-

Figure 1.
AA induces ferroptosis in hepatocytes at different levels. (a) L02 and (b) AML12 were treated with indicated AA for 24 h. The relative viability of cells was determined by crystal violet staining. Cell viability obtained after (c) L02 and (d) AML12 cells were pretreated with 1 μM Lip-1 for 6 h and then treated with the indicated AA for 24 h. The data are presented as mean ± SD. ** p < 0.01 versus the control group. # p < 0.05 versus the AA treatment group. Lip-1 blocked AA-induced upregulation of Fe2+, quantified in (e) L02 and (f) AML12 cells using the FerroOrange kit. Lip-1 blocked AA-induced upregulation of lipid peroxide levels, quantified in (g) L02 and (h) AML12 cells using the Liperfluo kit. Values are presented as mean ± SD. * p < 0.05, ** p < 0.01 and *** p < 0.001 versus the control group. # p < 0.05, ## p < 0.01 and ### p < 0.01 versus the AA treatment group.
-

Figure 2.
AA up-regulates TfR1 expression to transport more iron into cells, while also down-regulating GPX4. Western blotting analysis of TfR1 and GPX4 were performed 24 h after AA treatment in (a) L02 and (b) AML12 cells. The relative intensity of TfR1 was analyzed after AA treatment. Values are presented as means ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001 versus the control group.
-
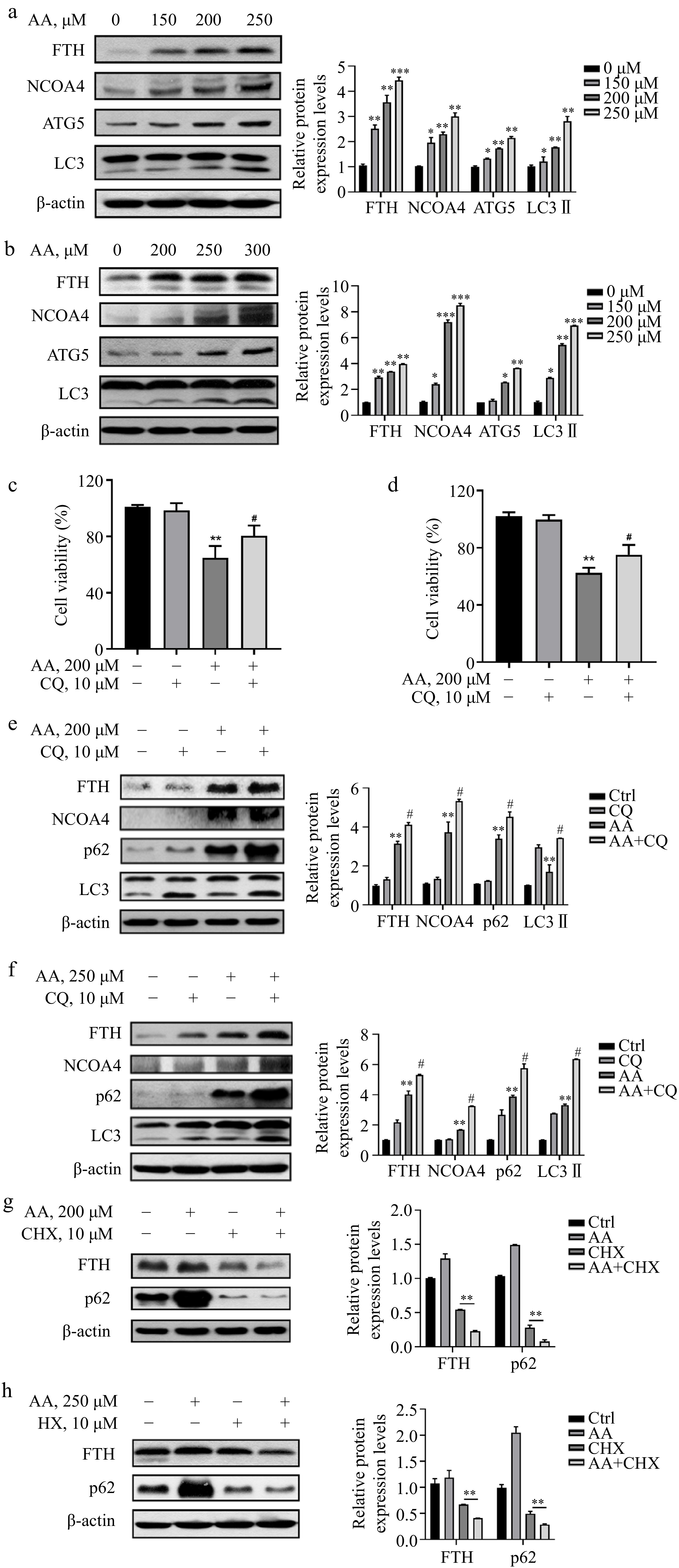
Figure 3.
AA increases free iron levels in labile iron pools by activating ferritinophagy. AA activated the expression of ferritinophagy-related proteins. The cells were treated with AA for 24 h, and the expression of FTH, NCOA4, ATG5 and LC3 in (a) L02 and (b) AML12 cells were detected by Western blotting. Inhibition of autophagy alleviated AA-induced ferroptosis. Cells were treated with AA and/or the autophagy flux inhibitor CQ, and the cell viability of (c) L02 and (d) AML12 was measured. Expression of ferritinophagy-related proteins. The cells were treated with AA and/or CQ for 24 h, and the lysates of (e) L02 and (f) AML12 cells were subjected to Western blot analysis to analyze the changes of FTH, NCOA4, p62 and LC3 protein expression. Expression of FTH and p62 after inhibition of protein synthesis. After the cells were treated with CHX, they were treated with AA for a certain period of time. Changes in FTH and p62 expression in (g) L02 and (h) AML12 cells were detected by Western blot to further demonstrate the activation of ferritinophagy. β-actin served as a loading control. The intensity of each protein expression band was quantified by densitometry normalized to β-actin. * p < 0.05, ** p < 0.01 and *** p < 0.001 versus the control group. # p < 0.05 and ## p < 0.01 versus the AA treatment group.
-
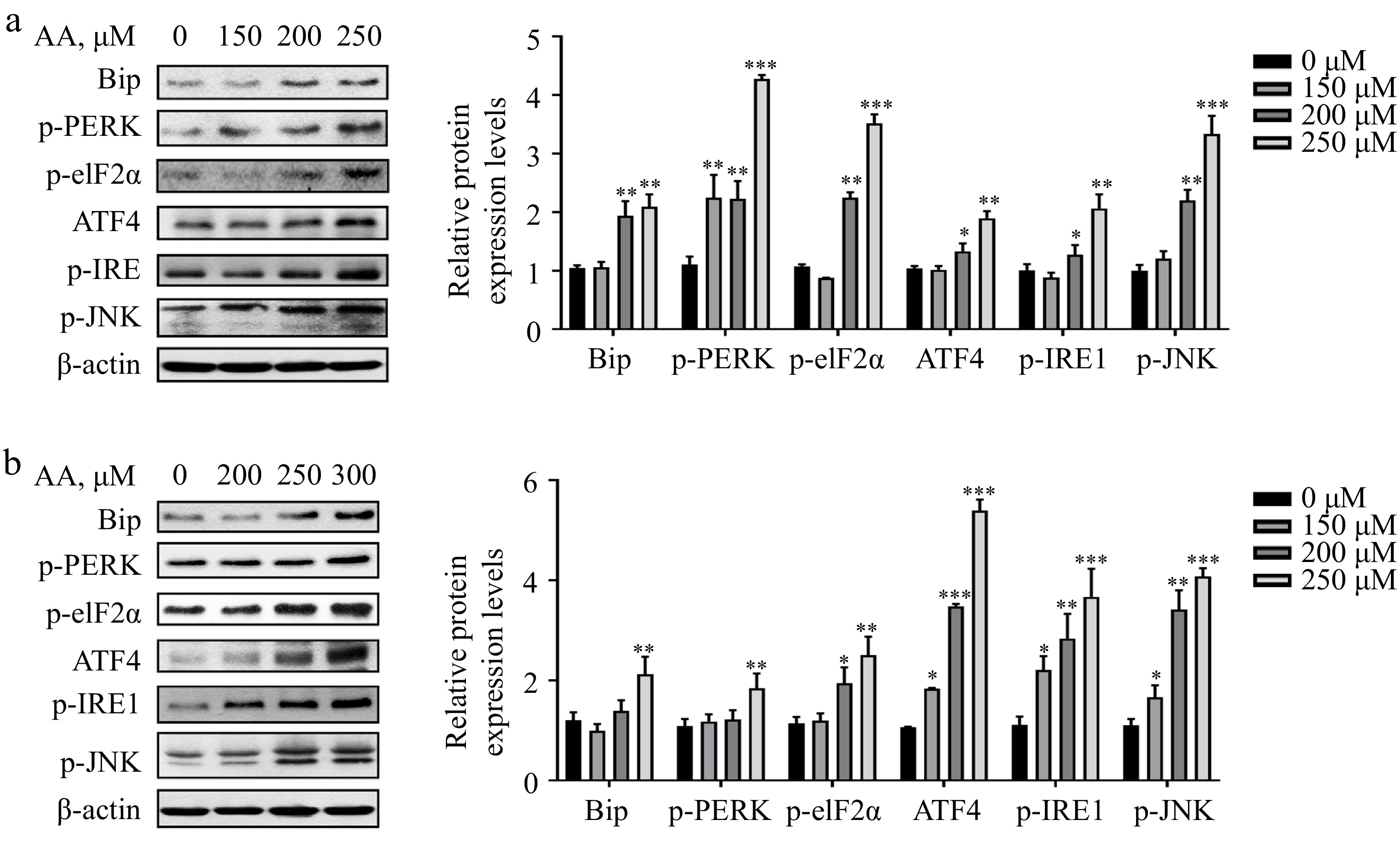
Figure 4.
Activated ER stress is involved in AA-induced ferroptosis. Western blotting analysis of Bip, p-PERK, p-eIF2α, ATF4, p-IRE1α and p-JNK were performed in (a) L02 and (b) AML12 cells. Bar chart shows relative quantitative levels of each protein. β-actin was used as a loading control. Data are presented as mean ± SD. * p < 0.05, ** p < 0.01 and *** p < 0.001 versus the control group.
-

Figure 5.
AA triggers lethal ferritinophagy by activating the PERK signaling pathway. Cell viability of (a) AML12 and (b) L02 cells pretreated with 1 μM GSK for 2 h and then stimulated with AA for 24 h. (c) Effect of knockdown of PERK on AA-induced ferroptosis in L02 cells. Western blotting analysis of p-eIF2α, ATF4, FTH, NCOA4, p62 and LC3 were performed in (d) AML12 and (e) L02 cells treated with GSK or PERK-si and AA. The data are presented as mean ± SD. ** p < 0.01 and *** p < 0.001 versus the control group. # p < 0.05 and ## p < 0.01 versus the AA treatment group.
-

Figure 6.
Inhibition of IRE1 instead activates the PERK signaling pathway, which in turn exacerbates AA-induced ferritinophagy. Cell viability of (a) AML12 and (b) L02 cells pretreated with 25 μM 4μ8C for 2 h and then stimulated with AA for 24 h. (c) Effect of knockdown of IRE1 on AA-induced ferroptosis in L02 cells. Western blotting analysis of p-eIF2α, ATF4, FTH, NCOA4, p62 and LC3 were performed in (d) AML12 and (e) L02 cells treated with GSK or PERK-si and AA. The data are presented as mean ± SD. ** p < 0.01 and *** p < 0.001 versus the control group. # p < 0.05 and ## p < 0.01 versus the GSK group or PERK-si group.
-

Figure 7.
Potential signaling pathways for AA-induced ferroptosis in hepatocytes. The polyunsaturated fatty acid AA upregulates the expression of TfR1 on the cell membrane surface and activates ferritinophagy, thereby increasing the level of Fe2+ in the cellular labile iron pool. In addition, AA-induced ER stress is located upstream of ferritinophagy, and its two branches, PERK and IRE1 signaling pathways, play different roles in regulating ferritinophagy.
Figures
(7)
Tables
(0)