-
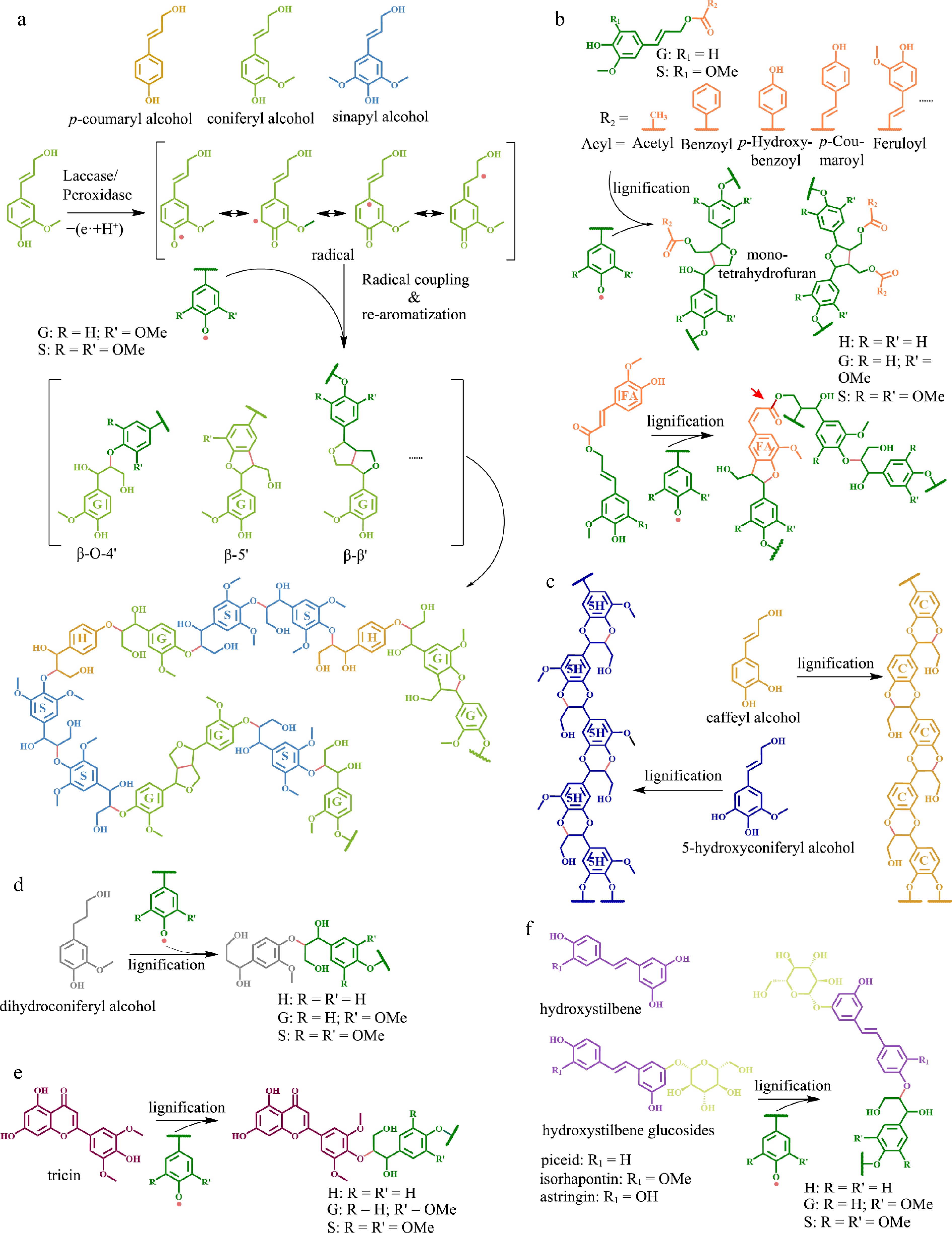
Figure 1.
Different types of monomers and corresponding lignin structures. (a) Conventional monolignols and their polymerization mechanism, which is mainly carried out by an 'end-wise' process that begins with single-electron oxidation of monolignols by laccase and/or peroxidase to generate activated radical intermediates. The radicals inter-couple or couple with a radical formed on the free-phenolic ends of a growing lignin polymer to form lignin with different linkages. (b) Acylated monolignols and the resulting lignin structure. Feruloyl esters, highlighted in orange color on feruloyl monomers, can be integrated into the lignin backbone. The red arrow indicate the presence of an alkali-labile linkage in the lignin structure. (c) Caffeyl alcohol and 5-hydroxyconiferyl alcohol (unconventional lignin monomers) and the resulting homogeneous linear lignin polymer. (d) Dihydroconiferyl alcohol and the corresponding lignin structure. (e) Tricin (a flavonoid) and the resulting lignin polymer. (f) Hydroxystilbene and hydroxystilbene glucoside, as well as their corresponding lignin structures.
-
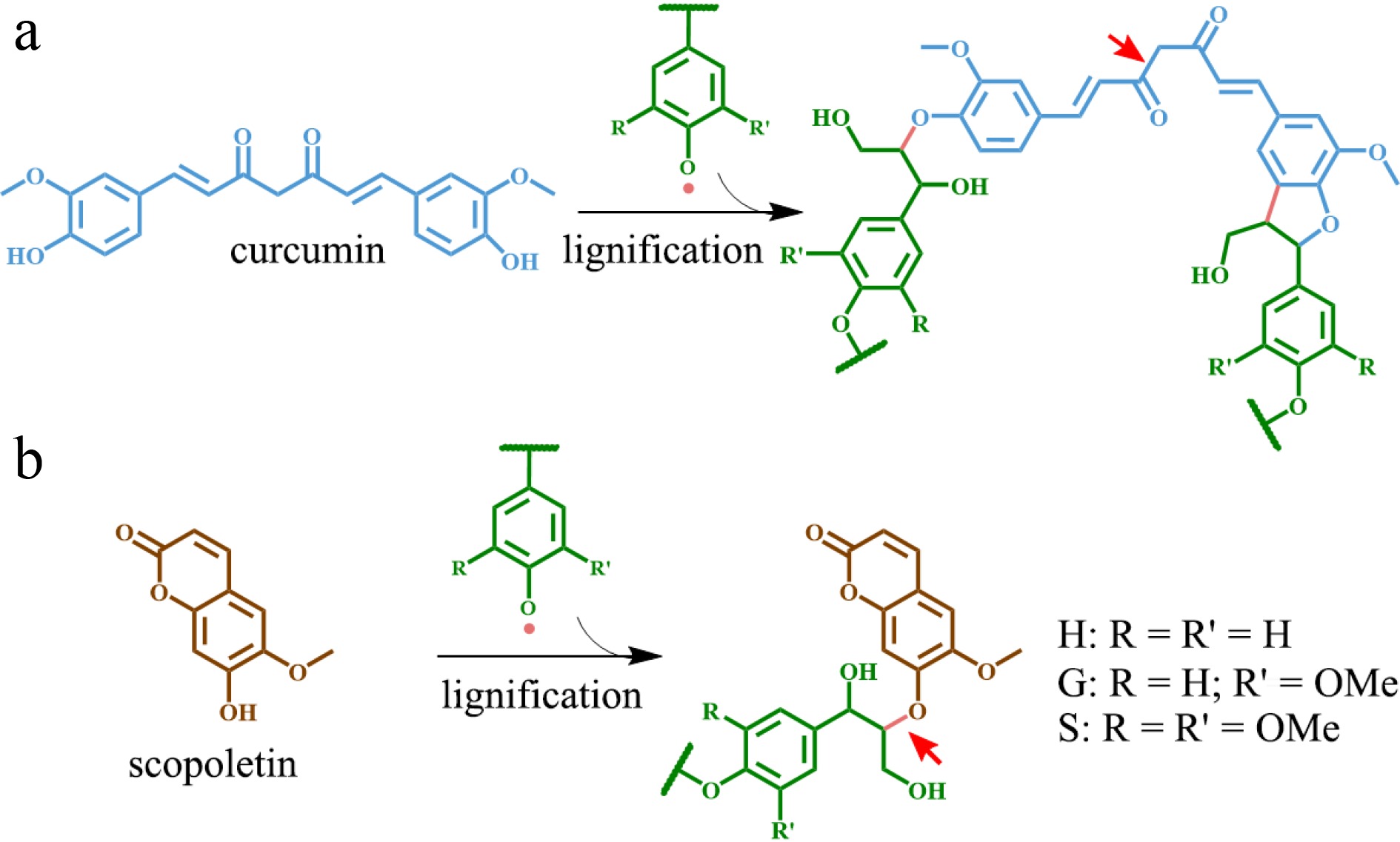
Figure 2.
Phenolic metabolites functioning as alternative monomers in lignin polymer. (a) Curcumin and the resulting lignin structure. (b) Scopoletin and corresponding lignin structure. The red arrow indicates the alkali-labile linkage on lignin.
Figures
(2)
Tables
(0)