-
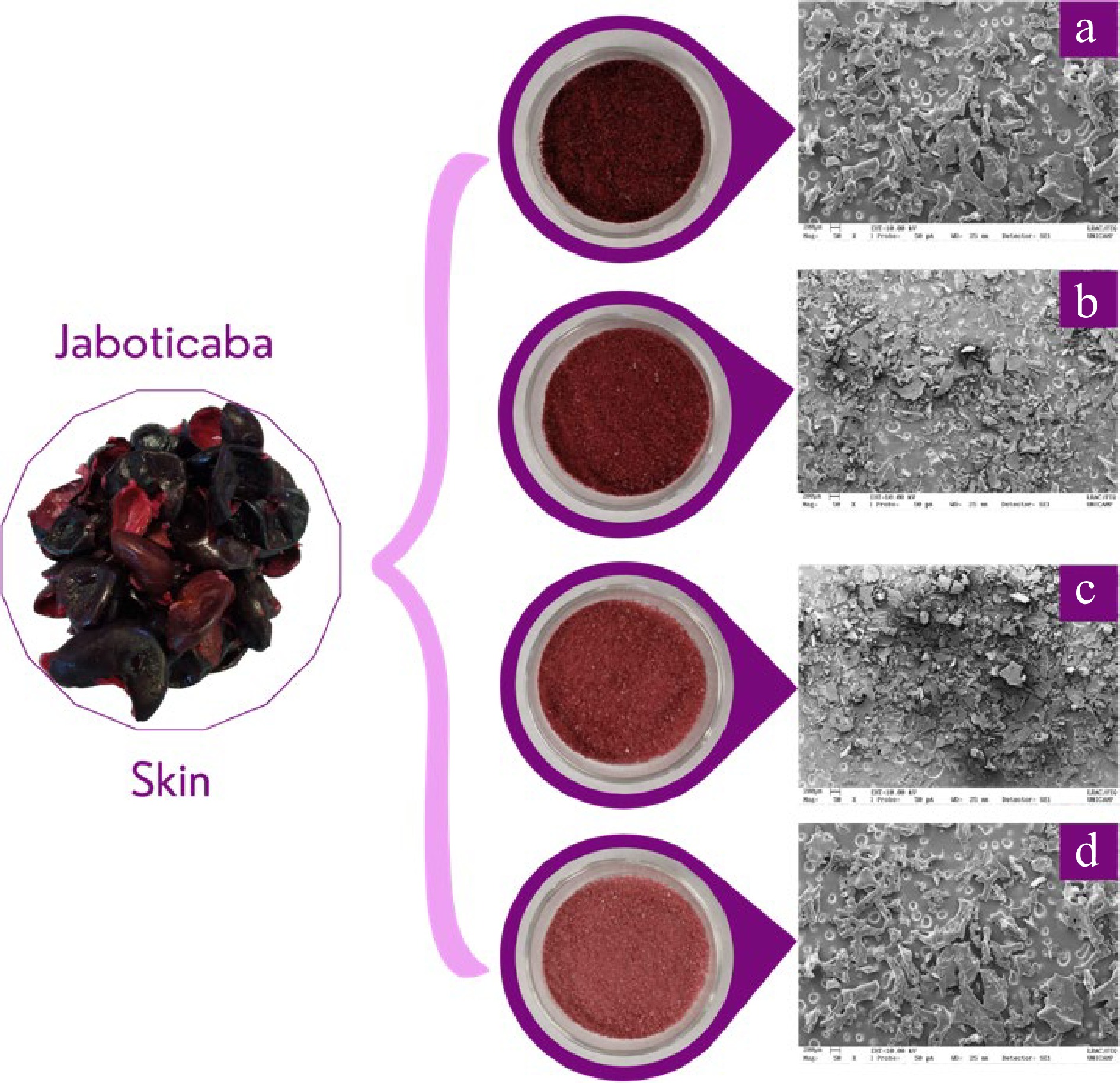
Figure 1.
Pictures of the four prepared formulations containing jaboticaba peel pulp and the OSA-modified starch Capsul® at different concentrations (w/w): containing 10%, 15%, 20%, and 25% of Capsul®.
-
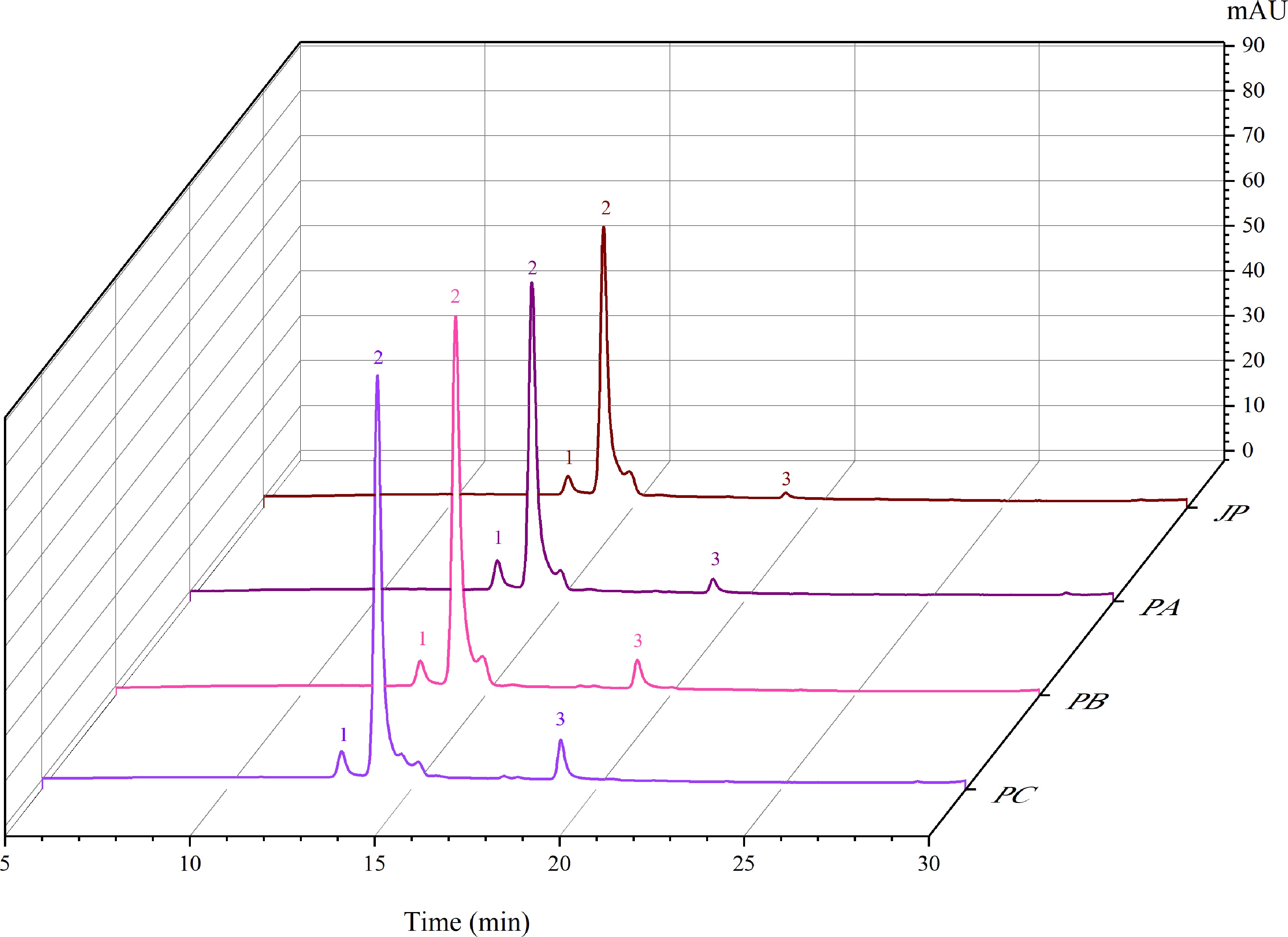
Figure 2.
Chromatograms represent the anthocyanins profile of the jaboticaba pulp (JP), jaboticaba powder with 10% of adjuvant (PA), jaboticaba powder with 15% of adjuvant (PB), jaboticaba powder with 20% of adjuvant (PC), jaboticaba powder with 25% of adjuvant (PD). Peak 1 refers to delphinidin 3-glucoside. Peak 2 refers to cyanidin 3-glucoside. Peak 3 is peonidin 3-glucoside.
-
Peaka Rt (min)b λmax (nm)b MS-MS
data (m/z)Identification 1 14.2 520 463 Delphinidin 3-glucoside 2 15.2 520 449 Cyanidin 3-glucoside 3 20.4 519 301 Peonidin 3- glucoside a Numbered according to the chromatograms shown in Fig. 1. b Solvent: gradient of 5% formic acid in methanol. Table 1.
Chromatographic and mass spectrometry characteristics of anthocyanins from jaboticaba skins using HPLC-DAD-MS/MS.
-
Samples Anthocyanins
expressed as
C3G* (µg/mL)Anthocyanins
expressed as
C3G* (µg/mL)
after digestion
in vitroBioaccessible anthocyanins**
(%)JP 257.95a ± 22.8 14.68b ± 0.75 6.03 PA 132.25b ± 5.4 12.20b ± 1.21 8.72 PB 119.54c ± 5.8 17.96a± 0.24 9.58 PC 110.16d ± 1.4 8.26c ± 0.77 5.30 PD 94.30e ± 4.1 9.53c ± 0.60 5.64 Different letters on the same column represent values different from each other (p < 0.05). * Cyanidin 3-glucoside. ** Bioaccessible anthocyanins (%) = Anthocyanins concentration after in vitro digestion × 100 / Initial concentration of anthocyanins. Table 2.
Determination of anthocyanins before and after the simulated digestion process (in vitro) of jaboticaba skins pulp (JP), jaboticaba powder with 10% of OSA modified-starch (PA), jaboticaba powder with 15% of OSA modified-starch (PB), jaboticaba powder with 20% of OSA modified-starch (PC), and jaboticaba powder with 25% of OSA modified-starch (PD).
-
Samples Initial Final Bioaccessible AA* (%) ABTS
(µM TE/g)PA 12.02a ± 1.47 4.85b ± 0.10 40.39 PB 11.48a ± 0.46 5.68a ± 0.67 49.46 PC 9.16b ± 0.90 4.03c ± 0.34 44.00 PD 9.99b ± 0.90 4.34c ± 0.54 43.49 ORAC
(µM TE/g)PA 122.92b ± 15.22 71.17c ± 9.56 57.90 PB 157.00a ± 12.07 122.62a ± 12.9 78.10 PC 132.48b ± 19.10 81.70c ± 6.54 61.67 PD 147.93ab ± 21.67 99.01b ± 5.43 66.93 Different letters on the same column represent values different from each other (p < 0.05). * Bioaccessible AA (%) = Antioxidant activity after in vitro digestion × 100 / Initial antioxidant activity. Table 3.
Determination of antioxidant activity before and after the simulated digestion process (in vitro) of jaboticaba skins pulp (JP), jaboticaba powder with 10% of OSA modified-starch (PA), jaboticaba powder with 15% of OSA modified-starch (PB), jaboticaba powder with 20% of OSA modified-starch (PC), and jaboticaba powder with 25% of OSA modified-starch (PD).
Figures
(2)
Tables
(3)