CoV-2 infection was decreased in Vero cells and T Calu-3 cells with IC50 values of 1.19 and 4.76 μM, respectively.SARS-CoV-2 is prevented from entering the host by redistributing membrane cholesterol and inhibiting TMPRSS2 activity to prevent membrane fusion. [95] Glycyrrhizin Glycyrrhiza species and
an edible brown seaweed (Hizikia fusiformis
(Harvey) Okamura)Molecular docking simulation and cell assay (HEK293, MASMC, 16HBE) Interacting with SARS-CoV-2 S1 subunit, KD = 0.87 uM; Disrupting the RBD/ACE2 interaction of SARS-CoV-2, IC50 = 22 μM. Disrupting the S-RBD/ACE2 interaction of SARS-CoV-2 and acting on membrane cholesterol, thereby affecting the entry of SARS-CoV-2 into cells. [89] Glycyrrhizin and isoliquiritoside Glycyrrhiza species and
an edible brown seaweed (Hizikia fusiformis
(Harvey) Okamura)Molecular docking simulation The binding energies of glycyrrhizin and isoglycoside to M Pro were −8.6 kcal/mol and
−7.9 kcal/mol, respectively.Binding to major proteases and inhibiting their activity, thus affecting SARS-CoV-2 replication. [90] Glycyrrhizin Same as above Cell assay (A549, NCI-H1299, BEAS-2B, SVGp12, U937 and Vero E6) Inhibiting the replication of SARS-CoV-2. Directly affects virus replication. [91] SPR: surface plasmon resonance, ACE2: angiotensin-converting enzyme 2, RBD: receptor binding domain, S: sipke, TMPRSS2: transmembrane serine protease 2, KD: the equilibrium disstociation constant value, Ki: inhibition constant, IC50: 50% inhibitory concentration, CC50: 50% of the cells were diseased at the drug concentration, EC50: 50% effective concentration, EC90: 90% effective concentration, EGCG: epigallocatechin gallate, TF: theaflavin, TSA: theanine A, TFDG: theaflavin 3,3'-di-o-gallate, MASL: Maackia amurensis seed lectin, H84T-BanLec: banana lectin and replace histidine 84 with a threonine; SJ-DSH: sulfated galactofucan, GN: glucuronmannan, GCG: (-)-gallocatechin gallate, LLPS: liquid–liquid phase separation, N: nucleocapsid, FRET: fluorescence resonance energy transfer, 3CL Pro: 3C-like protease, M Pro: major proteases, RdRp: RNA-dependent RNA polymerase, SIRT1: silencing information reg-ulator1, SI: selectivity index. Table 1.
Experimental assessment and mechanism of inhibition of SARS-CoV-2 entry and replication by functional components of edible plants.
-
Functional components Common source Experimental methods Biological activity Observations References Polyphenols Curcumin The rhizome of turmeric Population intervention experiments Regulating immunity and anti-inflammatory IL-17↓, IL-21↓, IL-23↓ , GM-CSF↓, Th17 cell number↓, Th17 cell frequency↓, mortality rate↓, discharge rate↑ [37] Nanocurcumin The rhizome of turmeric Cell assay (lung epithelial A549 cells and liver epithelial Huh7.5 cells) Anti-inflammatory IL-6↓, IL-8↓, IFNγ↓, IL-1β↓, IL-6↓, IL-8↓, CCL2↓, CCL3↓, CCL4↓, CCL5↓, TNFα↓, FGF-2 [38] Apigenin Parsley, celery and
chamomile teaCell assay (RAW264.7) and animal experiments (C57BL/6J mice) Anti-inflammatory MiR-155↓, SMAD2 and FOXO3a↑, TNF-α↓ [41] Tannins Quebracho and chestnut tannin extract Randomised placebo-controlled
trials (n = 124)Anti-inflammatory Inflammatory state↓, MIP-1α↓, [42] Polysaccharides GLP Ganoderma lucidum Cell assay (Vero E6) and animal experiments (hamster) Antiviral Excellent antiviral effect (2 μg/mL) and no cytotoxicity [53] Inulin Chicory and Jerusalem artichoke Cell assay (bone marrow cells) and animal experiments (BALB/c or C57BL/6, Ffar3 -/- and Ffar2 -/-,
Cd8 -/-, Ly5.1C57BL/6 mice)Regulating immunity, reducing tissue damage,
and anti-influenza,Bifidobacterium and bacteroidetes↑, CXCL1↓, CD8+ T cell response↑, the frequency of Ly6c− patrolling monocytes being released from the bone marrow↑ [56] Lectin Banana lectin(Musa acuminata) Banana animal experiments (Balb/c mice) Regulating immunity Total IgG levels↑ [66] Mannose binds lectin Beans, algae, etc. — Regulating immunity It acts as a key pattern recognition molecule in complement immunity, primarily as a proantibody, which is crucial for the host's first-line defense before antibody production. [67] Alkaloids Berberine Coptis and berberis Animal experiments (C57BL/6J mice) Anti-inflammatory and antiviral IFN-γ↓, TNF-α ↓, IL-4↓, mRNA expression of TLR7, MyD88 and NF-κB ↓ [77] Colchicine Daylily and other lily plants Population intervention experiments Anti-inflammatory Patients with lymphocytopenia ↓, time of clinical deterioration ↓ [81] Colchicine Daylily and other lily plants A single-center propensity score matched cohort study Anti-inflammatory Mortality rate↓, mean C-reactive protein↓ [82] PUFAs AA and ALA Walnut oil Animal experiments (C57BL/6J mice) Anti-inflammatory MPO activity↓, TNF-α↓, 1L-1β↓, FFAR4 levels↑, injury of colon↓, the cAMP-dependent mechanism↑ [84] AA, DHA and EPA Nuts, beans, vegetables,
seeds of oil crops, etc.Cell assay (human hepatocellular carcinoma cells ) Antiviral They showed effective anti-HCV activity at 100 μM. EC50 of AA is approximate 4 μM. [86] Terpenoids and saponins Crocin Saffron — Anti-inflammatory Saffron may reduce the expression of inflammatory cytokines such as TNF-α, IL-1, IL-2, and IL-6 by regulating NF-κB, MAPK, and Nrf2 pathways [96] Glycyrrhizin Glycyrrhiza species and seaweed Animal experiments (C57BL/6 mice, C57BL/10ScNJ TLR4 KO mice ) Anti-inflammatory and antiviral TNF-α↓, 1L-1β↓, 1L-6↓, IP-10↓, HMGB1↓ [97] Steroidal ginsenoside Ginseng Animal experiments (C57BL/6J mice, ICR mice) Plasma samples from patients infected with SARS-COV-2 Anti-inflammatory Effect of NETosis↓, inflammatory cytokine levels (L-1β, IL- 4, IL-6, IL-8, IFN-γ and TNF-α)↓, damage of tissue↓, NF-κB↓, ROS↓, SREBP2↓ [99] NOS: nitric oxide synthase, COX-2: Cyclooxygenase-2, NF-κB : nuclear factor kappa-B, STAT1: signal transducerand activator of transcription 1, IL-: interleukin-, MIP-1α: macrophage inflammatory protein-1α; MCP-1: macrophage chemoattractant protein-1, HO-1: Heme Oxygenase-1, Nrf2: nuclear erythroid 2-related factor 2, GM-CSF: granulocyte-macrophage colony-stimulating factor, Th17: T helper 17, SMAD2: smooth-muscle-actin and MAD-related 2, FOXO3a: Forkhead Box O3, TNFα: tumor necrosis factor α, GLP: Ganoderma lucidum polysaccharide, CXCL-: chemokine (C-X-C motif) ligand-, FGF-2: fibroblast growth factor 2; IgG: immunoglobulin G, TLR7: toll-like receptor 7 , MyD88: myeloiddifferentiationfactor88, PUFAs: polyunsaturated fatty acids, AA: arachidonic acid, LA: linoleic acid, ALA: α-linolenic acid, DHA: docosahexaenoic acid, EPA: eicosapentaenoic acid, MPO: myeloperoxidase, FFAR4: free fatty acid receptor 4, cAMP: cyclic adenosine monophosphate, EC50: effective concentration, IP10: interferon-inducible protein-10, IFN-γ: interferon γ, ROS: reactive oxygen species, SREBP2: Sterol-regulatory element binding protein 2. Table 2.
Experimental evaluation of the potential of edible plant functional components in inhibiting cytokine storms.
Figures
(2)
Tables
(2)