-
Dyes are mostly used in the food sector to prevent color loss during product preparation and storage. However, investigations on the safety of utilizing these colors in meals began in the 1950s and have raised concerns[1]. On the other hand, color is the central sensory element related to foodstuff appearance, affecting consumers' overall acceptance of a product. The literature has determined that color is responsible for 62%−90% of the assessment of a product by the food customer[2]. Hence, the search for the application of natural pigments in the food industry is emphasized. Still, some challenges are presented to this replacement on a large scale due to stability issues. The use of dyes aids in keeping homogeneous color, allowing the manufacture of products rich in shades. Besides promoting appealing appearance aspects of food after processing, natural pigments improve food safety and nutritional quality due to their proven biological effects[3].
Besides being known as bioactive compounds, Anthocyanins are also powerful pigments found in fruits, such as jaboticaba, and vegetables, and their regular ingestion is linked with a lower incidence of age-associated illness and neurodegenerative diseases[4]. In recent years, there has been a growing interest in clarifying the metabolism of bioactive compounds, particularly phenolics such as anthocyanins. Research has shown that the biological effects attributed to phenolic compound uptake are primarily due to the metabolites transformed during the digestion process[5−10]. Allied with that, changes in lifestyle and food consumption due to a growing awareness of the relation between diet and health have resulted in an increased search for new and healthier foodstuffs by the average consumer. More natural food containing natural bioactive biomolecules is a global trend related to food production[11].
Jaboticaba fruit is an excellent source of phenolic compounds and contains anthocyanins, which are known to possess antioxidant, anti-inflammatory properties, and anti-diabetic as well as other remarkable biological effects[7−10,12]. It is a native plant from Brazil, found uppermost in the Atlantic Forest Biome, which produces fruits suitable for fresh consumption and industrial processing. The use of jaboticaba fruits in the artisanal manufacture of sweets, liqueurs, fermented beverages, and vinegar is very popular. Yet, the offer of jaboticaba processed products in the Brazilian market is nevertheless restricted[13].
The jaboticaba skins are purple in color, thick, and have an astringent taste, covering a sweet and gelatinous white pulp. The skins have a composition of dietary fibers, minerals, sugars, free phenolic compounds such as ellagitannins and gallic acid derivatives, and high concentrations of anthocyanins[14,15]. However, jaboticaba skins are often discarded during food preparation as waste.
The transformation of anthocyanins from jaboticaba throughout the digestion process has not yet been extensively investigated, although some studies have already studied anthocyanin's bioaccessibility[7,16]. Therefore, studies to elucidate the jaboticaba behavior are desirable and could be conducted by researchers to apply more efficiently into food products to enhance nutritional and functional aspects.
The stability of anthocyanins and their color are related to environmental aspects such as light intensity, temperature increase, oxygen, and certain enzymes commonly associated with processing food. This way, some technologies have been reported as allies to stabilize these natural pigments[3].
The use of methods to encapsulate anthocyanins in a process that allows the transformation of a liquid or paste into a powder form facilitates their handling, transport, storage, dosage, application, and incorporation as ingredients into food[2]. Spray drying has been widely used to encapsulate thermally sensitive compounds since the evaporation of the water from the material occurs quickly, despite the use of relatively high temperatures during the process[17]. Freeze-drying is also suitable for microencapsulating bioactive compounds, leading to higher-quality products. The microencapsulation of material with high levels of anthocyanins by freeze-drying usually results in products with greater stability during storage, preserving their antioxidant properties[18−20].
As wall material, modified starch with octenyl succinic anhydride (OSA) is attractive due to incorporating a hydrophobic group into the starch molecule, promoting an interaction at the oil and water interface[20−24]. The OSA-modified starch is also capable of maintaining microbiological stability due to the low water activity of the powder's products[25,26]. In this context, Capsul® is a food-grade modified starch obtained from waxy maize and employed in the microencapsulation process of vitamins, condiments, and flavors. The chemical modification of this adjuvant consists of partial hydrolysis and the addition of octenyl succinate to the starch molecules.
Therefore, the main goal of the present study was to determine the bioaccessibility of anthocyanins in jaboticaba powder samples to evaluate the effects of the OSA-modified starch concentration (Capsul®) using a static in vitro model of digestion over the anthocyanin’s contents and their antioxidant activities.
-
Myrciaria jaboticaba (Vell.) O. Berg fruits, popularly known as Sabará, were manually peeled at room temperature. Afterward, the peels were triturated with water in a proportion of 50%/50% (w/w) using a blender (Philco PH900, 1200W, Brasil), filtered using a fabric filter, and frozen at −18 °C.
Four different formulations were produced by mixing the jaboticaba peel pulp and the OSA-modified starch Capsul® (EU classification: Food Additive E1450, complies with regulation EC 1333/2008, Ingredion Brasil Ingredientes, Brazil) at different concentrations (10%, 15%, 20%, and 25% (w/w)) using a magnetic stirrer (AJ Micronal, AJX-PA, Brasil), 5 min/100 g. The OSA-modified starch concentration was selected based on a previous preliminary test. The goal was to improve powder formation and quality using a low adjuvant concentration.
Freeze-drying process
-
The preparations were deposited in aluminum reservoirs and kept at −18 °C for 72 h (Freezer model CRD37, Consul, Brazil) before the freeze-drying procedure. The process was performed in a bench-scale freeze-drier at −30 °C and pressure of 0.5 mmHg for 72 h (L101 LIOTOP, São Carlos, Brazil). The powder obtained for each formulation was withdrawn from the aluminum reservoirs, crushed using a stainless-steel spatula, sieved using a domestic filter with a 1 mm opening, and kept in glass tubes for additional study at a temperature of −18 °C.
Bioaccessibility
-
The jaboticaba skin pulp (JP) and the jaboticaba powder samples with 10%, 15%, 20%, and 25% were digested according to the method of Chitchumroonchokchai & Failla[27]. The in vitro simulated digestion was begun with the mixture of 1 g of each sample with 10 mL of salt solution (NaCl: 120 mol·L−1, CaCl2 6 mmol·L−1, KCl 5 mmol·L−1) followed by the addition of 6 mL of artificial saliva solution containing α-amylase (106 U/mL) (Sigma® A3176), and the oral phase finished with incubation in an orbital shaker at 150 rpm, 37 °C for 10 min. Next, the gastric phase was started with the pH regulation for 2.5 with HCl 1 M, added 2 mL of pepsin (Sigma® P7000; 50,000 units·mL−1 in HCl 100 mM), and then complete the volume for 40 mL to incubated at 37 °C, 150 rpm for 1 h. Considering the intestinal and last phase, the pH was adjusted to 6.0 with 1 M NaHCO3. Subsequently, porcine and ovine bile solution (3 mL; Sigma® B8381; 40 mg·mL−1 in 100 mM NaHCO3), 4,000 U·mL−1 of porcine pancreatin (Sigma® P1750), and 1,000 U·mL−1 of lipase from porcine pancreas (Sigma® L3126) were added into the samples, modifying the pH for 6.5. The volume was completed until 50 mL afore the incubation at 37 °C, 150 rpm for 2 h. The final step was to submit the samples into a centrifuge for 1 h at 6,000 rpm and 4 °C since the acquired supernatant contained the bioaccessible anthocyanins. Samples from each stage of digestion were separated to quantify the anthocyanins and evaluate the antioxidant activity.
HPLC analysis of the anthocyanins
-
Anthocyanins were extracted from the jaboticaba skin pulp (JP) samples (5 g) and jaboticaba powder samples (2 g) formulated with 10%, 15%, 20%, and 25% of OSA-modified starch Capsul®. After the simulated digestion process, bioaccessible anthocyanins were also determined for all samples (anthocyanins from JP and each powder formulation). Acidified methanol (0.5% HCl), more precisely, 75 mL, together with an ultrasonic probe at 80 W of potency and for 3 min, were used to extract anthocyanins. After that, a vacuum bomb filtered the slurry, and a rotary evaporator concentrated the solution under 38 °C. The extracts were diluted in water containing 5% formic acid/methanol (85:15, v/v) before HPLC evaluation to adequate with the phase gradient used in the method.
The anthocyanin separation and identification were conducted as presented by De Rosso & Mercadante[28]. The anthocyanins from all samples, in triplicate, were quantified by HPLC-DAD established with optimized conditions of chromatography with C18 Shim Pack column at 28 °C, using a five-point analytical curve of cyanidin 3-glucoside (0.5−10.0 mg/mL), r2 = 0.998; the limit of detection was 0.05 mg/mL, and the limit of quantification was 0.1 mg/mL.
Antioxidant activity
-
To obtain the antioxidant extracts from the samples (same as the ones used to quantify anthocyanins), 30 mL of 80% cold acetone was added to each sample, followed by agitation in a magnetic stirrer for 15 min, filtration of the slurry. This process was repeated twice, and the samples were concentrated in a rotary evaporator under 40 °C. ORAC assay was performed to determine the antioxidant activity against the peroxyl radical (ROO∙), which is based on monitoring the fluorescence decay throughout the effect of the hydrophilic extract or standard (Trolox) on those results from ROO∙ induced oxidation of fluorescein[29]. The method was conducted using a 96-well microplate with fluorescein (61 μM) prepared in phosphate buffer 75 mM, pH 7.4, AAPH solution (19 mM) in phosphate buffer, hydrophilic extract, or Trolox (50 μM) in phosphate buffer. The microplate was preincubated for 10 min before adding AAPH. Afterward, the fluorescence signal was examined each minute at the reader (excitation: 485 ± 20 nm; emission: 538 ± 20 nm) for 1 h. ABTS+ radical assay was determined by monitoring the absorbances of the samples at 734 nm homogenized with a diluted solution of ABTS+ (7 mM) and compared with a Trolox standard curve[30] previously determined. The results were expressed as μmol of Trolox equivalent/g of sample.
Statistical analysis
-
All the analyses were realized in triplicate samples; the results were expressed as the mean ± standard deviation (SD); to enable comparisons, the ANOVA was utilized to detect differences among the samples, and the differences were considered significant at p < 0.05. Statistica 14.0 software was used to process the data analyses.
-
Four different powders were produced using jaboticaba skins pulp (Fig. 1) and OSA-modified starch Capsul® at different concentrations (10%, 15%, 20%, and 25% (w/w) (Fig. 1). Chromatographic and mass spectrometry characteristics of anthocyanins from jaboticaba skins used to produce the powders are presented in Table 1 and Fig. 2.
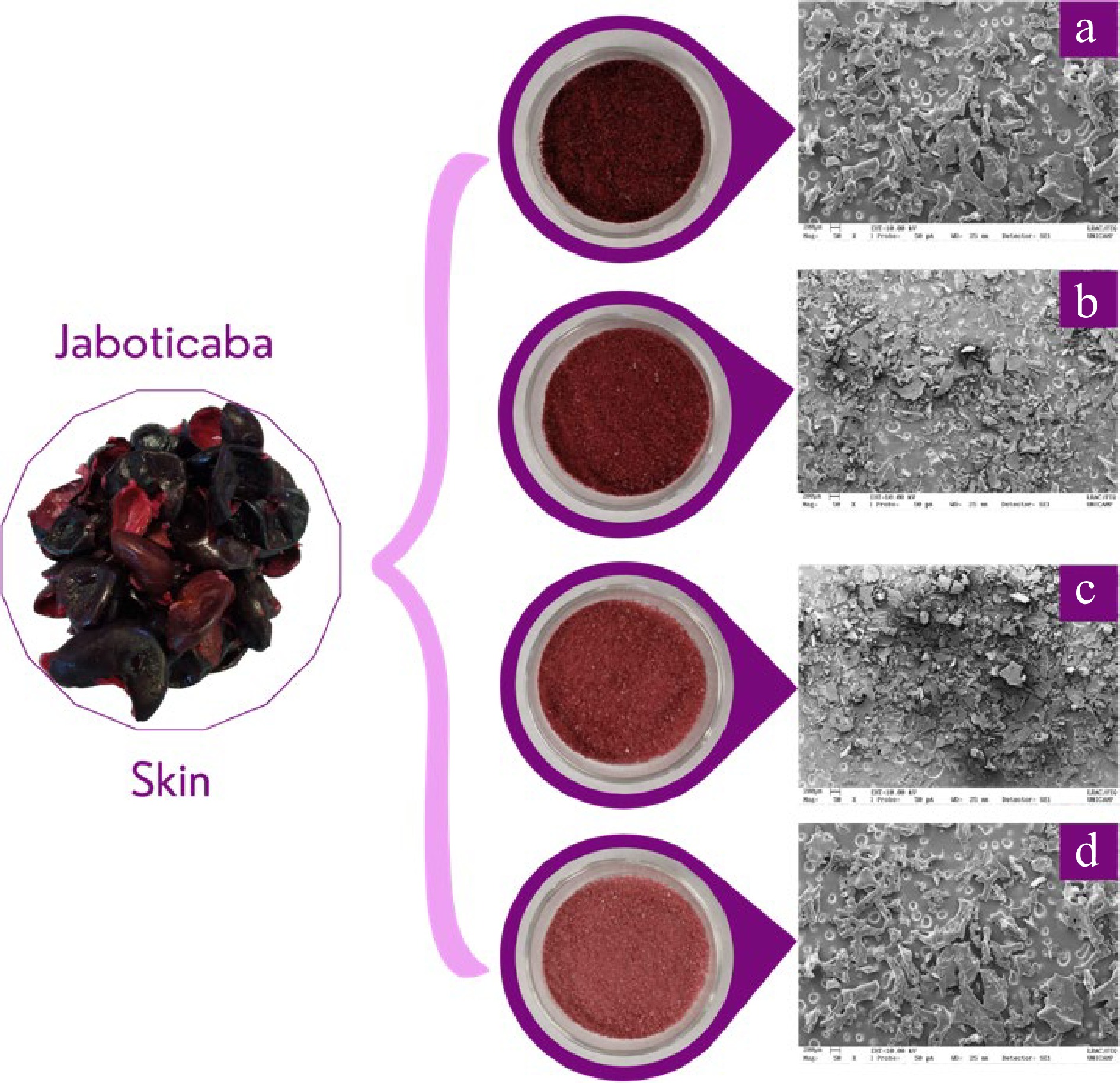
Figure 1.
Pictures of the four prepared formulations containing jaboticaba peel pulp and the OSA-modified starch Capsul® at different concentrations (w/w): containing 10%, 15%, 20%, and 25% of Capsul®.
Table 1. Chromatographic and mass spectrometry characteristics of anthocyanins from jaboticaba skins using HPLC-DAD-MS/MS.
Peaka Rt (min)b λmax (nm)b MS-MS
data (m/z)Identification 1 14.2 520 463 Delphinidin 3-glucoside 2 15.2 520 449 Cyanidin 3-glucoside 3 20.4 519 301 Peonidin 3- glucoside a Numbered according to the chromatograms shown in Fig. 1. b Solvent: gradient of 5% formic acid in methanol. 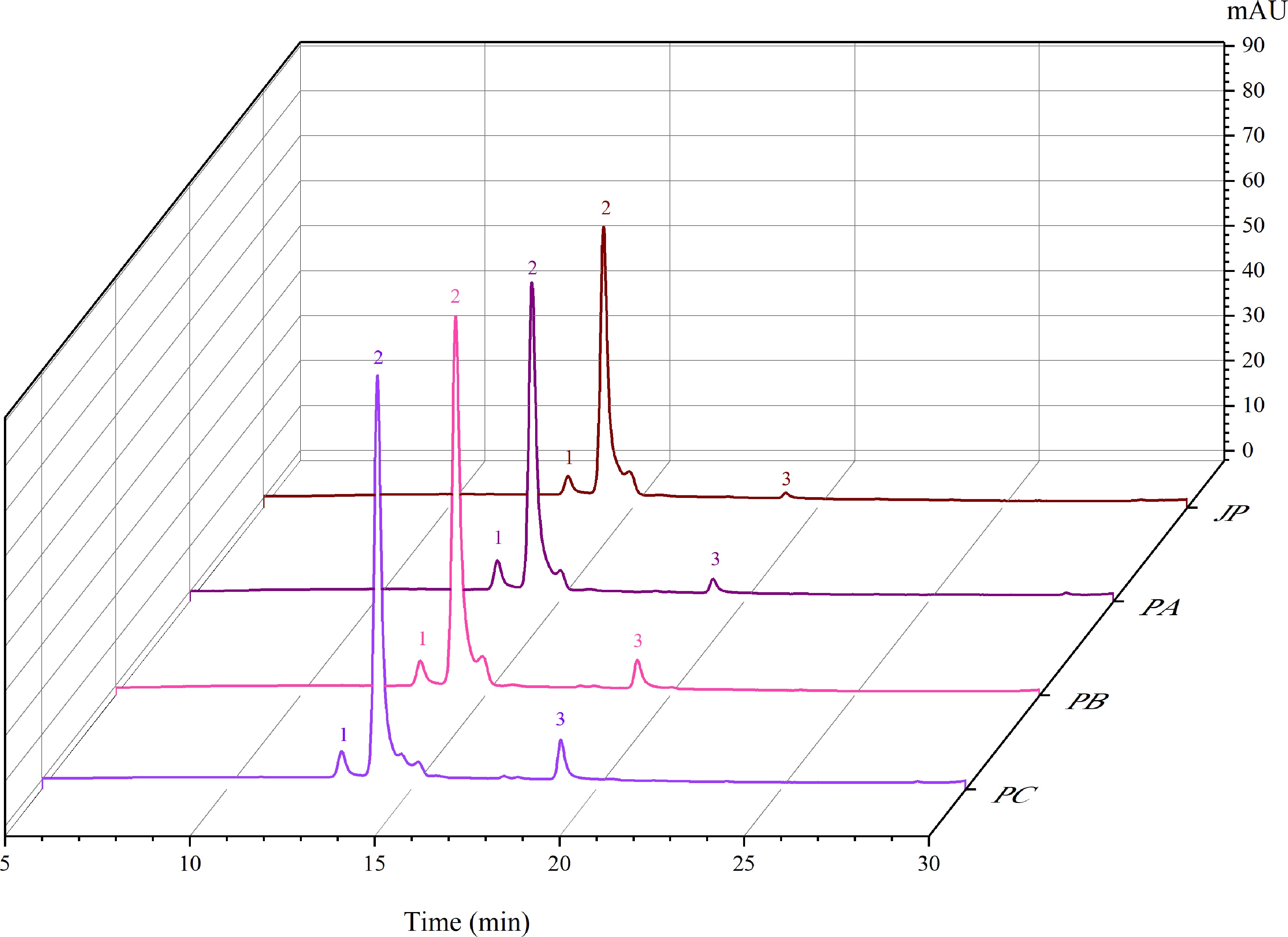
Figure 2.
Chromatograms represent the anthocyanins profile of the jaboticaba pulp (JP), jaboticaba powder with 10% of adjuvant (PA), jaboticaba powder with 15% of adjuvant (PB), jaboticaba powder with 20% of adjuvant (PC), jaboticaba powder with 25% of adjuvant (PD). Peak 1 refers to delphinidin 3-glucoside. Peak 2 refers to cyanidin 3-glucoside. Peak 3 is peonidin 3-glucoside.
Table 2 shows anthocyanins before and after the simulated digestion process (in vitro) of jaboticaba skin pulp (JP), jaboticaba powder with 10% of OSA modified-starch (PA), jaboticaba powder with 15% of OSA modified-starch (PB), jaboticaba powder with 20% of OSA modified-starch (PC), and jaboticaba powder with 25% of OSA modified-starch (PD).
Table 2. Determination of anthocyanins before and after the simulated digestion process (in vitro) of jaboticaba skins pulp (JP), jaboticaba powder with 10% of OSA modified-starch (PA), jaboticaba powder with 15% of OSA modified-starch (PB), jaboticaba powder with 20% of OSA modified-starch (PC), and jaboticaba powder with 25% of OSA modified-starch (PD).
Samples Anthocyanins
expressed as
C3G* (µg/mL)Anthocyanins
expressed as
C3G* (µg/mL)
after digestion
in vitroBioaccessible anthocyanins**
(%)JP 257.95a ± 22.8 14.68b ± 0.75 6.03 PA 132.25b ± 5.4 12.20b ± 1.21 8.72 PB 119.54c ± 5.8 17.96a± 0.24 9.58 PC 110.16d ± 1.4 8.26c ± 0.77 5.30 PD 94.30e ± 4.1 9.53c ± 0.60 5.64 Different letters on the same column represent values different from each other (p < 0.05). * Cyanidin 3-glucoside. ** Bioaccessible anthocyanins (%) = Anthocyanins concentration after in vitro digestion × 100 / Initial concentration of anthocyanins. Based on the results, it is possible to observe a decrease in anthocyanin content after the simulated digestive process. Before the digestion, the anthocyanin concentration values ranged from 94.30e ± 4.1 to 257.95a ± 22.8 µg/mL of C3G, the highest concentration was found on jabuticaba pulp (JP), and the lowest jaboticaba powder with 25% of OSA modified starch (PD). However, after the in vitro digestion, the loss of anthocyanins was more accentuated in the samples JP, PC, and PD. The bioaccessible anthocyanins variated from 5.30 (PC) to 9.58 (PB), therefore, the PB sample presented the best results among those studied in the present work.
The antioxidant activity results of the simulated digestion of all samples indirectly monitored the behavior of anthocyanin effects during this process, and the results are presented in Table 3.
Table 3. Determination of antioxidant activity before and after the simulated digestion process (in vitro) of jaboticaba skins pulp (JP), jaboticaba powder with 10% of OSA modified-starch (PA), jaboticaba powder with 15% of OSA modified-starch (PB), jaboticaba powder with 20% of OSA modified-starch (PC), and jaboticaba powder with 25% of OSA modified-starch (PD).
Samples Initial Final Bioaccessible AA* (%) ABTS
(µM TE/g)PA 12.02a ± 1.47 4.85b ± 0.10 40.39 PB 11.48a ± 0.46 5.68a ± 0.67 49.46 PC 9.16b ± 0.90 4.03c ± 0.34 44.00 PD 9.99b ± 0.90 4.34c ± 0.54 43.49 ORAC
(µM TE/g)PA 122.92b ± 15.22 71.17c ± 9.56 57.90 PB 157.00a ± 12.07 122.62a ± 12.9 78.10 PC 132.48b ± 19.10 81.70c ± 6.54 61.67 PD 147.93ab ± 21.67 99.01b ± 5.43 66.93 Different letters on the same column represent values different from each other (p < 0.05). * Bioaccessible AA (%) = Antioxidant activity after in vitro digestion × 100 / Initial antioxidant activity. Thus, comparing the different OSA modified-starch concentrations at the end of digestion concerning the behavior of the jaboticaba pulp. The PB sample showed a better remaining value (49.46 and 78.10 for ABTS and ORAC methods, respectively) than PA, PC, and PD, indicating a better performance of the Capsul® in protecting bioactive compounds contained in jaboticaba pulp when 15% of adjuvant was used.
-
The bioaccessibility of anthocyanins in jaboticaba powder samples to evaluate the effects of the OSA modified-starch concentration, using a static in vitro digestion model, over the anthocyanin’s contents was assessed in the present work. The process simulation involved several pH variations and enzymatic action, causing anthocyanins behavior and activity modification[31,32].
Anthocyanin pigments from edible plants, such as jaboticaba fruit, are composed of numerous individual molecules with distinctive substituent groups. Thus, indeterminate intermediate and digestive metabolites noticeably present overwhelming challenges during studying anthocyanins behavior from a chemistry standpoint. To consider the positive effects on human health from consuming foods rich in anthocyanins and other phenolic compounds, it is essential to verify the bioaccessibility after in vitro digestion to determine its metabolism and then strategize ways and processes to improve its beneficial properties[33].
Not only the freeze-drying process is reported in the literature as a methodology to improve the stability of bioactive compounds. Our research group evaluated the fermentation process and the formulation of nanocomposites as ways to preserve anthocyanin properties[3].
Similarly, the effects of fruit preservation using processes such as freeze-drying, osmotic dehydration, and convection drying related to antioxidant capacity, total polyphenols, and total anthocyanins were studied by Muñoz-Fariña et al.[34]. According to the authors, convection drying best preserved the total polyphenol contents and antioxidant capacity from the processes studied. While, freeze-drying and convection drying showed no significant differences in total anthocyanins. To determine the effects of the preservation techniques on the phenolic compounds/antioxidant capacity, their bioaccessibility was determined by a static model of in vitro gastrointestinal digestion. The results of the intestinal stage (ileum) showed that convection drying improved the preservation of antioxidant properties (DPPH), reaching values of 91.6% bioaccessibility (29.1 mmol Trolox equivalents TE/g DW) and 48.7 (9.4 mmol TE/g DW) for the freeze-dried berries. Meanwhile, osmotically dehydrated berries exhibited the lowest percentage of bioaccessibility of the antioxidant compounds at 27.6% (4.0 mmol TE/g DW). It was concluded that total polyphenols, anthocyanins, and antioxidant capacity are better preserved by convection drying, freeze-drying, and osmotic dehydration.
As expected, a decrease in anthocyanin content after the simulated digestive process was observed. This decrease suggests a potential degradation of anthocyanins or even transformation in other phenolics, which was predictable considering changes in pH and enzymatic activity during the digestive process[10,35]. Besides, anthocyanin metabolism involves its cleavage and consequent metabolization, often promoting the bioconversion of anthocyanins to protocatechuic acid, gallic acid, and p-coumaric acid, produced after the enzymatic action of gut microbiota bacteria on this compound[8,36,37].
Even though all samples presented a decrease in the anthocyanins content, the most expressive loss was observed in the sample without the adjuvant (Jaboticaba skins pulp). On the other hand, the sample with 15% of Capsul® (PB) maintained 9.58%, an increment of about 30% compared to the JP sample, showing potential as a great alternative to be used as a food ingredient to preserve anthocyanins. An evident interest has arisen in using octenyl succinic anhydride (OSA)-starch to increase the stability of bioactive compounds. Several cases were reported in the recently published review by Nhouchi et al.[26]. The interaction between these adjuvant and bioactive compounds promotes the protection of bioactive compounds against adverse digestion conditions, corroborating with the results of the present work.
The polyphenol bioaccessibility of juice and pomace of American elderberry after spray drying was studied using an in vitro digesting model by Ravichandran et al.[38]. Spray-dried particles formed from tapioca starch (TS) had considerably greater total polyphenol content (42−49 mg gallic acid equivalent/g sample), proanthocyanidin content (0.76−2.86 mg proanthocyanidin-B2/g sample), and anthocyanins (7.86−33.80 mg/g sample) than soy protein isolate (SPI) derived particles. Compared to non-encapsulated elderberry juice or TS-derived particles, particles of encapsulated elderberry juice or pomace extract with SPI demonstrated greater bioaccessibility. The authors concluded that spray drying American elderberry juice and pomace extract is a viable and long-term technique for developing new components for various culinary applications.
Other scenarios have also been reported in the latest literature. García-Perez et al.[39] studied the influence of pectin conformation over the bioaccessibility of cherry laurel polyphenols and gut microbiota distribution following in vitro gastrointestinal digestion and fermentation. The authors verified that pectin and pectinase altered the fecal microbiota profile after in vitro fermentation, with pectinase therapy enhancing bacterial diversity. The addition of pectins followed by pectinase showed varied effects on polyphenol bioaccessibility and gut microbial diversity, potentially affecting human health.
Regarding the present work and complementing the already discussed data, considering the antioxidant potential, the positively charged oxygen atom and distinct hydrogen-donating antioxidants equip anthocyanins with a potent antioxidant capacity. Two different methods also studied this biological effect. It was possible to establish that for both methods, higher maintenance of antioxidant activity was reached with 15% of Capsul®, confirming the positive interaction among the adjuvant and the bioactive compounds from jaboticaba. Similar results were achieved using the same adjuvants to maintain anthocyanins from blackberry pulp in work previously developed by our research group[24].
It is essential to highlight that a high percentage of antioxidant activity was reached even with a relatively high decrease in anthocyanins content throughout digestion. This phenomenon is probably related to the cleavage or transformation of anthocyanins in smaller compounds, such as phenolics, that also present antioxidant capacity[36,40].
As a next step considering the formulation of food containing microencapsulated anthocyanins, Czubaszek et al.[41] analyzed the quality of bread prepared with red cabbage, cornelian cherry, and chokeberry extracts microencapsulated in maltodextrin and inulin, as well as evaluated changes in anthocyanin content during in vitro digestion. Breads enhanced with extracts produced more bread with less volume than wheat bread treated with maltodextrin or inulin. Compared to wheat bread, breads prepared with microencapsulated extracts exhibited significantly greater levels of anthocyanins, ABTS, and FRAP antioxidant activity. The concentration of anthocyanins decreased during the simulated digestion, notably after the intestinal digestion stage. Red cabbage acylated anthocyanins were shown to be more digestible than cornelian cherry and chokeberry anthocyanins, however, this did not correlate with antioxidant activity.
The formulation of ingredients using starch-based to increase the stability of different compounds is increasing, not only to enhance bioactive compounds' properties but also to encapsulate flavor, delay the lipid oxidation process, and produce nanoparticles[42−44], all of these are initiatives to bring novelties to the food industry, amplifying the ways to meet consumers expectation of a more healthy and sustainable way to improve the overall healthiness in their lives.
-
The bioaccessible portion of anthocyanins in jaboticaba powder samples with four distinctive concentrations of the OSA-modified starch concentration (Capsul®) using a static in vitro digestion model was determined. The results showed a decrease in the content of anthocyanins. However, an increment of the recovery was reached using 15% of Capsul® as an adjuvant. It is essential to highlight that the antioxidant activity was maintained after the in vitro digestion process, probably due to the cleavage or transformation of anthocyanins in smaller compounds, such as phenolics, that also present antioxidant capacity. From the data presented, it is possible to affirm the potential of the OSA-modified starch as an ingredient to improve anthocyanins from jaboticaba properties to be used in foodstuff and the food industry as natural pigments, meeting with the profile behavior of modern consumers reaching for healthier processed food.
-
The authors confirm their contribution to the paper as follows: study conception and design: Braga ARC, Braga MB; draft manuscript preparation: Giaconia MA, Assis M, Moura MS and Braga ARC; analysis and interpretation of results: Braga ARC and Braga MB; data collection: Giaconia AM. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon request.
This work was supported by the 'Fundação de Amparo à Pesquisa do Estado de São Paulo' (FAPESP) (Process No. 2018/01550-8, 2019/26137-9, and 2020/06732-7).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Giaconia MA, Assis M, Moura MdS, Braga MB, Braga ARC. 2023. Bioaccessibility and antioxidant activity of anthocyanins from jaboticaba skins: the influence of OSA-modified starch concentration. Food Materials Research 3:33 doi: 10.48130/fmr-0023-0033
Bioaccessibility and antioxidant activity of anthocyanins from jaboticaba skins: the influence of OSA-modified starch concentration
- Received: 22 June 2023
- Accepted: 08 September 2023
- Published online: 04 December 2023
Abstract: In recent years, there has been a growing interest in clarifying the metabolism of bioactive compounds, particularly phenolics such as anthocyanins. The transformation of anthocyanins from jaboticaba throughout digestion has yet to be extensively investigated. Due to native-form anthocyanins' instability against environmental stress, bioactive compounds are not always as effective in improving human health as they could be. The microencapsulation of material with high levels of anthocyanins by freeze-drying usually results in products with greater stability during storage, preserving their antioxidant properties. Therefore, the main goal of the present study was to determine the bioaccessibility of anthocyanins in jaboticaba powder samples to evaluate the effects of the modified starch with octenyl succinic anhydride (OSA) concentration (10% of adjuvant (PA), jaboticaba powder with 15% of adjuvant (PB), jaboticaba powder with 20% of adjuvant (PC), jaboticaba powder with 25% of adjuvant (PD)) using a static in vitro model of digestion over the anthocyanin’s contents and their antioxidant activities. Based on the results, it is possible to observe a decrease in anthocyanin content after the simulated digestive process. Before the digestion, the anthocyanins concentration values ranged from 94.30e ± 4.1 to 257.95a ± 22.8 ug/mL of cyanidin 3-glucoside. The highest concentration was found on jabuticaba pulp (JP) and the lowest on jaboticaba powder with 25% OSA-modified starch (PD). However, after the in vitro digestion, the loss of anthocyanins was more accentuated in the samples JP, PC, and PD. The bioaccessible anthocyanins varied from 5.30 (PC) to 9.58 (PB). Therefore, the PB sample presented the best results among the studied in the present work. The interaction between adjuvant and bioactive compounds promoted the protection of bioactive compounds against adverse digestion conditions, considering the maintenance of antioxidant activity.
-
Key words:
- Bioaccessibility /
- Antioxidant /
- Activity /
- Anthocyanins /
- Jaboticabas












