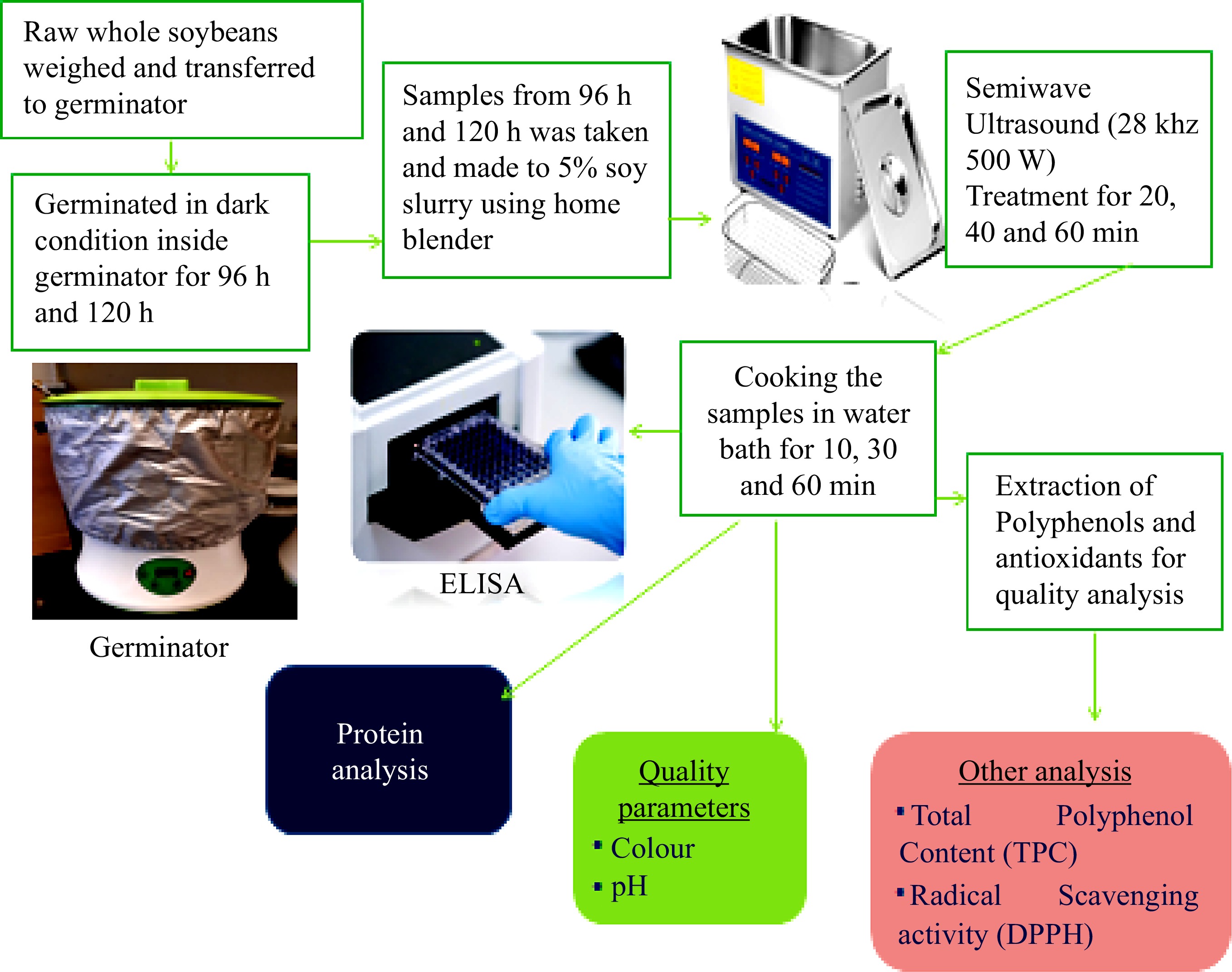
-
Soybeans are among the top eight food products causing allergies. The World Health Organization (WHO) listed eight allergen fractions in soybeans to be problematic: Gly m-1 to Gly m-8[1−4]. Among them β-Conglycinin (Gly m-5) and Glycinin (Gly m-6) are considered to be of major concern[5−9]. The majority of soybean proteins are storage proteins which perform diverse functions and serve as biological reserves for mineral and amino acid nutrients and contribute functionality to soy protein-based ingredients[10]. Soy products account for over 90% of food allergenic reactions[11]. Globular soy proteins are heat resistant and therefore reducing their immunoreactivity (IR) is difficult. Soy protein isolate (SPI) is a widely commercial protein in food preparations and has been a concern for people that are sensitive to soy allergens[12−15].
Generally, thermal processing is used to reduce the IR associated proteins. A successful moderated thermal processing procedure was suggested by Ravindran & Ramaswamy[16] for reducing the IR sensitivity of soybean allergens by a large margin. However, such a process can have significant influence on the resulting product quality. Recently, several nonthermal methods such as high pressure, germination, pulsed light, pulsed electric field, ultrasound etc., have gained attention as alternate processing methods to pasteurization, cooking and other heat based methods. Ultrasound has been used to reduce the allerginicity in shrimp[17], wheat[18], milk proteins and soy protein isolates[19]. Nonthermal treatments add to environmental friendliness, flavor maintenance, low energy consumption and nutrient retention[20]. Ultrasound technology, in combination with cooking can be effective in reducing the biological activities of certain proteins[21]. Cavitation or rapid formation of bubbles in ultrasound processing can result in disruption of molecular structure and functionality of macromolecules leading to efficient application of homogenization, enzyme inactivation, and molecular size reduction[22].
Germination enhances the seed nutritional quality through physiological enzyme activity elevating many required nutrients and also removing antinutrients like trypsin inhibitors[23,24]. Different factors like germination treatment time, temperature and light have been considered important factors influencing the quality of the sprouts[25]. The nature and duration of the germination process can also influence the seed palatability and IR in soybean allergen proteins[26]. Troszyńska et al.[27] reported a major reduction in IR of cowpeas and soybeans by germination in dark conditions. Protein hydrolysis has been identified as the reason for these IR reductions.
ELISA based allergen assays are simple to use, sensitive to allergen detection and accurate for quantitative estimations, and are commercially available. Sandwich ELISA is the most popular kit[28,29] and was used in our previous study[16]. FTIR spectroscopy techniques are commonly used for evaluating conformational changes in proteins as a result of process application and are widely accepted[30]. Amide bands which relates to carbon-carbon molecular disturbances are widely used and are considered to be most sensitive to protein modifications and influence on the secondary structure of proteins[31,32].
This study focused on evaluating the use of seed germination, ultrasound and conventional home cooking treatment of soy slurry either individually or in succession on the IR reduction. The influence of the combination procedure on the quality of soy slurry was tested based on their influence on total phenolic content and DPPH antioxidant activities. The allergen assay was based on the more contemporary sandwich ELISA method which are sensitive enough to detect soy allergen IR at micro- and nano-gram levels and FTIR analysis for conformational analysis.
-
Raw soybean (Glycine max), variety RD-714, with a high protein content (50.5%) was obtained from a commercial source (SG Ceresco Inc., Quebec, Canada). Raw soybeans (60 g) were weighed and distributed on to each tray of the germinator/sprouting machine (Kikiheim Automatic Bean Sprouts Machine, 25.5 cm × 34 cm; made in Liangzhou, Guangdong, China). Traditionally, soybeans require soaking for a long time and draining prior to cooking in order to eliminate the antinutritional compounds which help the seeds enter the sprouting process and initiate healthy growth. Several procedures are used for the soaking treatment, but most are based on overnight soaking in water[33]. In the current study, the water used during the germination treatment (up to 120 h) was changed every 24 h. The soy slurry was then processed through different steps as shown in Fig. 1. An unprocessed sample was prepared from soybeans soaked for 4 h in distilled water, drained with a resulting slurry of 5%.
Sonication treatment
-
The prepared 5% concentration soy slurry was ultrasound treated in a semi-wave ultrasound treatment chamber at 28 KHz [Ultrasound Cleaner, 10 L, 500 W capacity; Mophorn, Rancho Cucamonga, CA, USA]. Soy slurry samples of 30 mL were transferred to 50 mL conical flasks and immersed in the ultrasonic water. Table 1 explains the different ultrasound sonication treatment times and power and was carried out in the dark at room temperature (around 25 °C). After sonication, treated samples which did not require cooking were stored at 4 °C for ELISA analysis and others were frozen at −40 °C and freeze dried (Freezone Console Freeze dryer 12 L, −50 °C series; Labconco corporation, Kansas City, MO, USA) for FTIR and quality analysis. The experiments were carried out for each sample in triplicate.
Table 1. Treatment conditions of soy slurry samples.
Sample name Germination time in the dark (h) Ultrasound treatment time (min) Cooking time (min) Unprocessed 0 0 0 G96DUS0 96 0 G96DUS20 20 G96DUS40 40 G96DUS60 60 G96DUS20C10 20 10 G96DUS40C10 40 G96DUS60C10 60 G96DUS20C30 20 30 G96DUS40C30 40 G96DUS60C30 60 G96DUS20C60 20 60 G96DUS40C60 40 G96DUS60C60 60 G120DUS0 120 0 0 G120DUS20 20 G120DUS40 40 G120DUS60 60 G120DUS20C10 20 10 G120DUS40C10 40 G120DUS60C10 60 G120DUS20C30 20 30 G120DUS40C30 40 G120DUS60C30 60 G120DUS20C60 20 60 G120DUS40C60 40 G120DUS60C60 60 Sample notation: G(x)DUS(y)C(z); x is the germination duration in h, y is the ultrasound treatment time (min) and z is the cooking time (min). Cooking of ultrasound treated samples
-
Soy slurry samples from germinated soybeans and those which were ultrasound treated as detailed above were transferred to 15 mL centrifuge tubes and cooked in boiling water for different durations (10−60 min). After cooking, they were cooled to room temperature and stored at 4 °C for further experiments. Figure 1 shows the complete steps in the ultrasound cooking of the samples and Table 1 shows the nomenclature of the samples corresponding to each treatment. All tests were carried out in triplicate.
ELISA analysis
-
A 96-well Sandwich Soy ELISA kit was purchased from 3M Company, Canada and used for allergen IR analysis as detailed in Ravindran & Ramaswamy[16]. These were tested in untreated, germinated, sonicated and cooked samples both individually and in combination.
FTIR analysis
-
All samples were analyzed by Fourier transform infrared spectroscopy to study the conformational change in secondary structure of proteins. The procedure is detailed in Ravindran & Ramaswamy[16].
Quality analysis
Preparing samples for total phenolic content and antioxidant activity
-
The treated samples that were frozen were ground using a pestle and mortar, and used for extraction for total phenolic content (TPC) and antioxidant activity evaluations. The extraction process was carried out using the procedure detailed in Xu & Chang[34] with some modifications. Briefly, ~0.5 g of the grounded freeze dried samples were accurately weighed in a centrifuge tube, 5 mL of the extraction solvent (acetone : water : acetic acid = 70:9.5:0.5) was added. They were then shaken at 18× g at room temperature in an orbital shaker for 3 h and kept for another 12 h in the dark for further extraction. The supernatants were collected and stored at 4 °C for quantitative analysis.
Determination of total phenolic content (TPC)
-
The total phenolic content (TPC) was estimated using Folin-Ciocalteu assay[35] using gallic acid as the standard. TPC was expressed as milligrams of gallic acid equivalent (mg of GAE of the sample) with the help of the calibration curve.
Determination of radical DPPH scavenging activity
-
2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay was carried out according to the method of Guo et al.[36]. 0.2 ml of the extract of each sample was added to 3.8 mL of absolute alcohol solution of DPPH (0.025 g/L). Absolute alcohol of DPPH solution with no sample was taken as the control (A1). The absorbance of each sample (A2) was recorded at 515 nm using UV/VIS Spectrophotometer (VWR, Model V-3100PC, Mississuaga, Canada). Results were expressed as percentage of inhibition of DPPH Radical using the following equation:
$ {\text% }{\rm{ DPPH\; Inhibition}} = \left(\frac{A1-A2}{A1}\right)\times 100 $ Statistical analysis
-
The data were analyzed by One-way analysis of variance (ANOVA) and Tukey's test (p < 0.05) were used for data analysis using an SPSS analytical software (SPSS Inc., Chicago, USA). All tests were carried out in triplicate.
-
The IR of samples gradually reduced from the highest value (377 mg/L) in the raw unprocessed control sample to the lowest (192 mg/L) value when germination and ultrasound treatment was combined (Table 2). The results were significantly different from each other as the different treatments were combined in the samples (p < 0.05) (Table 2). About 50% reduction soy allergen IR was observed when ultrasound treatment of 60 min was used in slurries prepared from soybeans subjected to both 96 and 120 h germination period. Between the germinated samples at 96 and 120 h, the IR levels were nearly the same (302 and 303 mg/L) showing no statistical difference (p > 0.05) but demonstrated a 20% reduction in IR levels as compared with unprocessed slurry prepared from soaked soybeans without germination. This may be due the degradation of soy proteins during the germination time as a similar study carried out with whole soybeans reported an absence of major allergens in a 3-day germination period[26].
Table 2. Immunoreactivity of soy slurry samples with germination and ultrasound treatment.
Sample name Soy allergen immunoreactivity (mg/L) Percentage reduction in soy allergen immunoreactivity (%) Unprocessed 377.35 ± 2a G96DUS0 302.3 ± 0.57b[0.10] 19.9 ± 0.3b[0.10] G120DUS0 303.4 ± 9.26b[0.09] 19.6 ± 1.99b[0.09] G96DUS20 264.71 ± 4.96A[0.15] 29.9 ± 0.91A[0.15] G96DUS40 250.57 ± 0.76CD[0.18] 33.6 ± 0.58CD[0.18] G96DUS60 189.6 ± 4.22E[0.30] 49.8 ± 1.41E[0.30] G120DUS20 289.84 ± 0.23BC[0.11] 23.2 ± 0.5BC[0.11] G120DUS40 248.3 ± 9.97CD[0.18] 34.2 ± 2.26CD[0.18] G120DUS60 192.3 ± 9.57E[0.29] 49.1 ± 2.24E[0.29] Values are presented as mean ± SD (n = 3). Values with different superscripts are significantly different (p < 0.05). Lowercase letters represent immunoreactivity and percentage reduction in immunoreactivity for germination. Uppercase letters represent combinations of germination and ultrasound treatment. The values given in [ ] are the log reduction in immunoreactivity of samples. Sample notation: G(x)DUS(y)C(z); x is the germination duration in h, y is the ultrasound treatment time (min) and z is the cooking time (min). The ultrasound treatment time had a linear relationship with the allergen protein IR and therefore, as the treatment time increased, an increase in the reduction of IR was observed (IR reduction increased from 23% to 50%) solely with the help of ultrasound energy. The fact that energy from ultrasound can significantly reduce the degradation of molecules that induce IR has been earlier observed in shrimp when an ultrasound treatment with 800 W ultrasonication was used[37]. Therefore, for soy slurry samples those that were exposed to low ultrasound treatment time of 20 min showed a lower reduction when compared with that with a higher time interval of 60 min. These results are in accordance with the reports of Wu et al.[26] and Troszyńska et al.[27] who also reported similar reductions in IR with germination in cowpeas and soybeans and ascribed it to possible hydrolysation or modification of allergen proteins. Ultrasound has been used to reduce the IR of several other food products[17−19].
Influence of ultrasound - cooking on IR
-
In this study, the IR values of the soy slurry samples had a steady decreasing trend with the germination and sonication process with subsequent cooking process which increased with the cooking time (Table 3). The main difference between normal cooking and this ultrasound combination cooking process is that ultrasound can enhance the emulsifying properties of soy proteins by increasing the surface hydrophobicity of free sulfhydryl groups[38]. Due to the globular nature, the hydrophobic areas of soy proteins are embedded inside the structure making it resistant to treatments[39]. Sonication can also greatly influence the protein's structure and its aggregation characteristics[40]. The ultrasonic waves can reduce the size of these globular proteins making them susceptible to further process steps like cooking. When the molecules breakdown the hydrophobic sites that can induce IR they become more visible and easy for normal thermal treatments like cooking to lose its epitope integrity[39].
Table 3. Immunoreactivity of soy slurry samples with a combination of germination ultrasound treatment and cooking.
Sample
nameCooking
time (min)Soy immunoreactivity
(mg/L)Logarithmic cycle reduction in soy
immunoreactivity
Log(10)Unprocessed 0 377.35 ± 2.16a G96DUS20C10 10 29.67 ± 0.15b 1.10 G96DUS40C10 20.46 ± 0.03de 1.27 G96DUS60C10 19.74 ± 0.02e 1.28 G96DUS20C30 30 9.05 ± 0.06f 1.62 G96DUS40C30 4.71 ± 0.08l 1.90 G96DUS60C30 3.08 ± 0.01m 2.09 G96DUS20C60 60 7.37 ± 0.07ij 1.71 G96DUS40C60 1.92 ± 0.08n 2.29 G96DUS60C60 1.53 ± 0.02n 2.39 G120DUS20C10 10 26.56 ± 0.15c 1.15 G120DUS40C10 20.4 ± 0.18de 1.27 G120DUS60C10 18.14 ± 0.01e 1.32 G120DUS20C30 30 6.77 ± 0.03k 1.75 G120DUS40C30 3.88 ± 0.03m 1.99 G120DUS60C30 1.15 ± 0.02op 2.52 G120DUS20C60 60 6.02 ± 0.01k 1.80 G120DUS40C60 1.81 ± 0.06n 2.32 G120DUS60C60 1.03 ± 0.01op 2.57 Values are presented as means ± SD (n = 3). Values with different superxscripts are significantly different (p < 0.05). Log reduction: Log (unprocessed – processed). Sample notation: G(x)DUS(y)C(z); x is the germination duration in h, y is the ultrasound treatment time (min) and z is the cooking time (min). As can be seen from Fig. 2, for all ultrasound treatments done for 20, 40 and 60 min, a higher reduction in the level of IR was observed producing a cumulative reduction above 90%. The highest reduction was nearly 99% observed when the 60 min ultrasound treated sample was further subjected to 60 min cooking. This makes it easier to understand that the cavitation process is induced by ultrasonic energy. There are several reports which emphasis the importance of high intensity ultrasonic energy that can alter the protein structure and physicochemical characteristics of vegetable proteins especially in soy protein isolate[42−45]. These magnitudes of reduction in allergen reactivity is somewhat masked when considered in terms of percentage reduction as there is little difference between 99.0% and 99.9%. In our previous study[16], the IR reduction in soy slurry after cooking the samples for 10 to 60 min was reported along with those under intense thermal processing conditions. Cooking alone for 60 min helped to reduce the IR by 97%. This value is also only relatively slightly lower that the 99.0% or 99.9% values shown in Fig. 2. As with microbial activity when these are considered in terms of logarithmic cycle reduction, the differences become more apparent. It is advisable to consider the logarithmic scale since even small traces of allergen can cause allergenic symptoms. The overall reduction in IR was over 2.57 logarithmic cycles (99.9%) when all treatments are considered, while the reduction is about less than one cycle reduction after two treatments (germination and with ultrasound ~50%) and less than 0.3 cycle reductions (~20%) after just the germination treatment. Cooking alone for 60 min results in 1.53 long reductions, thus the combined treatment effects can be better recognized with this log reduction approach.
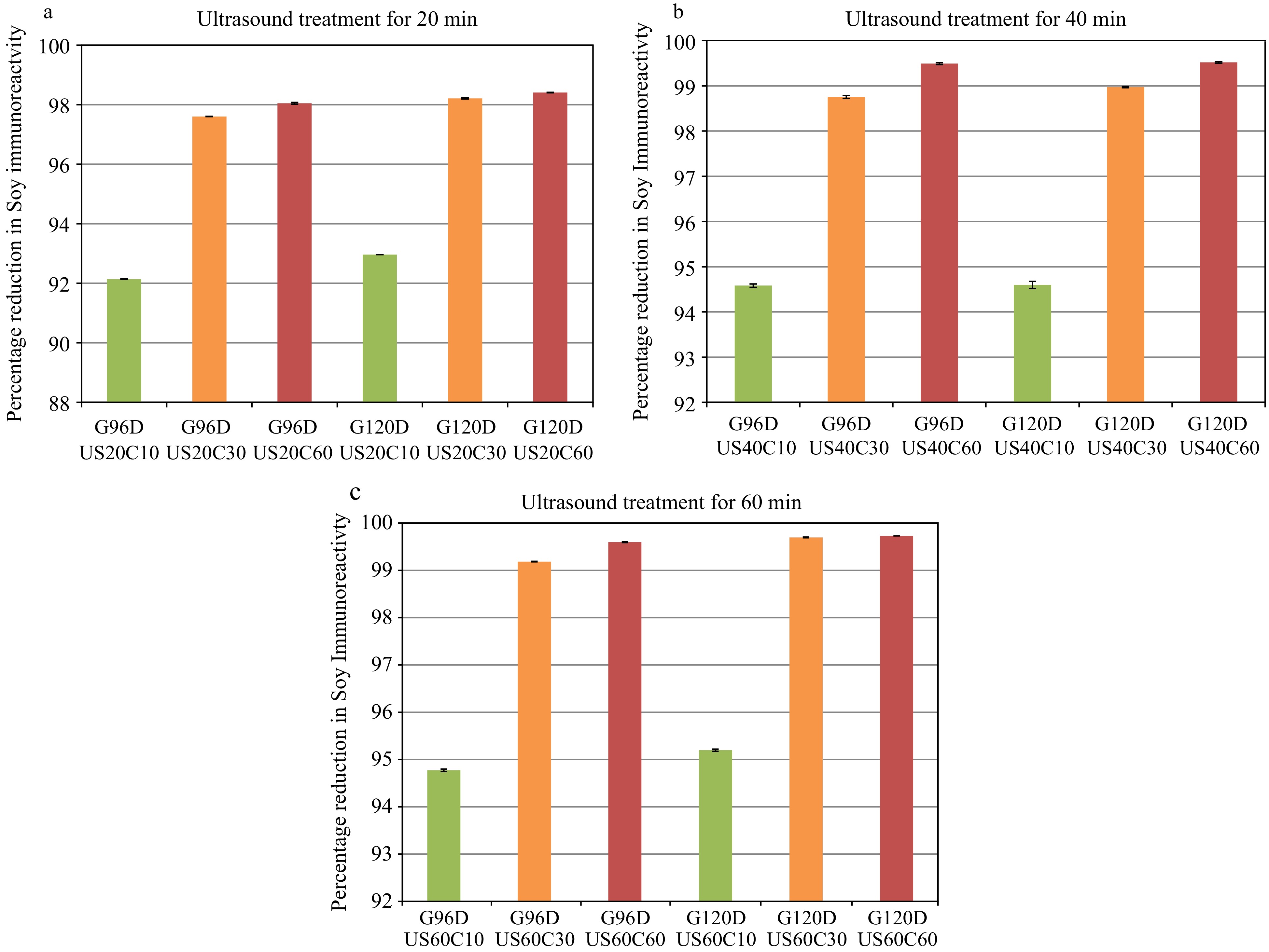
Figure 2.
(a) Percentage reduction in soy IR in samples germinated for 96 or 120 h (G96D, G120D), ultrasound treated for 20 min and cooked for 10, 30 and 60 min (C10, C30 and C60). (b) Percentage reduction in soy IR in samples germinated for 96 or 120 h (G96D, G120D), ultrasound treated for 40 min and cooked for 10, 30 and 60 min (C10, C30 and C60). (c) Percentage reduction in soy IR in samples germinated for 96 or 120 h (G96D, G120D), ultrasound treated for 60 min and cooked for 10, 30 and 60 min (C10, C30 and C60).
Overall effect of combination treatments on soy allergen IR
-
The lowest IR value of 1.03 mg/L was found with the sample obtained after 120 h dark germination plus an ultrasound treatment of 60 min at 28 kHz and final cooking at 100 °C for 60 min. The allergen activity based on IR reduced from 370 mg/L to 303 mg/L after 120 h germination time (about 19% reduction), but reduced to 192 after 60 min ultrasound treatment (representing 48% from the fresh sample; 36% from the germinated sample), and further down to 1 mg/L after 60 min cooking [(99.7% from the fresh sample; 99.7% from the germinated sample and 99.5% from the germinated ultrasound treated sample]. Thus germination by itself only accounts for a small reduction (~20%) the added successive ultrasound and cooking treatments bring them close to a 99.7% overall reduction. Thus ultrasound treatment and cooking have a great impact on allergen reactivity reduction. The difference between the allergen IR reduction between 96 and 120 h germination was relatively small and often mixed when different ultrasound treatment and cooking times were included.
Overall, when all ultrasound treatment times were compared, there was a somewhat linear relationship between allergen protein IR reduction and the treatment time, the reduction increasing with treatment time. An increase in the reduction of IR as a result of increase in ultrasound treatment time ranged from 20% to 50% on average only with the help of elevating the ultrasound energy. The energy from ultrasound can therefore significantly influence the impact on protein IR[37]. However, it was necessary to add the cooking step to increase these values to over 99% IR reduction levels. These cooking steps are basic and can easily be accomplished in home kitchens prior to consumption of these products. As previously mentioned, cooking time has a major influence on the reduction of IR of allergens.
Effect of processing on the secondary structure of proteins
-
Researchers have always focused on the evaluation of the protein-peptide groups of protein molecules with several unique absorption bands in the amide A and B, and amide I - VII specific bands[26, 46, 47]. FTIR spectra is usually used to characterize the secondary structure of proteins by demonstrated absorptions in these bands corresponding to -CO-NH- bonds with each type of secondary structure providing different C=O stretching. All soy slurry samples that showed a reduction in IR above 95% were taken for secondary structure quantification (Fig. 3).

Figure 3.
(a) FTIR spectrum of unprocessed, germinated for 96 and 120 h (G96D and G120D) and ultrasound treated for 20 min (US20) and cooked for 30 and 60 min (C30, C60). (b) FTIR spectrum of unprocessed, germinated for 96 and 120 h (G96D and G120D) and ultrasound treated for 40 min (US20) and cooked for 30 and 60 min (C30, C60). (c) FTIR spectrum of unprocessed, germinated for 96 and 120 h (G96D and G120D) and ultrasound treated for 60 min (US20) and cooked for 30 and 60 min (C30, C60).
Notable differences were specifically observed in the intensity and shape of bands near 1050 cmˉ1 for all treated samples. These were attributed to the vibration of covalent [C–O and C–C vibrations] glycosidic bonds and pyranoid ring[47]. Generally, the intensity peak and variations in these bands provide demonstration of conformational changes associated with the proteins which are assumed to arise from the denaturation of proteins from the germination, cooking and/or cooking process. High intensity ultrasound treatments did not show significant change in the secondary structure of proteins as found with cooked samples which reduced IR by nearly 99%. Based on Fig. 4, it is clear that the primary structure (α-helix) of the soy proteins were gradually reduced as the processing severity was increased. Germination for 96 and 120 h had similar profiles and had a low β-sheet percentage demonstrating similar degradation of certain proteins[26]. The percentage of random coil structure varied but generally had a higher value during the germination stages (96 and 120 h).
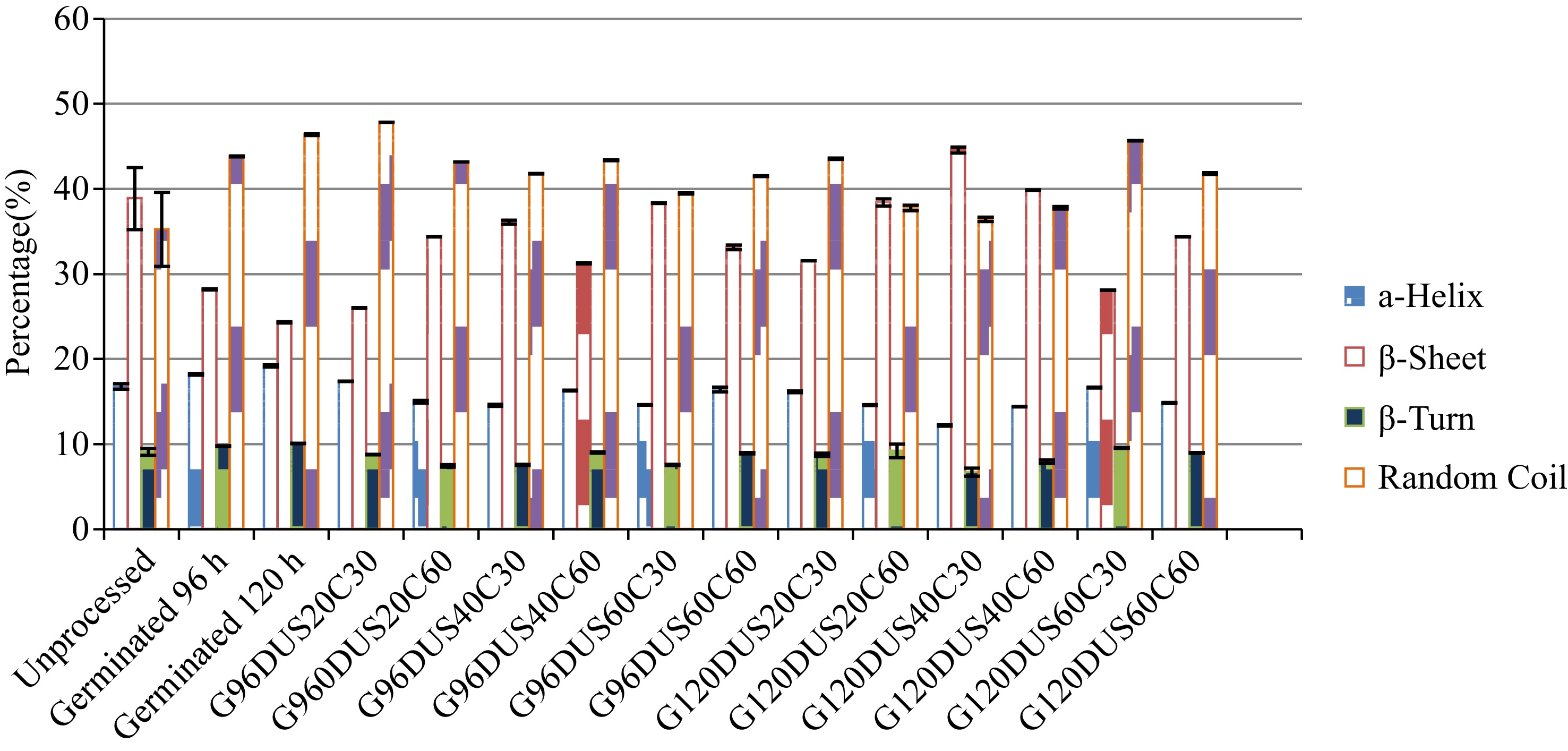
Figure 4.
Percentage of secondary structure of soy slurry samples after different treatments: unprocessed, germinated for 96 and 120 h (G96D and G120D) and ultrasound treated for 20 min (US20), 40 min (US40), 60 min (US60) and cooked for 30 and 60 min (C30, C60).
Effect of germination and sonication treatment on the TPC
-
The total phenolic content (TPC) significantly increased from 2.53 to 12.7 mg of GAE/g in germinated soy slurry samples (96 and 120 h) without any processing steps (Table 4). Further, an increase in the level of TPC was observed as the ultrasound treatment was induced making the highest total phenolic content in samples to be nearly 15 mg of GAE/g (14.7 ± 0.64) when 60 min of ultrasound sonication was provided to slurry made from soybeans germinated for 120 h in the dark. Addition of cooking process made the TPC levels to drop to 6.2 mg as reflected in the sample that was cooked for the maximum cooking time of 60 min. The values of TPC varied and moreover showed a decreasing trend across the different samples previously treated with ultrasound.
Table 4. Total phenolic and DPPH inhibition values of soy slurry samples.
Sample Germination time (h) Ultrasound treatment Cooking time at 100 °C
(min)TPC
(mg of GAE/g)DPPH radical
scavenging activity (%)Unprocessed 0 0 0 2.53 ± 0.03a 20.9 ± 0.03a Germinated 96 h 96 0 0 12.5 ± 0.3b 54.4 ± 0.14b Germinated 120 h 120 0 0 12.7 ± 0.11b 55.2 ± 0.07bc G96D US20 96 20 0 14.3 ± 0.07ab 57.2 ± 0.05d G96D US40 40 0 14.4 ± 0.06ab 59.8 ± 0.05e G96D US60 60 0 14.5 ± 0.03ab 60.8 ± 0.04f G96D US20C10 20 10 9.3 ± 0.07cd 45.8 ± 0.06h G96D US40C10 40 10 9.2 ± 0.27cd 43.3 ± 0.78i G96D US60C10 60 10 9.9 ± 0.02e 43.1 ± 0.04i G96D US20C30 20 30 7.6 ± 0.02 48.9 ± 0.15j G96D US40C30 40 30 7.5 ± 0.04gh 43.9 ± 0.01i G96D US60C30 60 30 7.3 ± 0.03gh 33.1 ± 0.07k G96D US20C60 20 60 7.3 ± 0.17gh 36.6 ± 0.04kl G96D US40C60 40 60 7.2 ± 0.04gh 36.4 ± 0.06kl G96D US60C60 60 60 7.6 ± 0.44gh 33.4 ± 0.01k G120D US20 120 20 0 14.6 ± 0.11ab 56.4 ± 0.01bc G120D US40 40 0 14.6 ± 0.22ab 59.4 ± 0.03e G120D US60 60 0 14.7 ± 0.64ab 66.6 ± 0.15g G120D US20C10 20 10 10.6 ± 0.24ef 37.4 ± 0.05ij G120D US40C10 40 10 9.2 ± 0.06cd 33.3 ± 0.03k G120D US60C10 60 10 9.1 ± 0.01cd 26.7 ± 0.01m G120D US20C30 20 30 8.8 ± 0.09g 34.5 ± 0.01klm G120D US40C30 40 30 8.5 ± 0.02g 28.2 ± 0.04n G120D US60C30 60 30 7.5 ± 0.16gh 26.4 ± 0.07m G120DUS20C60 20 60 8.5 ± 0.06g 29.1 ± 0.04no G120D US40C60 40 60 7.3 ± 0.05gh 26.2 ± 0.01m G120D US60C60 60 60 6.2 ± 0.03h 24.7 ± 0.01op Values are presented as means ± SD (n = 3). Values with different superscripts are significantly different (p < 0.05).
Sample notation: G(x)DUS(y)C(z); x is the germination duration in h, y is the ultrasound treatment time (min), and z is the cooking time (min).The increase in value of TPC in germinated legumes has been previously reported in several studies[26,36,48]. In a study of cowpeas the TPC content was increased from 12% to 136% by germination under dark conditions[48]. During germination, certain endogenous enzymes are activated and can enhance the polyphenol contents in legumes. Even though the slurry from germinated soybeans was very dilute (5%) the increase in the TPC content was observed more than 100%. The influence of processing methods like ultrasound can impact the bioactive content. Ultrasound processing is widely used for extraction of phenolic content in food processing industry[49]. High intensity ultrasound energy greater than 28 kHz can increase the functional and quality properties of the vegetable proteins making them available and finally increasing its level inside the soy slurry solution. This can be due to the cavitations process occurring that brings out embedded polyphenols inside the protein molecules. In case of soy, large amounts of polyphenol were extracted when ultrasound treatment combined with normal solvent extraction process was used[50].
Germination and sonication treatment on the radical scavenging activity
-
The radical scavenging activity was found similar to TPC values as it increased to a 50% level in germinated samples and later on increased when ultrasound treatments of 20, 40 and 60 min were applied (Table 4). A sudden decrease in the value of polyphenol was observed even with a cooking time of 10 min and the level remained the same across the cooking time of 30 to 60 min. The DPPH inhibition rate decreased from a value of 60.8% to the lowest of 24.7% as cooking process was added. Germination can result in the enhancement of several antioxidants in legume seeds due to secondary metabolites like anthocyanins and flavanoids. It also increases the potent properties of the bioactive compounds already present in the product[51,52]. When ultrasound is added to this step the all the bioactive compound embedded in the storage molecules comes out causing the level to increase. Ultrasound is an environmentally friendly technology which can promote high yield of antioxidant extraction.
-
The combined effect of germination, ultrasound treatment and cooking reduced the soy allergen IR to ~1 mg/L levels. Since the accepted range of soy allergens in food is 50 to 80 mg/L, this processing maybe useful in bringing the concentration to within acceptable IR levels. Germination alone reduced the IR only by 20% but resulted in a 150% increase in TPC and radical scavenging activity. When combined with ultrasound treatment, IR reduction increased to 50% but also showed a major increase in the total phenolic and DPPH activity values. When followed by cooking, the overall reduction in IR reached nearly 99%. Soy protein are usually added to food in a low amount (up to 3%) and at these levels, the suggested treatments could bring the IR to sufficiently low levels and reduce the allergen risk.
The actual amount of residual allergen to cause allerginicity may vary depending on an individuals' allergen sensitivity. Therefore, it is desirable that these results be confirmed with animal or human trials to test their influence on allerginicity before adoption in food formulations. Nevertheless, processing concepts that reduce the allergen concentrations to low levels is a step in the right direction for further studies on allerginicity.
This research was supported by a grant from the Natural Sciences and Engineering Research Council of Canada - Collaborative Research Development Grant with Industry.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Ravindran A, Ramaswamy HS. 2023. Effect of sonication - cooking on the immunoreactivity of soy slurry from germinated soybeans. Food Innovation and Advances 2(2):60−68 doi: 10.48130/FIA-2023-0008
Effect of sonication - cooking on the immunoreactivity of soy slurry from germinated soybeans
- Received: 10 January 2023
- Accepted: 20 February 2023
- Published online: 04 April 2023
Abstract: Soy proteins are globular in nature and are resistant to denaturation with lower intensity thermal treatments like cooking. Likewise, germination can also alter the protein structure through the activity of various enzymes and sonication can disrupt the molecular structure through cavitation and other ultrasound effects, and contribute to some reduction in immunoreactivity (IR) of allergens. This study evaluated the effects of germination and sonication pretreatment in combination with common cooking on lowering the soy allergen IR. Germination was carried out for up to 120 h and ultrasound sonication treatments were given for 20, 40 and 60 min at room temperature. Cooking at 100 oC was carried out for 10 to 60 min. The soy allergen IR was evaluated using a commercial sandwich ELISA kit. The combined action of germination, sonication and cooking helped to reduce the soy allergen IR to single digit mg/L levels from the nearly 400 mg/L initial level in the 5% soy slurry (> 99% reduction). These levels are lower than the reported threshold values of soy allergens in foods. In addition, the germination and ultrasound process was shown to reduce the anti-nutritional properties and enhance the phenolic and radical scavenging activity by over 50%.
-
Key words:
- Allergen /
- Soybean /
- ELISA /
- Ultrasound /
- Cooking Immunoreactivity /
- Quality /
- FTIR