-
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a major global threat. The virus is highly infectious and spreads mainly by breathing[1]. In the early stages, symptoms of infection include coughing, fever, tiredness, headaches, myalgia, and diarrhea. When symptoms are severe, infected people may experience respiratory distress and hypoxemia, and even cytokine storm, which may cause multi-organ failure[2] (Fig. 1d). At present, large quantities of new coronavirus vaccines which may prevent infection, disease or transmission have been produced and administered[3]. However, individuals differ in their exposure to viruses and response to vaccination, both depend on multiple factors, such as age, sex and race. Some vaccine recipients have experienced mild to severe adverse systemic reactions after the vaccine, including fatigue, joint discomfort, and headaches[4]. In addition, recent papers have revealed that the virus could constantly mutate and evolve into more infectious variants[5]. Similarly, a variant discovered for the first time in India may be more transmissible and have greater pathogenic potential than the existing variants[6]. As the virus evolves genetically, it may mutate further, making the virus more infectious, which could weaken the effects of existing vaccines and antibody therapies.
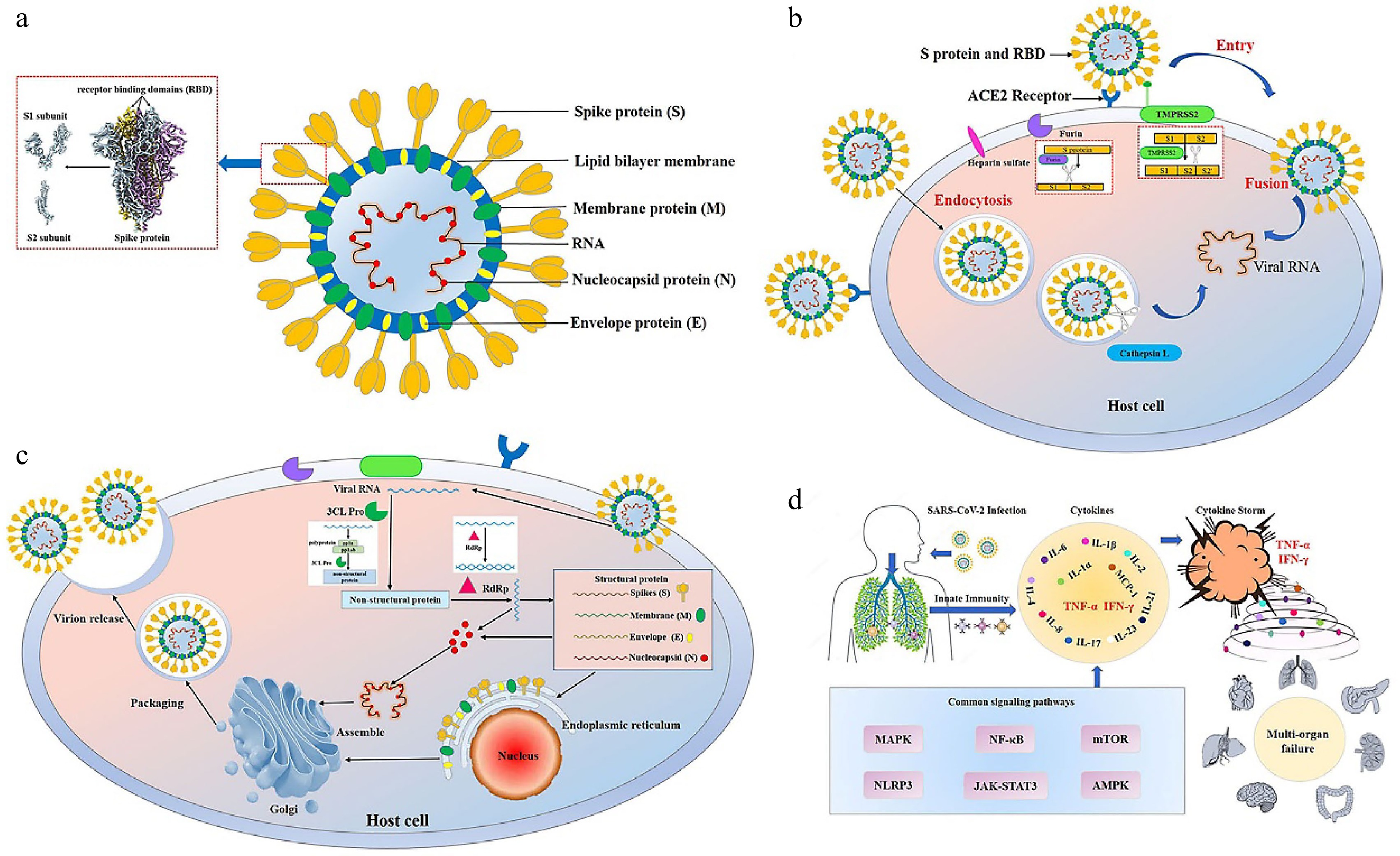
Figure 1.
Structure, infection and pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). (a) The structure of SARS-CoV-2. SARS-CoV-2 has four main structural proteins, namely spike (S), envelope (E), membrane (M) and nucleocapsid (N). The S protein of SARS-CoV-2 includes S1 and S2 subunits. The receptor binding domain (RBD) is located on the S1 subunit[111]. (b) Entry of SARS-CoV-2 into the host cell. SARS-CoV-2 entry into the host cell depends mainly on the action of host proteases, such as angiotensin-converting enzyme 2 (ACE2) (responsible for recognizing the virus S protein), transmembrane serine protease 2 (TMPRSS2) (responsible for endocytosis-independent entry and spike protein cleavage at the S2 site), cathepsin L (responsible for endocytosis-dependent entry) and furin (responsible for spike protein cleavage at the S1/S2 site). The S1 protein binds to and recognizes the host receptor ACE2, and then the host TMPRSS2 targets the S2 site for cleavage, releasing the spike fusion peptide and promoting virus entry into the target cell. (c) Replication of SARS-CoV-2 in the host cell. After entering and unhulling, genomic RNA acts as a transcript and is translated to produce multi-protein pp1a and pp1ab. Under the action of 3C-like protease (3CL Pro), pp1a and pp1ab are cleaved to produce 15-16 non-structural proteins with various functions. Under the action of RNA-dependent RNA polymerases (RdRp), genomic RNA generates double strands and then unwinds to generate new plus strands, helping the genome to replicate. Transmembrane structural proteins (S, M, and E) and some membrane-related helper proteins are translated in the endoplasmic reticulum, while N proteins are translated by cytoplasmic free ribosomes. The virion is then assembled in the golgi apparatus, transported in smooth-walled vesicles, and transported by the secretory pathway for release into the extracellular domain by exocytosis. (d) Cytokine storm. The infection of SARS-CoV-2 leads to the release of a large number of cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), interferon γ (IFN-γ) and monocyte chemotactic protein-1 (MCP-1) by immune cells, resulting in acute respiratory distress syndrome and multiple organ failure.
The SARS-CoV-2 life cycle entails getting into the host, genome transcription and replication, structural protein translation, virus assembly, and release of viral descendants. In the early stages of a virus's life cycle, entry into the host is a crucial phase. SARS-CoV-2 is dependent on host proteases to enter host cells through endocytosis or an endocytosis-independent mechanism, such as transmembrane serine protease 2 (TMPRSS2) (responsible for endocytosis-independent entry), cathepsin L (responsible for endocytosis-dependent entry) and furin (responsible for the cleavage)[7]. SARS-CoV-2 has the spike (S) protein, membrane protein, envelope protein and nucleocapsid protein. And the S protein includes S1 and S2 subunits (Fig. 1a). The S1 subunit recognizes the angiotensin-converting enzyme 2 (ACE2), while the S2 subunit anchors the S protein to the membrane and releases the spike fusion peptide, which promotes virus entry into the target cell[8] (Fig. 1b). ACE2 is the primary target for SARS-COV-2 virus protein recognition and entry into the host. In addition, the function of numerous important enzymes, including papaya-like cysteine protease (PL Pro), and 3C-like protease (3CL Pro), and RNA-dependent RNA polymerase (RdRp), is crucial for SARS-CoV-2 replication in the host (Fig. 1c). Therefore, blocking the recognition and replication of viral proteins by inhibiting key enzymes plays an important role in the prevention of COVID-19. In addition, SARS-CoV-2 triggers strong immune responses that lead to the cytokine storm, an excessive release of cytokine. The cytokine storm, which aggravates inflammatory states and leads to organ injuries, is considered an important signal of transition to severe and critical illness in ordinary patients and a leading cause of death from COVID-19[9]. Therefore, inhibition of the inflammatory response is equally critical to reduce the degree of inflammation in COVID-19.
Dietary botanical therapy has attracted widespread attention in recent years. The regulation of dietary composition is critical for the prevention and recovery of COVID-19. Plants contain polysaccharides, polyphenols, alkaloids, aldehydes, fatty acids, saponins and other components, which are considered to have the potential to enhance the body's immunity and antiviral and anti-inflammatory activities. Natural edible polysaccharides, such as mushroom polysaccharides, algae polysaccharides and fruit polysaccharides, can regulate immune responses through a variety of biochemical pathways and have anti-inflammatory and antiviral effects. Moreover, due to their easy binding to cellular receptors, they could be employed as an adjuvant in the creation of anti-SARS-CoV-2 vaccines[10]. Fruits, vegetables, tea and other natural plants contain a mass of polyphenols, such as tannins, phenolic acids, flavonoids, and anthocyanins. These functional components show important antioxidant and antiviral properties. Natural polyphenols have been demonstrated to suppress important proteins linked to SARS-CoV-2 infection, including ACE2, PL Pro, and 3CL Pro, which are expected to be targeted to reduce transmission of the virus[11,12]. In addition, fruits and vegetables are rich in vitamins and minerals, which can scavenge free radicals, reduce inflammation and enhance immune function[13]. A large number of unsaturated fatty acids are present in oil crops, such as eicosapentaenoic acid, docosahexaenoic acid, linolenic acid and so on. They are potential antiviral agents because they can regulate the phagocytosis of macrophages and other immune cells and inactivate enveloped viruses[14]. The prevention of COVID-19 through plant-based food has been considered a feasible plan by most researchers.
Herein, we reviewed the effects of functional components of plant-derived foods (polyphenols, polysaccharides, lectins, alkaloids, polyunsaturated fatty acids, terpenoids, and saponins) on key enzymes in virus recognition and replication, on SARS-COV-2 itself and on cytokine storms, and present the prospects and challenges of edible plants as vaccine adjuvants. This review aims to provide a comprehensive understanding of the potential preventive effects of functional components in edible plants on COVID-19.
-
Polyphenols are the most abundant functional components in plant-based foods. As natural antioxidants, polyphenols have beneficial effects, such as anti-inflammatory and antibacterial activities, prevention of cancer and cardiovascular disease and regulation of metabolic disorders[15]. Numerous studies have demonstrated the potency of polyphenols in edible plants against COVID-19 because they can inhibit SARS-CoV-2 fusion and entry into the host and inhibit SARS-CoV-2 replication, reduce inflammation, and prevent the occurrence of cytokine storms[16].
The potential of polyphenols in the inhibition of SARS-CoV-2 fusion and entry
-
Inhibiting the action of enzymes such as TMPRSS2 and furin proteinase, or blocking mutual recognition between the S protein receptor binding domain (RBD) and human ACE2, are the main ways that polyphenols impede viral entrance (Table 1).
Table 1. Experimental assessment and mechanism of inhibition of SARS-CoV-2 entry and replication by functional components of edible plants.
Functional components Common source Experimental methods Observations Mechanisms References Polyphenols Quercetin, kaempferol and puerarin Fruits, vegetables, spices and medicinal food homologous plants Network pharmacology, molecular docking simulation and SPR The binding free energies of quercetin, puerarin and kaempferol to ACE2 were −7.92 kcal/mol, −7.46 kcal/mol and −7.21 kcal/mol, respectively. Quercetin showed high binding affinity for SARS-CoV-2 RBD with KD value of 2.21x10-6 M. The interaction between S protein RBD and ACE2 was almost completely eliminated by quercetin. It binds ACE2 and RBD to eliminate the interaction between S protein RBD and ACE2 and prevent the virus from recognizing the host and entering. [17] Luteolin and quercetin Same as above Enzyme kinetics and fluorescence experiment Luteolin showed the highest inhibitory effect on ACE2 activity with IC 50 value of 23 μM, followed by quercetin with IC 50 value of 43 μM. By inhibiting ACE2 [18] Hesperidin In the peel of citrus fruits Molecular docking simulation Hesperidin binds to S protein and its receptor ACE2 with a binding energy of -8.99 kcal/mol. Binding ACE2 and S protein makes the binding structure of ACE2 and S protein unstable and prevents SARS-CoV-2 from entering host cells through ACE2. [19] Resveratrol Grapes, berries, peanuts, etc Molecular docking simulation Good affinity (> −7 kcal/mol) The highly stable binding conformation with the viral protein-ACE2 complex affects the role of S protein and inhibits viral entry. [20] EGCG and TF Green tea and black tea Molecular docking simulation The binding fractions with RBD were −9.7 kcal/mol and −11.6 kcal/mol, respectively. The binding fractions with ACE2 were −8.5 kcal/mol and −8.0 kcal/mol, respectively. Virus recognition is inhibited by binding to virus S protein and ACE2. [21] EGCG, TSA and TFDG Tea Cell assay (VeroE6/TMPRSS2 cells) Three compounds strongly block the binding between ACE2 and RBD. It interacts with RBD and prevents the interaction between RBD and ACE2. [22] Epigallocatechin and epicatechin Tea Molecular docking simulation The binding energies of epigallocatechin and epicatechin with furin were
−7.7 kcal/mol and −7.1 kcal/mol, respectivelyFurin activity is inhibited by binding to the active site of furin. [23] GCG Tea Cell assay (A549, A549-hACE2-Flag, H1299, HEK293T) Viral titers were dramatically inhibited, IC50 = 44.4 uM; the selective index (ratio of CC50 to IC50) was 3.5. It inhibits SARS-CoV-2 replication by disrupting the LLPS of N. [24] EGCG Tea Network pharmacology, molecular docking simulation, SPR and FRET KD = 6.17 μM, IC50 = 0.847 μM. Binding to 3CL Pro and inhibiting 3CL Pro activity, thereby affecting viral replication. [26] EGCG Tea Cell assay (RD cells were infected with HCoV-OC43 and HCoV-229E) EGCG treatment decreased the 3CL Pro activity of HCoV-OC43 and HCoV-229E in a dose-dependent manner, with IC50 of 14.6 μM and 11.7 μM, respectively. EGCG treatment aslo reduced viral cytotoxicity, plaque formation, viral RNA and protein expression. It interferes with coronavirus replication by inhibiting 3CL Pro activity and reducing viral RNA and viral protein production. [27] EGCG, curcumin, resveratrol, quercetin
and ellagic acid— Molecular docking simulation, SPR and enzyme kinetics IC50 = 13.9, 11.9, 16.9, 23.4 and 11.8 µM;
KD = 311 ± 69 μM (ellagic acid).Binding to 3CL Pro and inhibiting 3CL Pro activity, thereby affecting viral replication [33] Naringenin In the peel of citrus fruits Molecular docking simulation and cell assay (Vero E6) Naringin showed moderate activity against SARS-CoV-2 at non-cytotoxic concentrations in vitro, with a significant selectivity index(CC50/IC50 = 178.748/28.347 = 6.3) By inhibiting the activity of M Pro and SARS-CoV-2. [29] Rutin and hesperidin Rue leaves, dates,
apricots, tomatoes,
buckwheat flowers and citrus fruitsMolecular docking simulation The binding free energy of rutin was −9.55 kcal/mol and that of hesperidin was -9.02 kcal/mol. By tightly combining 3CL Pro. [31] Luteolin Pepper, celery and other vegetables; honeysuckle, perilla and other natural Chinese herbal medicine Molecular docking simulation The binding energy of luteolin with 3CL Pro was −5.37 kcal/mol, forming five hydrogen bonds with GLN-189, LEU-4, ASN-142 and THR-26. Virus replication is inhibited by tightly combining 3CL Pro. [30] Silybin Silybum marianum Molecular docking simulation and enzyme kinetics The binding energy of silybin with 3CL Pro was −8.9 kcal/ mol , IC50 = 47.11 mg /L. Virus replication is inhibited by good binding of 3CL Pro and inhibiting enzyme activity. [28] Resveratrol Grapes, berries, peanuts, etc Cell assay (Vero E6) Inhibiting the replication of SARS-CoV-2,
EC50 = 4.48 μM.Viral infection is inhibited by inhibiting the replication of SARS-CoV-2 and activating SIRT1 signal in cells. [35] Resveratrol Same as above Cell assay (MRC5 and
Vero E6)Inhibiting the replication of HCoV-229E,
EC50 = 4.6 uM, CC50 = 210 µM, SI = 45.65.
Inhibiting SARS-CoV-2 replication,
EC90 = 11.42 µM, EC50 = 10.66 µM.The virus titer was reduced by direct inhibition of SARS-CoV-2 replication. [36] Polysaccharides Fucoidans Edible seaweed Molecular docking simulation, SPR and cell assay (Vero cells) EC50 = 8.3 ± 4.6 μg/mL (equivalent to 83 nM) Sulfated polysaccharides bind tightly to S protein of SARS-CoV-2 in vitro, thus interfering with the binding of S protein to heparin sulfate co-receptors in host tissues and inhibiting virus entry. [45] SJ-DSH and GN — SPR, NMR, cell assay (HEK293T) SJ-DSH binding to heparin competing virus pseudotype particles (A and B), IC50 = 27 nM; GN binding to heparin competing virus pseudotype particles (C and D), IC50 = 231 nM By inhibiting the interaction between S protein and heparin, S protein contact ACE2 was reduced. [46] κ- carrageenan Seaweed Molecular docking simulation The binding free energy of caffeine was
−14.37 kcal/mol; Ki = 29.35 pM.Virus replication is inhibited by good binding of 3CL Pro. [47] Sulfated polysaccharide Caulerpa lentillifera Cell assay (HeLa cell) IC50 = 48.48 μg/mL SARS-COV-2 activity is inhibited. [48] Lectin FRIL Hyacinth beans (Lablab purpureus) Glycan array analysis,
cell assay (MDCK and
Vero E6), animal experiment (BALB/c mice)EC50 = 0.80 μg/mL (7.15 nM); FRIL closely bound to the recombinant S protein at the concentration of 10 ng/mL. By effectively neutralizing SARS-COV-2 and binding to S protein with complex N-glycan (natural glycosylation), the virus can be influenced to recognize hosts. [63] MASL Maackia amurensis seed Cell assay (HSC-2 cell) ACE2 mRNA, ACE2 protein expression, glycosylation expression, fruin and ADAM17 mRNA were significantly decreased. Virus recognition and fusion are affected by inhibition of ACE2 and furin activity. [65] H84T-BanLec Musa acuminata Cell assay; animal experiment and human lung tissue in vitro SARS-CoV-2 infection significantly decreased. By targeting viral entry, binding to the mannose site of S protein and competitive binding to ACE2 [64] Alkaloids Bis-benzylisoquinoline alkaloids Poppies Cell assay (293T-ACE2, Calu-3, A549 and
Vero E6)Effectively protect different cells from coronavirus infection; The virus-induced cytotoxicity and viral RNA levels were partially inhibited. To some extent, SARS-CoV-2 can be directly inhibited or Ca2+ -mediated fusion and virus entry can be inhibited by blocking host calcium channel. [71] Caffeine and
theophyllineCocoa beans, kola nuts, tea and coffee beans, etc Molecular docking simulation The binding free energy of caffeine was −7.95 kcal/mol and that of theophylline was −8.91 kcal/mol. Virus replication is inhibited by good binding of 3CL Pro. [72] Berberine Coptis and berberis Cell assay (Vero E6 and Vero FM) It was effective against SARS-CoV-2 in Vero E6 cells at low micromolar concentrations, EC50 = 9.1 µM. The level of SARS-CoV-2 RNA in supernatant of the nasal epithelial cell model was inhibited, EC50 = 10.7 µM. By acting on the later stage of the virus life cycle, it slightly affected viral RNA synthesis and reduced the infectious virus titer, thus inhibiting SARS-CoV-2 replication in primary target cells. [73] Berbamine
hydrochlorideBerberis Cell assay (Vero E6 and Caco2 cell) In Vero E6 cells: EC50 = 1.732 μM, CC50 = 66.88 μM; In Caco2 cells: EC50 = 1.887 μM,
CC50 = 31.86 μMBy inhibiting S-mediated cell-cell fusion and targeting the stage of viral entry. [75] Terpenoids and saponins Ginseng saponin Ginseng Molecular docking simulation and cell assay (HEK293, MASMC and 16HBE) KD(Ra2) = 9.44 μM, KD( Rb1) = 0.47 uM, KD(Rb3) = 1.00 μM. By acting on the S1 subunit, it disrupts the S-RBD/ACE2 interaction of SARS-COV-2, thereby inhibiting virus entry. [89] limonin and limonin glycosides In the peel of citrus fruits Molecular docking simulation The binding energies with fruin were
−8.7 kcal/mol and −7.8 kcal/mol, respectively.
The binding energies with TMPRSS2 were
−8.3 kcal/mol and −8.1 kcal/mol, respectively.Binding to the active site of furin and TMPRSS2, thus inhibiting the activity of furin and TMPRSS2 and affecting virus fusion and entry. [23] Platycodon D Platycodon grandiflorum Cell assay (H1299, HEK293T, A549, MRC-5, Caco2 Vero and Calu-3) Inhibition of pSARS-CoV-2 into
ACE2/TMPRSS2 cells, IC50 = 0.72 μM;
Inhibition of sIPSC frequency;
SARS-CoV-2 infection was decreased in Vero cells and T Calu-3 cells with IC50 values of 1.19 and 4.76 μM, respectively.SARS-CoV-2 is prevented from entering the host by redistributing membrane cholesterol and inhibiting TMPRSS2 activity to prevent membrane fusion. [95] Glycyrrhizin Glycyrrhiza species and
an edible brown seaweed (Hizikia fusiformis
(Harvey) Okamura)Molecular docking simulation and cell assay (HEK293, MASMC, 16HBE) Interacting with SARS-CoV-2 S1 subunit, KD = 0.87 uM; Disrupting the RBD/ACE2 interaction of SARS-CoV-2, IC50 = 22 μM. Disrupting the S-RBD/ACE2 interaction of SARS-CoV-2 and acting on membrane cholesterol, thereby affecting the entry of SARS-CoV-2 into cells. [89] Glycyrrhizin and isoliquiritoside Glycyrrhiza species and
an edible brown seaweed (Hizikia fusiformis
(Harvey) Okamura)Molecular docking simulation The binding energies of glycyrrhizin and isoglycoside to M Pro were −8.6 kcal/mol and
−7.9 kcal/mol, respectively.Binding to major proteases and inhibiting their activity, thus affecting SARS-CoV-2 replication. [90] Glycyrrhizin Same as above Cell assay (A549, NCI-H1299, BEAS-2B, SVGp12, U937 and Vero E6) Inhibiting the replication of SARS-CoV-2. Directly affects virus replication. [91] SPR: surface plasmon resonance, ACE2: angiotensin-converting enzyme 2, RBD: receptor binding domain, S: sipke, TMPRSS2: transmembrane serine protease 2, KD: the equilibrium disstociation constant value, Ki: inhibition constant, IC50: 50% inhibitory concentration, CC50: 50% of the cells were diseased at the drug concentration, EC50: 50% effective concentration, EC90: 90% effective concentration, EGCG: epigallocatechin gallate, TF: theaflavin, TSA: theanine A, TFDG: theaflavin 3,3'-di-o-gallate, MASL: Maackia amurensis seed lectin, H84T-BanLec: banana lectin and replace histidine 84 with a threonine; SJ-DSH: sulfated galactofucan, GN: glucuronmannan, GCG: (-)-gallocatechin gallate, LLPS: liquid–liquid phase separation, N: nucleocapsid, FRET: fluorescence resonance energy transfer, 3CL Pro: 3C-like protease, M Pro: major proteases, RdRp: RNA-dependent RNA polymerase, SIRT1: silencing information reg-ulator1, SI: selectivity index. Quercetin, kaempferol and puerarin are three kinds of flavonoids with a high content in nature and are important dietary antioxidants. Surface plasmon resonance and molecular docking simulations were employed to forecast the binding capacity between active compounds and ACE2. The results showed that three polyphenols all had a binding affinity to the ACE2 target, and quercetin was the best binding with a binding score of −7.92 kcal/mol. In the same study, quercetin was also discovered to interact with S protein, showing a strong affinity for the RBD, and the equilibrium dissociation constant value was 2.21 × 10-6 M. These results indicated that quercetin can not only react with the RBD but also block virus neutralization[17]. Similarly, the interactions of various polyphenols, such as quercetin, rutin, isorhamnetin, campholol, epicatechin, and luteolin, with ACE2 were studied in detail. According to the findings, quercetin was the most effective inhibitor of rhACE2 among the common polyphenols assessed, and the 50% inhibitory concentration (IC50) value was 4.48 μM[18].
Hesperidin and naringin are dihydroflavonoids with the highest content in the peel of citrus fruits and have antiviral and anti-inflammatory activities. Studies have shown that hesperidin could noncompetitively bind to ACE2 and bind to the binding site between S protein and ACE2, resulting in an unstable binding structure. Therefore, hesperidin prevented viruses from entering into the host by blocking the ACE2, thus preventing viral infection[19]. Resveratrol, the most abundant polyphenol in the peel of red wine grapes, has shown similar effects. Resveratrol could form a highly stable conformation with a complex of viral proteins and ACE2 receptors and stop viruses getting into the body[20].
Theaflavin and epigallocatechin gallate (EGCG) are abundant in tea. Studies have shown that tea polyphenols hold promise for alleviating COVID-19. Theaflavin and EGCG could prevent the RBD from attaching to the ACE2, thus blocking the viruses from getting into the host[21]. In vitro cell experiments, the results also confirmed that three kinds of tea polyphenols, EGCG, theanine A and theaflavin 3,3'-di-o-gallate, significantly inactivate SARS-CoV-2. They reduced viral contagiousness and inhibited viral RNA replication and the formation of secondary viruses in cells[22]. In addition, molecular docking analysis demonstrated that green tea polyphenols could also target the active sites of furin protease, thus inhibiting the activity of host protease and affecting the entry of the virus[21,23]. However, there are few studies on polyphenols targeting furin and TMPRSS2 and even fewer cell experiments.
In conclusion, polyphenols primarily bind to the S protein and ACE2 receptor to inhibit virus entry and stop viral infection. However, it should be noted that ACE2 is responsible for the degradation of angiotensin II and has an important negative regulatory effect on the renin-angiotensin system. Therefore, prevention of COVID-19 by inhibiting the function of ACE2 is not desirable. Targeting this enzyme needs to be carefully assessed to ensure that its physiological functions are not affected. Furthermore, high concentrations of polyphenols may be cytotoxic, and their safe dose should be determined.
The potential of polyphenols in the inhibition of SARS-CoV-2 replication
-
Plenty of papers have demonstrated that polyphenols could stop SARS-CoV-2 from replicating in the host (Table 1). The nucleocapsid protein regulates viral genomic RNA assembly and influences the host's antiviral defense. Some scientists have shown that the inhibition of nucleocapsid protein is related to liquid–liquid phase separation (LLPS). During coronavirus replication, the nucleocapsid protein combines with genomic RNA and is subsequently concentrated into protein-RNA complexes that initiate virion assembly. A study using (−)-gallocatechin gallate (GCG) to treat cells infected by SARS-CoV-2 indicated a significant inhibition of the virus titer. The main reason might be that GCG interfered with the LLPS of nucleocapsid protein, which prevented SARS-CoV-2 from replicating. Consequently, polyphenols that target nucleocapsid protein-RNA condensation may be a possible COVID-19 preventive[24].
3CL Pro is the primary protease of SARS-CoV-2 and can cut the translated polyprotein into multiple active proteins[25]. Existing studies have shown that polyphenols mainly acted on 3CL Pro to inhibit SARS-CoV-2 replication. EGCG could effectively inhibit 3CL Pro, and the IC50 value was 0.87 μM. The surface plasmon resonance assay revealed that the KD value between EGCG and 3CL Pro was close to 6.17 μM, indicating a high affinity. This indicated that EGCG had a strong interaction with 3CL Pro[26]. In addition, EGCG treatment reduced the 3CL Pro activity of two coronaviruses (HCOV-OC43 and HCOV-229E) and reduced the RNA and protein levels in the medium of coronavirus-infected cells[27]. Similar effects were also observed with naringin, carvonol, rutin, hesperidin, luteolin, silybin and other polyphenols. Molecular docking simulation analysis and in vitro experiments have confirmed that polyphenols had inhibitory effects on 3CL Pro[28−32]. In a recent study, Bahun et al.[33] found that polyphenols had a significant inhibitory effect on 3CL Pro. For EGCG, curcumin, resveratrol, quercetin and ellagic acid, IC50 values were obtained of 13.9, 11.9, 16.9, 23.4 and 11.8 µM, respectively. In addition, some polyphenols acted on RdRp. RdRp is vital in the viral RNA cycle. It is encoded by RNA viruses and catalyzes the target mRNA to synthesize dsRNA and then cleaves it to produce siRNA for cyclic replication. Common flavonols, such as flavonoid, biorobytin, myricetin, astragaloside IV, kaempferol, quercetin, and quercetin 3-O-glucoside, are potential inhibitors of RdRp because they have shown strong binding affinities to RdRp and have strong effects on its active site[34].
Based on cell experiments, the polyphenols' capacity to stop viral multiplication in the host was again demonstrated. Vero cells were given treatments with various resveratrol doses after infection, and viral replication was analyzed after infection for 2 d. In this study, it was discovered that resveratrol notably suppresses virus replication and the EC50 value was 4.48 μM. The results suggested that resveratrol mainly activated intracellular SIRT1 signaling, thus playing a role in protection against viral infection[35]. Another in vitro study also observed that resveratrol reduced viral titer and cytotoxicity and significantly reduced reproduction at concentrations up to 25 µM[36].
Polyphenols restricted SARS-CoV-2 reproduction mainly by inhibiting the replication of key enzymes, inhibiting structural proteins and viral resistance signaling pathways, thus displaying excellent promise for preventing COVID-19. However, most research on the inhibition of key enzymes remain at the level of molecular docking simulation analysis, so it is a lack of more in-depth and more effective data to directly prove the effect of polyphenols.
The potential of polyphenols in the inhibition of cytokine storm
-
Polyphenols may have an advantage in suppressing cytokine storms because they have powerful anti-inflammatory and immune-regulating abilities (Table 2). Due to their powerful antioxidant function, polyphenols could scavenge free radicals and reduce the quantity of intracellular reactive oxygen species, thus inhibiting inflammation to a certain extent. In addition, polyphenols inhibited inflammation by regulating immune cells, limiting inflammatory factor synthesis and controlling nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways. For example, luteolin effectively inhibited the levels of cyclooxygenase-2, nitric oxide, and inducible nitric oxide synthase in RAW264.7 cells infected with the pseudorabies virus. It inhibited inflammation by preventing signal transducer and activator of transcription 1/3-dependent NF-κB activation and promoting nuclear factor erythroid 2-related factor 2-mediated heme oxygenase 1 production[36]. One study showed a population experiment in which nanocurcumin therapy reduced the activation of T helper cell 17 (Th17) cells and associated inflammatory cytokines in COVID-19 patients, preventing excessive inflammation and disease progression caused by the increased frequency and overactivation of Th17 cells[37]. In addition, a recent study suggested that nanocurcumin could effectively inhibit the release of cytokines, chemokines and growth factors associated with SARS-CoV-2 spike-induced liver Huh7.5 and lung A549 epithelial cell injury and control hyperinflammatory responses. It had the potential to prevent lung and liver injury associated with SARS-CoV-2 spike-induced cytokine storms[38]. Polyphenols could also affect the intestinal barrier function and change the composition of intestinal microbiota through their interaction with the intestinal microbiota, thus showing an anti-inflammatory effect[39,40]. In addition, studies have shown that polyphenols played anti-inflammatory and immune regulatory roles by affecting miRNA expression. By lowering the expression of miR-155 which was generated by lipopolysaccharide (LPS), apigenin effectively inhibited inflammation in vivo and restored immunological balance[41]. In a randomised placebo-controlled trial, 124 patients were randomised into an oral tannin group and a placebo group. The results showed that after 14 d, patients in the oral tannin group had a significantly reduced inflammatory status. There was also a significant reduction in macrophage inflammatory protein-1α, an inflammatory factor positively correlated with IL-1β and TNF-α, suggesting that polyphenol intake had a beneficial effect on the development of cytokine storm in COVID-19 patients[42].
Table 2. Experimental evaluation of the potential of edible plant functional components in inhibiting cytokine storms.
Functional components Common source Experimental methods Biological activity Observations References Polyphenols Curcumin The rhizome of turmeric Population intervention experiments Regulating immunity and anti-inflammatory IL-17↓, IL-21↓, IL-23↓ , GM-CSF↓, Th17 cell number↓, Th17 cell frequency↓, mortality rate↓, discharge rate↑ [37] Nanocurcumin The rhizome of turmeric Cell assay (lung epithelial A549 cells and liver epithelial Huh7.5 cells) Anti-inflammatory IL-6↓, IL-8↓, IFNγ↓, IL-1β↓, IL-6↓, IL-8↓, CCL2↓, CCL3↓, CCL4↓, CCL5↓, TNFα↓, FGF-2 [38] Apigenin Parsley, celery and
chamomile teaCell assay (RAW264.7) and animal experiments (C57BL/6J mice) Anti-inflammatory MiR-155↓, SMAD2 and FOXO3a↑, TNF-α↓ [41] Tannins Quebracho and chestnut tannin extract Randomised placebo-controlled
trials (n = 124)Anti-inflammatory Inflammatory state↓, MIP-1α↓, [42] Polysaccharides GLP Ganoderma lucidum Cell assay (Vero E6) and animal experiments (hamster) Antiviral Excellent antiviral effect (2 μg/mL) and no cytotoxicity [53] Inulin Chicory and Jerusalem artichoke Cell assay (bone marrow cells) and animal experiments (BALB/c or C57BL/6, Ffar3 -/- and Ffar2 -/-,
Cd8 -/-, Ly5.1C57BL/6 mice)Regulating immunity, reducing tissue damage,
and anti-influenza,Bifidobacterium and bacteroidetes↑, CXCL1↓, CD8+ T cell response↑, the frequency of Ly6c− patrolling monocytes being released from the bone marrow↑ [56] Lectin Banana lectin(Musa acuminata) Banana animal experiments (Balb/c mice) Regulating immunity Total IgG levels↑ [66] Mannose binds lectin Beans, algae, etc. — Regulating immunity It acts as a key pattern recognition molecule in complement immunity, primarily as a proantibody, which is crucial for the host's first-line defense before antibody production. [67] Alkaloids Berberine Coptis and berberis Animal experiments (C57BL/6J mice) Anti-inflammatory and antiviral IFN-γ↓, TNF-α ↓, IL-4↓, mRNA expression of TLR7, MyD88 and NF-κB ↓ [77] Colchicine Daylily and other lily plants Population intervention experiments Anti-inflammatory Patients with lymphocytopenia ↓, time of clinical deterioration ↓ [81] Colchicine Daylily and other lily plants A single-center propensity score matched cohort study Anti-inflammatory Mortality rate↓, mean C-reactive protein↓ [82] PUFAs AA and ALA Walnut oil Animal experiments (C57BL/6J mice) Anti-inflammatory MPO activity↓, TNF-α↓, 1L-1β↓, FFAR4 levels↑, injury of colon↓, the cAMP-dependent mechanism↑ [84] AA, DHA and EPA Nuts, beans, vegetables,
seeds of oil crops, etc.Cell assay (human hepatocellular carcinoma cells ) Antiviral They showed effective anti-HCV activity at 100 μM. EC50 of AA is approximate 4 μM. [86] Terpenoids and saponins Crocin Saffron — Anti-inflammatory Saffron may reduce the expression of inflammatory cytokines such as TNF-α, IL-1, IL-2, and IL-6 by regulating NF-κB, MAPK, and Nrf2 pathways [96] Glycyrrhizin Glycyrrhiza species and seaweed Animal experiments (C57BL/6 mice, C57BL/10ScNJ TLR4 KO mice ) Anti-inflammatory and antiviral TNF-α↓, 1L-1β↓, 1L-6↓, IP-10↓, HMGB1↓ [97] Steroidal ginsenoside Ginseng Animal experiments (C57BL/6J mice, ICR mice) Plasma samples from patients infected with SARS-COV-2 Anti-inflammatory Effect of NETosis↓, inflammatory cytokine levels (L-1β, IL- 4, IL-6, IL-8, IFN-γ and TNF-α)↓, damage of tissue↓, NF-κB↓, ROS↓, SREBP2↓ [99] NOS: nitric oxide synthase, COX-2: Cyclooxygenase-2, NF-κB : nuclear factor kappa-B, STAT1: signal transducerand activator of transcription 1, IL-: interleukin-, MIP-1α: macrophage inflammatory protein-1α; MCP-1: macrophage chemoattractant protein-1, HO-1: Heme Oxygenase-1, Nrf2: nuclear erythroid 2-related factor 2, GM-CSF: granulocyte-macrophage colony-stimulating factor, Th17: T helper 17, SMAD2: smooth-muscle-actin and MAD-related 2, FOXO3a: Forkhead Box O3, TNFα: tumor necrosis factor α, GLP: Ganoderma lucidum polysaccharide, CXCL-: chemokine (C-X-C motif) ligand-, FGF-2: fibroblast growth factor 2; IgG: immunoglobulin G, TLR7: toll-like receptor 7 , MyD88: myeloiddifferentiationfactor88, PUFAs: polyunsaturated fatty acids, AA: arachidonic acid, LA: linoleic acid, ALA: α-linolenic acid, DHA: docosahexaenoic acid, EPA: eicosapentaenoic acid, MPO: myeloperoxidase, FFAR4: free fatty acid receptor 4, cAMP: cyclic adenosine monophosphate, EC50: effective concentration, IP10: interferon-inducible protein-10, IFN-γ: interferon γ, ROS: reactive oxygen species, SREBP2: Sterol-regulatory element binding protein 2. From what has been discussed above, we concluded that SARS-CoV-2 proliferation and entrance and cytokine storms could be prevented by polyphenols. However, the majority of the existing research on the impact of plant-derived polyphenols on SARS-CoV-2 are computer simulations and experiments in vitro, lacking supporting data from animal and human experiments. Furthermore, attention should be given to the cytotoxicity and bioavailability of polyphenols in the inhibition of SARS-CoV-2, and using polyphenols as a dietary adjuvant therapy for COVID-19 is advised.
-
Polysaccharides are made up of ten or more monosaccharides jointed together via glycosidic linkages. The polysaccharides are rich in fruits, vegetables, mushrooms and herbs, and possess biological activities, such as antioxidant, anti-inflammatory, immunomodulatory, and antiviral effects[10]. Previous studies have shown that natural plant polysaccharides, such as sulfated xymannan, xylan, pectin, and rock algae polysaccharides, could inhibit viral infection by interfering with the virus life cycle or directly stimulating the immune response[43]. Herein, we focus on polysaccharides' ability to reduce SARS-CoV-2 infection.
The potential of polysaccharides in the inhibition of SARS-CoV-2 replication.
-
The ability of polysaccharides from edible plants to inhibit virus entry and replication has been studied. The suppression of SARS-CoV-2 entrance and replication by various polysaccharides from plant-based functional foods was summarized in Table 1.
The RBD bound with ACE2 and heparan sulfate (HS). HS is a glycosaminoglycan, which is distributed on mammalian cells and mediates many pathological processes, such as cancer, inflammation and viral infection, by interacting with proteins. HS is considered as a co-receptor. The viruses use HS as the initial anchor site on the surface of host. This initial contact is made through a weak, reversible interaction force[44]. Therefore, targeted inhibition of S protein-HS interactions may have the potential to attenuate viral infection.
Previous studies have shown that the S protein bound firmly to fucoidans derived from edible sulfated seaweed polysaccharides in vitro, interfering with the S protein's ability to connect to the heparin sulfate co-receptor and preventing viral infection[45]. In the same research, the low cytotoxicity of polysaccharides in vitro was demonstrated. Antiviral studies have shown that an EC50 value of 8.3 μg/mL polysaccharides was more effective than remdesivir, which was an urgent medication authorized to treat COVID-19 infection. Jin et al.[46] subsequently carried out a similar study and found that sulfated galactofucan and glucuronmannan strongly hinder the S protein's combination with heparin, having IC50 values of 27 and 231 nM, respectively. Polysaccharides might make good SARS-CoV-2 preventive options.
Sulfated polysaccharides may be able to stop SARS-CoV-2 replication because of their high affinity for 3CL Pro. κ-carrageenan could bind with 3CL Pro, exhibiting the greatest binding score (−14.37 kcal/mol), lower inhibition constant (Ki = 29.35 pM) and a higher docking score than common antiviral medications[47]. A recent study found that a sulphated polysaccharide from an edible seaweed (Caulerpa lentillifera) was effective in protecting HeLa cells from SARS-CoV-2 infection with an IC50 value of 48.48 µg/mL, using a virus neutralisation assay[48]. Regardless, plant-derived natural sulfated polysaccharides, due to their negative charge, may interfere with the electrostatic interaction of viral glycoproteins and inhibit virus recognition, fusion, and transmission. In addition, sulfated polysaccharides cause macrophages to produce cytokines, which has a strong immunomodulatory effect, and it might be a viable option for COVID-19 prevention[49].
The potential of polysaccharides in the inhibition of cytokine storm
-
The anti-inflammatory effect of polysaccharides in plant functional foods has been demonstrated in many studies (Table 2). Polysaccharides have the potential to inhibit cytokine storm. Natural polysaccharides from edible fungi and Chinese herbs have shown strong anti-inflammatory and antiviral activities[50]. Ganoderma lucidum polysaccharide has been revealed to be anti-inflammatory in various studies[51]. Ganoderma lucidum polysaccharide reduced obesity by regulating intestinal flora and activated immune cells such as lymphocytes and neutrophils, thus regulating host immune function. Polysaccharides also regulate host immune function by regulating natural killer cell signaling pathways or by inhibiting overactivation of the immune response caused by stimulation[52]. By evaluating the viral strain's cytopathic impact on VeroE6 cells, Jan et al. demonstrated that Ganoderma lucidum polysaccharide had an excellent anti-SARS-CoV-2 virus efficacy (2 μg/mL)[53]. Polysaccharides of edible fungi regulated immunity by recognizing and activating membrane receptors of corresponding signaling pathways, such as NF-κB and MAPK, and by stimulating the development and release of related factors (tumor necrosis factor-α, nitric oxide, reactive oxygen species, interleukin-6 and interleukin-1β). Moreover, they influenced immunological organs and gut bacteria to control immunity[54].
Pectin is the most important polysaccharide substance in fruits and vegetables. Numerous papers have demonstrated the anti-inflammatory and immunomodulatory properties of pectin, and its anti-inflammatory ability is affected by the molecular weight of the side chain and the composition of the neutral polysaccharides. Therefore, the structure of polysaccharide itself is an important factor affecting its inhibition of SARS-CoV-2[55]. Inulin, a natural soluble polysaccharide mainly derived from chicory, has attracted much attention in recent years. It is a common and readily available dietary fiber that has been shown to defeat influenza A via increasing the production of Ly6c (−)-patrolling monocytes through CD8+ T cell and hematopoiesis cell metabolism[56]. Hu et al. also showed that inulin could be used as a prebiotic to improve host immune function and had the potential for systemic antiviral activity[57].
Being a type of natural plant material with high biocompatibility, sustainability, and low toxicity, polysaccharides have various biological activities, such as enhancing immune function and inhibiting viruses. They might be a good candidate for upcoming studies on COVID-19 alternative therapies, particularly the development and application of polysaccharides as vaccine adjuvants and drug delivery systems[58]. In addition, recent research has shown the use of polysaccharides as adhesive materials for prophylactic nasal sprays reduced SARS-CoV-2 damage to the upper respiratory lining, which was another potential application of polysaccharides in preventing the spread of viruses[59].
-
Lectins are glycoproteins or glycobinding proteins purified from various plants, invertebrates and higher animals. Commonly used lectins are phytoagglutin, such as peanut agglutinin, concanavalin A, wheat germ agglutinin, and soybean agglutinin. Lectins have antiviral and immunomodulatory activities and may be used to combat COVID-19[60].
The potential of lectins in the inhibition of SARS-CoV-2 entry and replication
-
Lectins could act on the highly glycosylated coronavirus S protein and block the S protein-mediated entrance of the virus. Lectins exerted antiviral activity by interfering with the presence of human N-glycans on the surface of the virion envelope to prevent viral infection (Table 1). The highly glycosylated S protein was modified by complex N-glycans or high-mannosyl-type N-glycans. Specific lectins from plants, algae and fungi preferentially recognized these glycans and interfered with the entry of viruses. Moreover, steric hindrance produced by the interaction of specific lectins with high mannose or complex N-glycans located near the RBD region could interfere with the receptor recognition process, which might interfere with the coronavirus entrance[61]. A recent study demonstrated that a tetrameric lectin, fms-like tyrosine kinase 3 receptor interacting lectin (FRIL), which was from hyacinth beans (Lablab purpureus), bound to S protein at quantities of 10 ng/mL, which can hinder the entry of the virus into the host cells. Furthermore, FRIL was found to attach to complex-type N-glycans more strongly when they had 1-3 or 1-4 fucosylated subterminal GlcNAc, indicating that FRIL has a larger inhibitory effect on viruses that have complex-type N-glycans[62]. In addition to directly interfering with the S protein, lectins also lowered the level of fruin and ACE2. The expression and glycosylation of ACE2 in HSC-2 cells were shown to be inhibited by more than 50% after 12 h of treatment with 1,925 nM Maackia amurensis seed lectin (MASL). MASL reduced ACE2 mRNA level by nearly 50% at 770 nM in HSC-2 cells. In addition, both 770 nM and 1,925 nM MASL could reduce the mRNA levels of furin and metalloprotease 17 in HSC-2 cells by 20% and 40%[63]. In conclusion, studies have confirmed that MASL might prevent the spread of COVID-19. Similarly, in a study by Chan et al.[64], a banana-derived lectin was found to prevent Middle East respiratory syndrome coronavirus and SARS-CoV-2 infections in vivo, which may be attributed to the lectin binding to multiple high-affinity high-mannose sites on the S protein of SARS-CoV-2, thereby inhibiting virus entry. In addition, lectins attacked the S protein's sugar portion (glycoprotein), interfering with the virus's attachment to S protein, which may be regarded as an inhibitor of entry of the virus and can be used to prevent COVID-19[65]. However, no research has demonstrated that lectins could influence virus replication, and whether lectins might serve as virus replication blockers remains to be explored.
The potential of lectins in the inhibition of cytokine storm
-
Lectins are crucial for controlling immunity, especially for suppressing complement immune activation in vitro (Table 2). Some scientists have found that banana lectins could regulate immune cells in vitro. Oral banana lectins have been shown to react with mucous membranes and trigger antibody formation in in vivo mouse experiments[66]. In the complement immune system, mannose-binding lectin (MBL) acts as a key pattern recognition molecule and mainly as a preantibody, which is crucial for the host's first-line defense before antibody production. However, it should be noted that when highly pathogenic coronaviruses excessively activate the complement immune system through the lectin pathway, this can lead to the recruitment of proinflammatory cytokines, leading to tissue damage and multiple organ failure[67]. Therefore, the use of MBL to prevent coronaviruses from activating the complement immune system in vitro blocked the occurrence of cytokine storms.
In short, plant lectins could prevent the entry of SARS-CoV-2 in vitro, thus reducing viral infection. MBL (such as legume lectins and algae lectins) could recognize mannose glycans, so it is a prospective candidate for SARS-CoV-2 viral particle-targeting glycan probes. Plant lectins would have important prospects for blocking and detecting virus infections[68]. However, some lectins have toxic effects, for example, eating raw lectin-rich kidney beans may cause symptoms such as diarrhea and vomiting. When using lectins for the treatment of COVID-19, we must pay attention to the safe dosage and toxicity of lectins.
-
Alkaloids are nitrogen-containing substances but are not peptides proteins, or amino acids. They are mostly found in the kingdom of plants[69]. They can inhibit the entry and replication of viruses and can also reduce inflammation. Therefore, we firmly believe that alkaloids have certain potential for use in SARS-CoV-2 prevention.
The potential of alkaloids in the inhibition of SARS-CoV-2 entry and replication
-
The inhibition of virus entry by alkaloids has been demonstrated in cell experiments (Table 1). Bis-benzylisoquinoline alkaloids commonly found in poppy could be used as pancoronavirus entry inhibitors. These substances prevented virus entry by blocking host calcium channels, which prevented calcium ion-mediated membrane fusion. Bis-benzylisoquinoline alkaloids were proven to effectively protect cell lines from coronaviruses and its variant infections in vitro[70].
According to several recent investigations, alkaloids have been revealed to be able to prevent the SARS-CoV-2 virus from replicating (Table 1). Alkaloids could block 3CL Pro and prevent virus replication and transcription. Researchers found that theophylline as well as caffeine, which were present in cocoa beans, tea and other plants, showed a good binding affinity with the His (41) and Cys (145) residues of 3CL Pro. Caffeine and theophylline may be potential inhibitors of 3CL Pro[71]. Berberine is an isoquinoline alkaloid, which is rich in coptis and berberis. One study in which 20 µM berberine was used to treat Vero E6 cells, indicated that berberine could effectively reduce the titer of infectious viruses by 1.5 logs and increase the ratio of particles to plaque forming unit in viral titers. In addition, berberine significantly inhibited the level of SARS-CoV-2 RNA in nasal epithelial cell model's supernatant and lowered the virus particles' infectiousness[72]. The reason why berberine inhibits the virus might be that berberine interfers with the capsid protein-viral RNA interaction, leading to abnormalities in nucleocapsid construction or disassembly and fewer infectious virus particles[73]. Another reason might be that berberine targets several cellular pathways, such as the MAPK pathway, mammalian target of rapamycin (mTOR) pathways, NF-κB and so on[74]. As a result, SARS-CoV-2 cannot upregulate these pathways to maximize the use of cellular resources for replication. A recent study found that berberamine hydrochloride, a bisbenzylisoquinoline alkaloid, effectively inhibited SARS-CoV-2 infection in Vero E6 and Caco2 cells in a dose-dependent manner with EC50 values of 1.732 and 1.887 μM, while CC50 values of 66.88 and 31.86 μM, respectively. In addition, berberamine hydrochloride was found to inhibit SARS-CoV-2 infection mainly by targeting the virus entry phase and inhibiting S-mediated cell-cell fusion[75].
The potential of alkaloids in the inhibition of cytokine storm
-
Alkaloids have a significant effect on suppressing inflammation and cytokine storm (Table 2). The production of cytokines that promote inflammation and the infiltration of inflammatory cells could both be inhibited by berberine. And the inflammatory response would be alleviated. Berberine could reduce inflammation by activating adenosine 5‘-monophosphate-activated protein kinase (AMPK) pathway, inhibiting NF-κB and pathway and inhibiting activator protein 1[76,77].
Tea contains the methylxanthine alkaloid, which includes caffeine, theobromine, and theophylline. Caffeine could inhibit monocyte chemotaxis, neutrophils, and tumor necrosis factor-α in the blood. Theophylline had anti-inflammatory effects on bronchial airways, and theobromine also had similar activities[78]. They both had purine nucleoside structures that inhibited the function of adenosine receptors and had a variety of effects on the central nervous system[79]. γ-aminobutyric acid, the primary neurotransmitter of the central nervous system, has the ability to regulate inflammatory cytokines[80]. Methylxanthine could influence γ-aminobutyric acid by crossing the blood-brain barrier. This may have a positive effect in alleviating the central nervous system dysfunction caused by COVID-19.
In addition, epidemiological survey results showed that colchicine treatment could significantly improve the time to clinical worsening[81]. In another single-center propensity score-matching cohort study, colchicine treatment was shown to be associated with a higher discharge rate and a decrease in mortality in COVID-19 patients who were critically unwell on the 28th day[82]. However, the role of colchicine remained controversial, with the results of a retrospective study based on big data analysis suggesting that colchicine was not a treatment for COVID-19. However, it is undeniable that alkaloids show important advantages in preventing SARS-CoV-2 entrance and reproduction. It is expected to become the most promising source of treatment and preventive agents for COVID-19.
-
Common polyunsaturated fatty acids (PUFAs) include linolenic acid, docosahexaenoic acid, eicosapntemacnioc acid, arachidonic acid, and α-linolenic acid. PUFAs in plants are mainly derived from various nuts, beans, vegetables, and seeds of oil crops. It must be mentioned that plants do not directly produce docosahexaenoic acid and eicosapntemacnioc acid and can only be used as indirect supplementary sources and obtained through transformation in the body. Previous studies have confirmed that PUFAs had obvious anti-inflammatory and antiviral functions (Table 2), but their efficacy in preventing COVID-19 remains to be studied.
PUFAs have well-proven anti-inflammatory ability and are of great benefit in the treatment of chronic diseases. Research has suggested that n-3 PUFAs could produce specialized pro-resolving mediators. Specialized pro-resolving mediators inhibited cytokine storm and proinflammatory lipid mediators, such as eicosanoids, by downregulating NF-κB and some inflammasomes, thereby playing a role in reducing inflammation[83]. Data from a set of animal experiments showed that the use of walnut oil rich in linolenic acid and α-linolenic acid significantly improved colon damage in mice with colitis induced by dextran sodium sulfate and lowered the damaged tissue's score while considerably recovering ion transport and colon wall permeability[84].
PUFAs killed microorganisms and enhanced the phagocytosis of white blood cells and macrophages mainly through the following methods: (1) direct activity on the cell membrane of microorganisms; (2) enhancing the production of free radicals; (3) increasing the formation of cytotoxic lipid peroxides; and (4) increasing the formation of biologically active metabolites (such as prostaglandins, lipoxins, decomposing factors, and protective factors). The beneficial effects of PUFAs may reduce the risk of pneumonia[85].
In terms of antiviral activity, PUFAs also showed great potential. Evidence has shown that several PUFAs, including arachidonic acid, docosahexaenoic acid and eicosapntemacnioc acid, could use the hepatitis C virus (HCV) subgenomic RNA replicon to exert anti-HCV activity[86]. Both linolenic acid and arachidonic acid could inactivate herpes, flu, Sendai and Sindbis viruses within minutes[85]. In addition, Das et al. suggested that arachidonic acid and other bioactive lipids influenced cell membrane fluidity, thereby regulating ACE2 receptor and influencing virus entrance, which may contribute to the therapy of COVID-19[31]. However, there is no clear research of PUFAs on SARS-CoV-2.
Furthermore, the proportion of n-6 to n-3 PUFAs is controversial. Excessive n-6 PUFA consumption, such as linolenic acid, leads to the formation of prostaglandins, leukotrienes and so on, which might amplify inflammation and aggravate the growth of SARS-CoV-2[87]. Therefore, attention must be paid to the ratio of different unsaturated PUFA intake.
-
Terpenoids, saponins and sterols in edible plants are also of research significance. Licorice is a popular medicinal plant and can also be used as a food ingredient. It has nutritional and therapeutic value. It is one of the key ingredients in the prescription of Chinese medicine for COVID-19[88]. Glycyrrhizin is the most important functional substance in licorice. It was a non-hemolytic saponin that could block ACE2 binding to RBD[89]. It caused lipid rafts to disintegrate in a cholesterol-dependent manner at the membrane level, thereby affecting the entry of coronavirus into cells. The latest research through molecular docking simulation also confirmed that licorice had a great potential to stop virus infection because it could react with the main protease of SARS-CoV-2[90]. In cell experiments, glycyrrhein prevented the freeing of high mobility group box 1 (HMGB1) and suppressed SARS-CoV-2 replication[91]. Ginseng is another major ingredient in Chinese prescriptions in addition to licorice. It has been used as one of the drugs to successfully treat SARS patients[92]. In addition, ginseng has many beneficial activities, includes antioxidation, antiaging, and anticancer activities[93]. The saponins contained in ginseng also have the ability to treat viral diseases, like COVID-19, HIV, HSV and rotavirus[94]. Molecular docking showed that ginsenosides might influence the association of ACE2 with RBD, which could result in anti-SARS-CoV-2 activity[88]. Compounds such as limonin and limonin glycosides are common triterpenoids in citrus. Studies have shown that they could attach to the furin and TMPRSS2 active sites to prevent the virus from entering host cells[23]. Platycodon D is a triterpenoid saponin as well as a common medicinal and dietary plant. It could prevent membrane fusion by redistributing membrane cholesterol, effectively limiting the virus's ability to infect the host[95]. Table 1 provide an overview of these additional bioactive components' effects against SARS-CoV-2 and their modes of action.
The anti-inflammatory impact of terpenoids, saponins, sterols and other bioactive compounds in edible plants cannot be ignored, which a great number of research have supported (Table 2). Carotenoids refer to a class of important natural pigments and are tetraterpene compounds with multiple biological activities, such as antioxidation, immune regulation and antiaging activities. Common carotenoids include β-carotene, crocin, lycopene, and zeaxanthin. As an example, crocin is the main component of saffron, a dual-purpose plant for medicine and food, and is an uncommon water-soluble carotenoid (dicarboxylic acid polyene monosaccharide ester). According to studies, crocin decreases inflammation by blocking the NOD-like receptor thermal protein domain associated protein 3 (NLRP3) and NF-κB pathway and activating the nuclear factor erythroid 2-related factor 2 pathway, thereby reducing the production of cytokines and oxidative stress. Furthermore, crocin could also significantly reduce the occurrence of cytokine storm involving immune cells such as macrophages, which might prevent the acute effects of COVID-19 and reduce mortality[96]. Glycyrrhizin from licorice could directly reduce inflammatory cytokines levels and oxidative stress that depended on HMGB1, preventing cytokine storms[97]. In addition, a recent review suggested that ginsenosides could exert anti-inflammatory effects by inhibiting the initiation of canonical and non-canonical inflammatory vesicles such as NLRP1, NLRP3, absent in melanoma 2 and cystathione-11 in inflammatory responses and diseases, thereby suppressing cytokine storms[98].
In addition, recent research has proposed using PEGylated albumin nanoparticles as a carrier to load steroidal ginsenosides with therapeutic effects against COVID-19. The data indicated that this complex could observably alleviate tissue damage and cytokine storm in animal experiments. In addition, it dramatically reduced systemic inflammation in SARS-CoV-2 patients[99]. Therefore, plant functional components and their mixtures loaded onto nanoparticles have potential applications in treating COVID-19.
Medicinal and edible plants and fungi are important sources of terpenoids and steroidal saponins, which are crucial in blocking the spread of the virus. These functional components mainly affect the replication and entry of the virus by influencing key enzymes and the distribution of membrane cholesterol. However, research on the functions of medicinal and edible plants has shown that usually the effect is not the result of a single compound, and characterization of specific active components is lacking. In order to defeat COVID-19, we should consider both the specific function of a single functional component and the synergistic effect between the functional components.
-
Vaccine adjuvants are a group of substances that play a complementary role in enhancing the body's immune response to antigens. They allow for an improved immune response, thus minimising the amount of vaccine used and reducing production costs[100]. In recent years, edible plant functional components have been reported to show potential as natural vaccine adjuvant candidates, such as plant polysaccharides, polyphenols and proteins. Natural plant polysaccharides are the most promising natural vaccine adjuvants due to their immunomodulatory, biocompatible, biodegradable, low toxicity and safety characteristics. Common edible polysaccharides that can be used as vaccine adjuvants include inulin and herbal polysaccharides such as shiitake mushroom polysaccharide, wolfberry polysaccharide, ganoderma lucidum polysaccharide and astragalus polysaccharide. Previous studies have shown that the δ form (semi-crystalline) of inulin stimulated strong humoral and cellular immune responses when bound to various antigens, and its immune adjuvant properties have been used to enhance the immunogenicity of many antigens[101]. Advax, the latest generation of δ inulin-based adjuvants developed by the NIH Adjuvant Development Program, has been tested in a variety of animal models against a wide range of pathogens, including malaria, HIV, influenza, tetanus toxoid, hepatitis B virus and others. Advax has shown a high safety profile and good tolerability[102,103]. Chinese herbal polysaccharides have likewise been shown to have potential as vaccine adjuvants, as they can enhance cytokine expression and promote immune cell activity, resulting in a strong immunomodulatory effect and promoting the immune function of the body[104]. Previous studies have shown that astragalus polysaccharides, lycium barbarum polysaccharides, ganoderma lucidum polysaccharides and shiitake mushroom polysaccharides were able to induce the maturation of dendritic cells, suggesting that they could be effective adjuvants for dendritic cell-based vaccines[105]. In addition, herbal polysaccharides have shown adjuvant effects comparable to aluminium hydroxide for vaccines such as H5N1, H1N1 influenza and hepatitis A, suggesting that herbal polysaccharides are potential vaccine adjuvant candidates for human vaccines. Lectins can induce mucosal and systemic immune systems by acting as adjuvants and are ideal candidates for vaccine formulations. It has been found that lectins derived from pineapple honey have been used as adjuvants in protozoan parasite vaccines. The lectin induced a helper T cell 1 immune response mainly by stimulating interleukin-12 production by macrophages and dendritic cells[106]. Another recent study found that IgG responses were significantly enhanced when mannose-conjugated lectins from garlic were administered to BALB/c mice along with antigenic ovalbumin[107]. Lectins could be used to develop highly effective COVID-19 vaccines and drugs. Plant polyphenols also have immunomodulatory properties. A study found that green tea extract and EGCG exhibited strong adjuvant effects by increasing neutralising antibody titres and protective efficacy. In addition, EGCG induced antibody-dependent cell-mediated cytotoxicity (ADCC)-mediated antibodies, which induced IgG2a and IgG1 antibodies associated with ADCC activity, thereby increasing the efficacy of protection[108]. EGCG could be used as a novel adjuvant for the development of safe and effective vaccines. Notably, the use of saponins from edible plants as vaccine adjuvants has been widely studied. Previous studies found that saponin microemulsion adjuvant co-administered with SARS-CoV-2 S1-Fc vaccine produced very high titre-specific neutralizing antibodies against live SARS-CoV-2 infection in macaques[109]. In addition, the efficacy of the saponin-based vaccine Matrix-M adjuvant against COVID-19 has been extensively studied. The Matrix-M adjuvanted combination vaccine was found to produce a strong anti-S response and virus neutralisation in mice[110]. All these findings indicate that edible plant functional ingredients have the potential to become natural vaccine adjuvants, and that the application of edible functional ingredients in the development of vaccine adjuvants is of great research value.
-
Plant-based food functional ingredients are important sources for enhancing human resistance to COVID-19. Combined with recent studies, we summarized the effects of several common functional components of plant-derived foods (such as polyphenols, polysaccharides, lectins, alkaloids, polyunsaturated fatty acids, terpenoids, and saponins) on COVID-19. We discovered that the biological components of diets produced from plants are mostly responsible for treating and preventing COVID-19 in the following ways: (1) Inhibition of virus entry: Various functional ingredients act on ACE2, TMPRSS2 and furin or bind to the viral S protein, thereby preventing the RBD in combination with ACE2 and the subsequent membrane fusion, ultimately stopping the virus's entrance (Fig. 2a); (2) Inhibition of virus replication: By inhibiting the N structure protein and the activity of replication-related enzymes, mainly 3CL Pro and RdRp, the proliferation and spread of the virus can be inhibited (Fig. 2b); (3) Inhibition of cytokine storm: Phytochemical components prevent SARS-CoV-2 from triggering a strong immune response by regulating immunity and anti-inflammatory effects, avoiding excessive inflammation, and preventing the aggravation of COVID-19 symptoms (Fig. 2c).

Figure 2.
The effect of functional components from edible plants on COVID-19. (a) Bioactive components inhibit virus entry into host cells. Various functional ingredients act on angiotensin-converting enzyme 2 (ACE2), transmembrane serine protease 2 (TMPRSS2) and furin or bind to the viral spike (S) protein, thereby preventing the interaction between the receptor binding domain (RBD) of the virus S protein and the host ACE2 and the subsequent membrane fusion, ultimately preventing the virus from entering the host cell. (b) Bioactive components inhibit virus replication in host cells. Bioactive components inhibit virus replication and spread of the virus by inhibiting the liquid phase condensation of the nucleocapsid (N) structure protein and the activity of replication-related enzymes, mainly 3C-like protease (3CL Pro) and RNA-dependent RNA polymerases (RdRp). (c) Bioactive components inhibit cytokine storm. Bioactive components can inhibit the infection of SARS-CoV-2, regulate immunity and inhibit the increase of inflammatory factors, so as to prevent cytokine storm.
Nevertheless, using edible plants to prevent COVID-19 still has some limitations and unresolved problems. First, the cytotoxicity and bioavailability of the functional ingredients of edible plants has always been widely studied. We must clarify safe dosages when using these components and find the best inhibitory concentration with a noncytotoxic dosage. In addition, improving the bioavailability of functional ingredients is still an important and unresolved difficulty[69]. At present, some studies have used nanoparticles as carriers loaded with plant functional components for treating diseases and have achieved good results. This may be a solution we need to focus on. Second, the impacts of physiologically active components in edible plants on COVID-19 performed by computer simulations and cell experiments. However, animal experiments and human clinical experiments are still lacking and difficult to carry out. The third point is that intake of plant-derived food usually does not equate to the ingestion of a single ingredient. We need to clarify the specific biological functional components of plant-derived foods that work and consider that a synergistic intake may have a stronger preventive and therapeutic effect. Finally, since most of the plant functional ingredients are effective at inhibiting the entry of viruses, we believe that some current studies on the prevention of COVID-19 using nasal sprays should consider adding plant functional ingredients to guard against SARS-CoV-2 encroachment[59]. In short, functional components of edible plants can enhance human resistance to COVID-19, which have feasibility and broad application prospects for the prevention of COVID-19.
This work was supported by the National Natural Science Foundation of China (No.32172270).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Pu Y, Chen L, He X, Ma Y, Cao J, et al. 2023. Potential beneficial effects of functional components of edible plants on COVID-19: Based on their anti-inflammatory and inhibitory effect on SARS-CoV-2. Food Innovation and Advances 2(1):44−59 doi: 10.48130/FIA-2023-0006
Potential beneficial effects of functional components of edible plants on COVID-19: Based on their anti-inflammatory and inhibitory effect on SARS-CoV-2
- Received: 27 September 2022
- Accepted: 11 January 2023
- Published online: 15 March 2023
Abstract: COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a major public health threat. Edible plants are rich in bioactive components, with a variety of functions, such as enhancing immunity, antiviral, anti-inflammatory and so on. Thus, the intake of edible plants to boost the body's resistance to COVID-19 is a promising and possibly affordable strategy. This review revisits the effects of functional components from edible plants (such as polyphenols, polysaccharides, lectin, alkaloids, polyunsaturated fatty acids, terpenoids, and saponins) on COVID-19. The inhibitory effects of bioactive components on the virus's entrance and replication, anti-inflammatory and immune enhancement are discussed. And finally, we present the prospects of using edible plant functional ingredients as vaccine adjuvants and the prospects and problems in the use of edible plant functional components for the prevention of COVID-19. Functional components of edible plants interacted with structural proteins of SARS-CoV-2 virus and key enzymes in virus recognition and replication, thereby inhibiting virus entry and replication in the host. Meanwhile, these bioactive components had anti-inflammatory effects and could inhibit cytokine storms. Therefore, we believe that functional components from edible plants can enhance human resistance to COVID-19 and can be applied in the development of new therapies.
-
Key words:
- SARS-CoV-2 /
- Edible plants /
- Functional components /
- Cytokine storms /
- COVID-19












