-
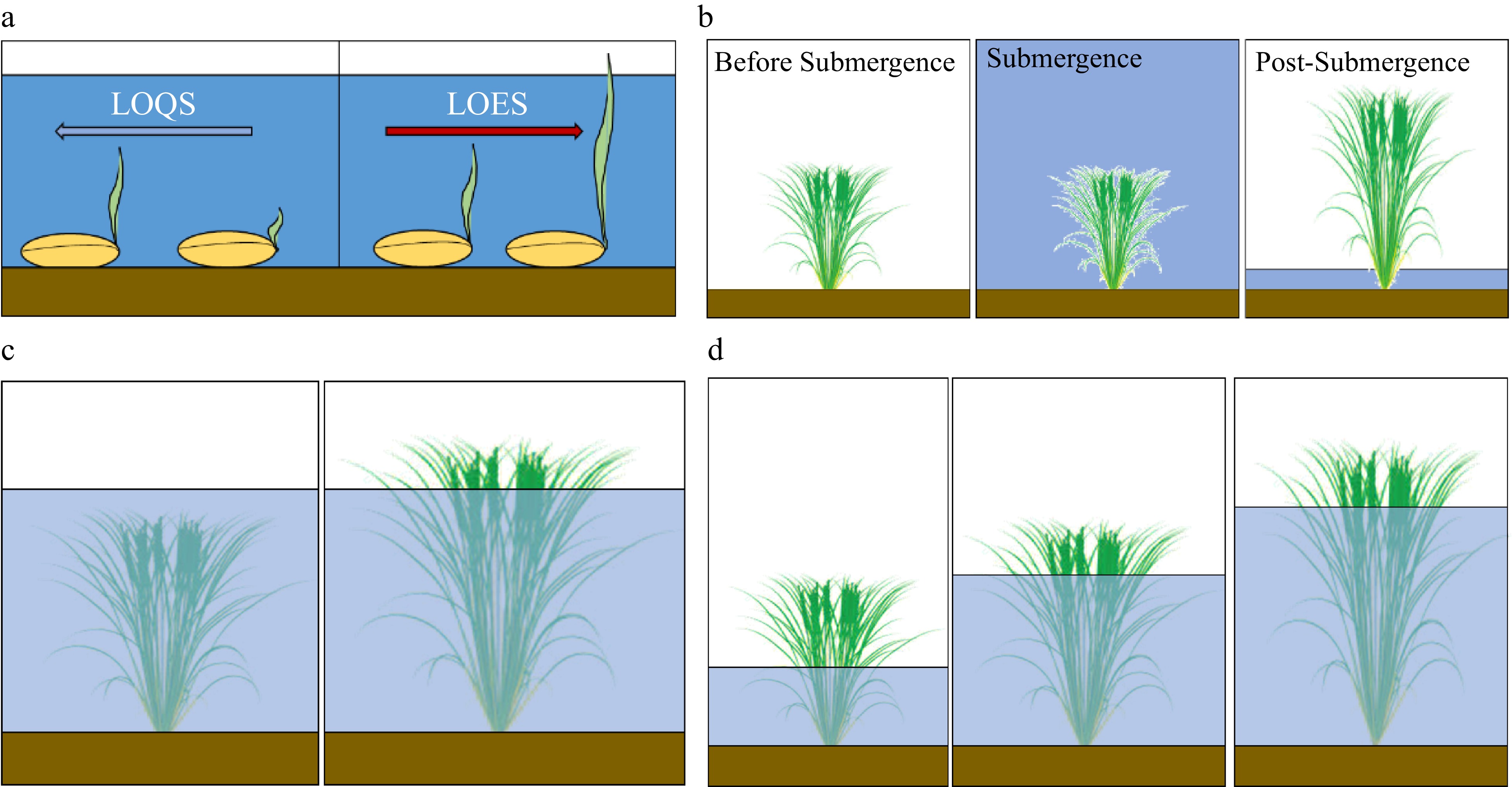
Figure 1.
Complete submergence: Rice coping mechanism. (a) Flash flooding at germination. Low O2 escape (LOES) and low O2 quiescence (LOQS) strategies[117]. Oxygen is one of the important factors that affects germination. Under submergence, the root formation is inhibited. Sensitive cultivars exhibit LOQS strategy, that inhibits coleoptile elongation. Tolerant varieties exhibit faster coleoptile elongation. (b) Flash flooding (< 2 weeks). Under complete submergence, shoot elongation of tolerant varieties is inhibited (quiescence), conserving carbohydrate reserves and allowing survival under water. After water subsided, growth and rejuvenation is resumped[118]. (c) Severe flash flooding (< 2 weeks). The response to flooding in rice is a function of the SNORKEL (SK) locus, responsible for stem elongation[119]. The expression of these genes promotes elongation of the rice internodes, enabling plants to elongate. (d) Deepwater (slow rise stagnant flooding). Rice plants tolerant to deepwater flooding, where water depth is from 50 cm to around 4 m, show significant stem elongation as water levels rise. The deepwater-flood-tolerant rice becomes flattened, and generates new roots and tillers[120].
-

Figure 2.
Drought tolerance mechanism in sugarcane. A tropical crop having a C4 photosynthetic metabolism is sugarcane. Under moderate water stress, stomatal conductance (gs), transpiration rate (E), internal CO2 concentration (Ci), and photosynthetic rate decrease, primarily because of stomatal limitations. When sugarcane plants experience mild to moderate dryness, this is the most frequent initial adaptation, along with the suppression of stalk and leaf growth. But non-stomatal restrictions brought on by water stress have also been implicated in sugarcane photosynthesis suppression, whenever there is a lot of tension or stress[121].
-
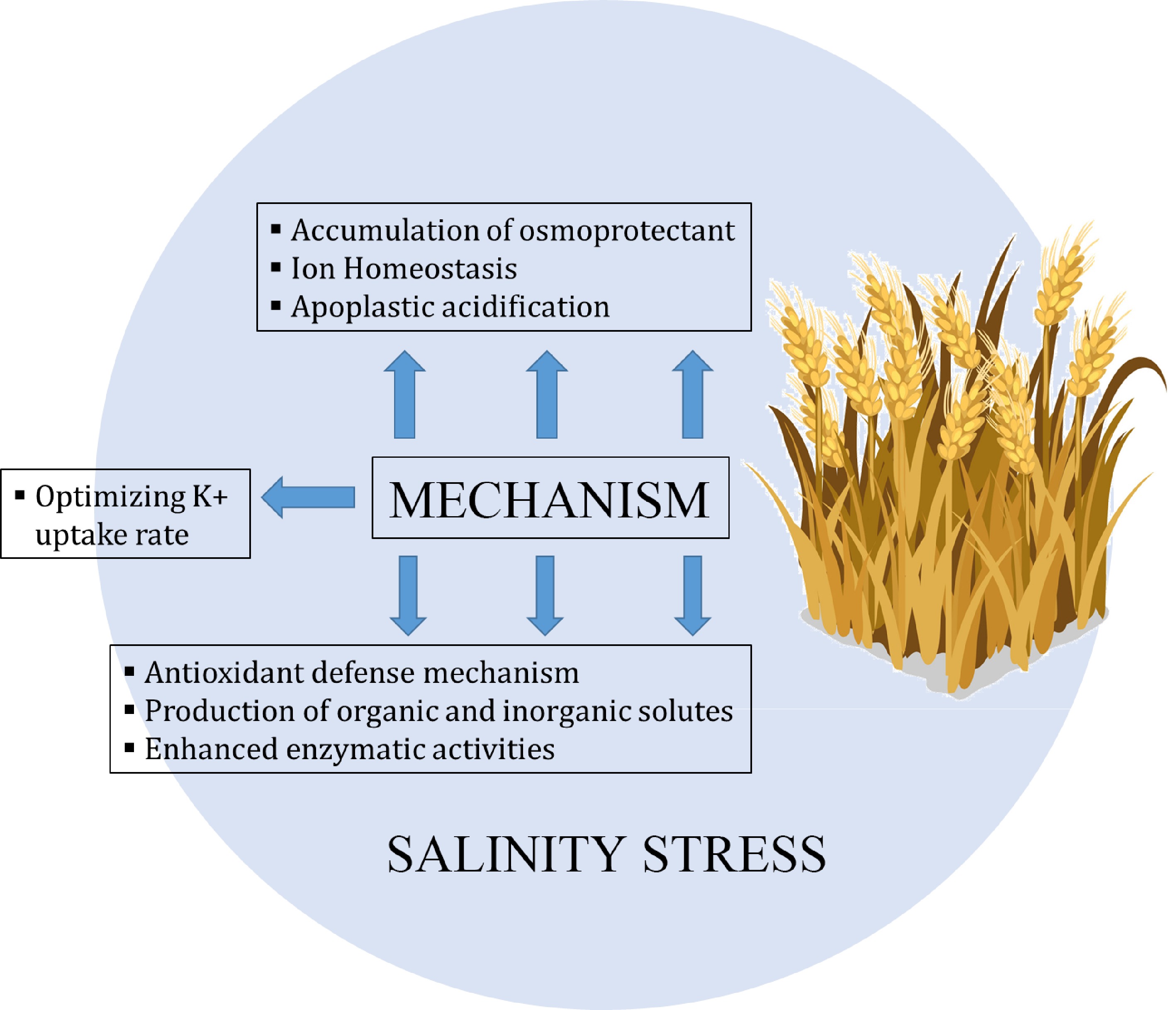
Figure 3.
Saline stress response mechanism in wheat[122]. The defensive system against anti-oxidant species is triggered by osmoregulation, which also controls the water interaction between plants. The molecular weight of osmoprotectants is low, they have no net charge, and they are hydrophilic in nature. The salt-tolerant cultivars of bean plants have lower protein levels and higher levels of proline and other amino acids than the salt-sensitive variants. In order to maintain a low Na+ ion concentration and increase the K+ concentration, ionic homeostasis is a crucial function that controls ion flux. The cytosolic activity of the different enzymes depends on the control of intracellular Na+ and K+ ions (homeostasis), which also regulates membrane potential and cell volume. Excessive salt buildup in the root zone of plants causes the development of reactive oxygen species (ROS), severe osmotic stress, and ion toxicity in plants. The breakdown of proteins and changes to DNA sequencing are both brought on by ROS in plants. The superoxide dismutase and catalase enzymes, among others, are activated by salt stress in plants to create an anti-oxidative mechanism.
-
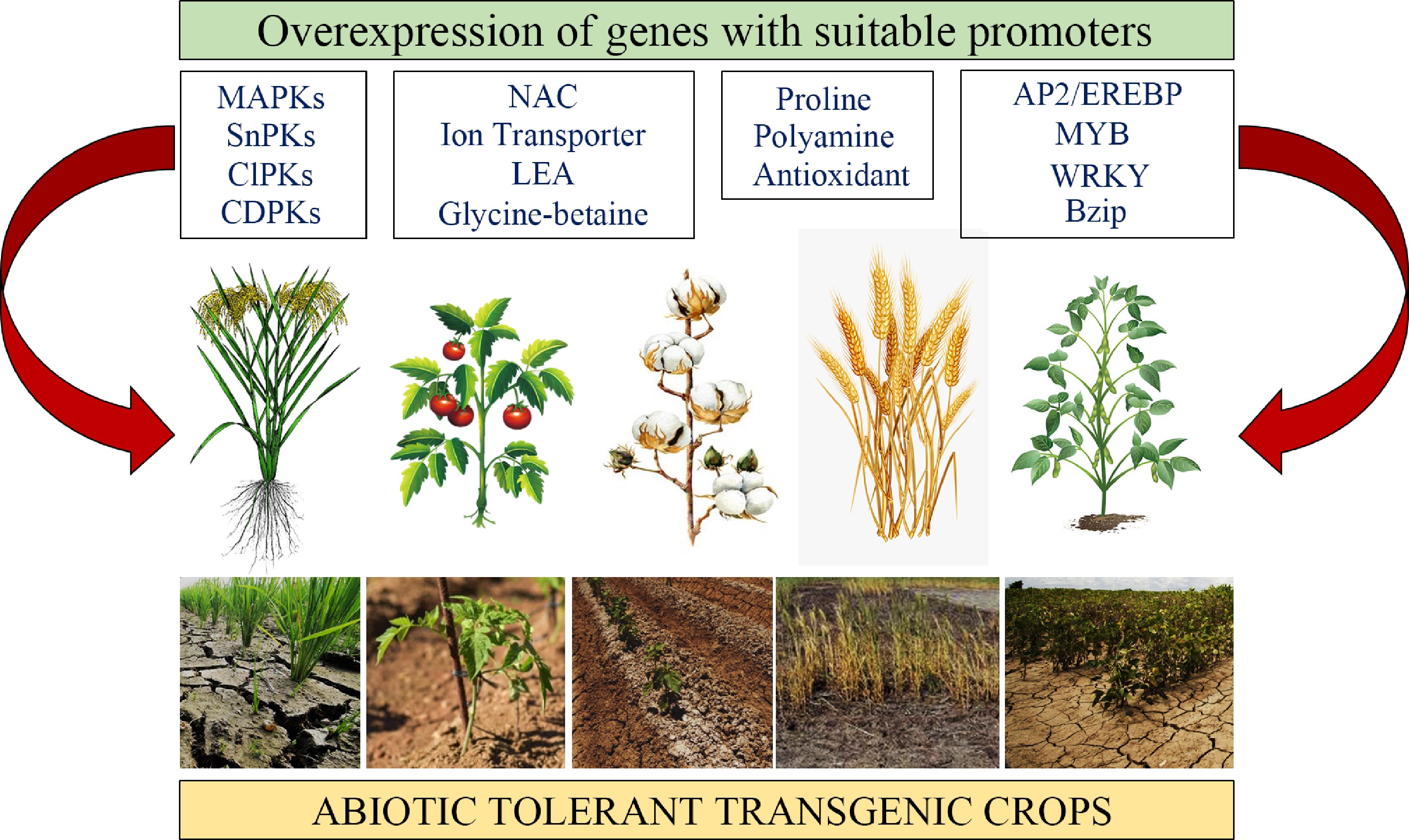
Figure 4.
Transgenic approach. Transgenic strategies by overexpressing different groups of genes, including regulatory and functional genes with suitable promoters (stress inducible) to improve abiotic tolerance in crops[91].
-
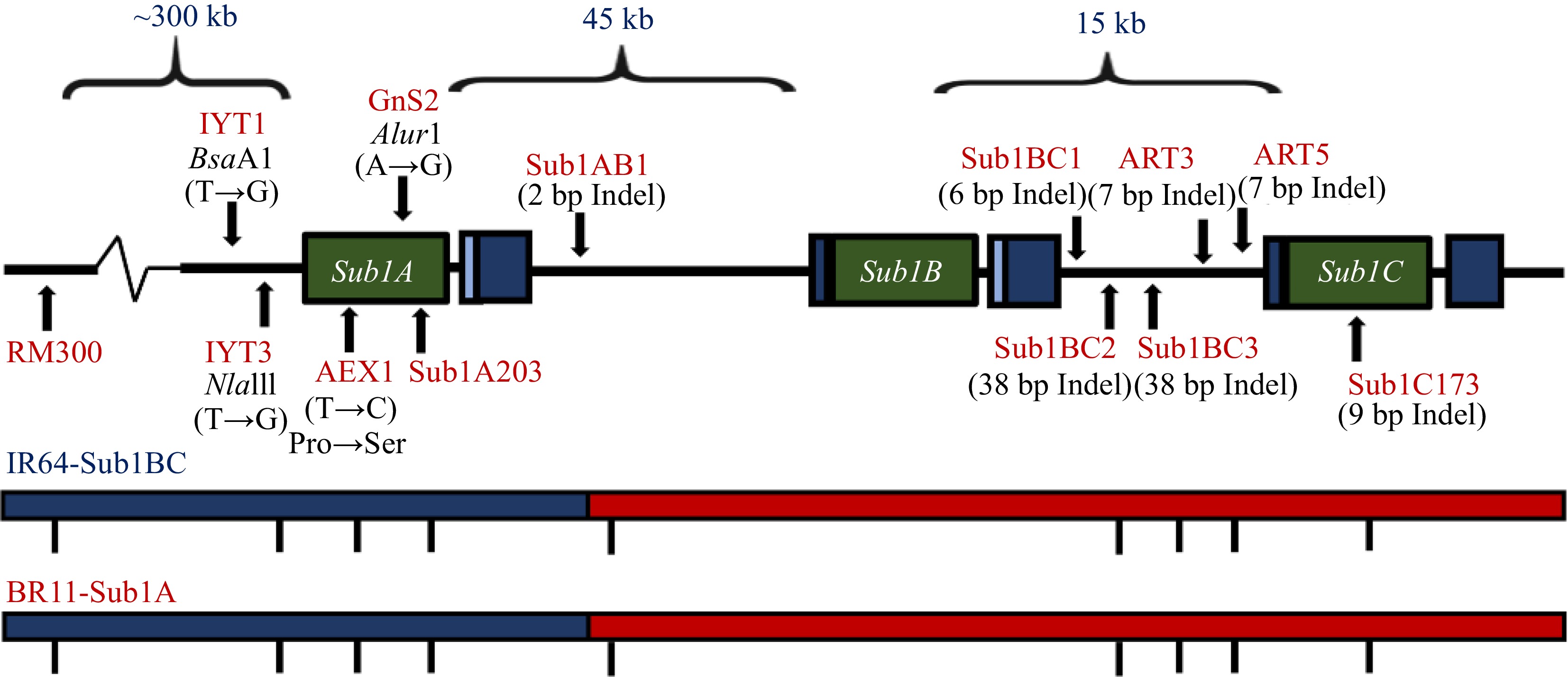
Figure 5.
The Sub1 QTL conferring submeregnce tolerance. Relative location of Sub1 QTL region and SC3 (RM8300) and ART5 foreground markers in chromosome 9 of the rice genome, conferring tolerance to complete submergence[104].
-
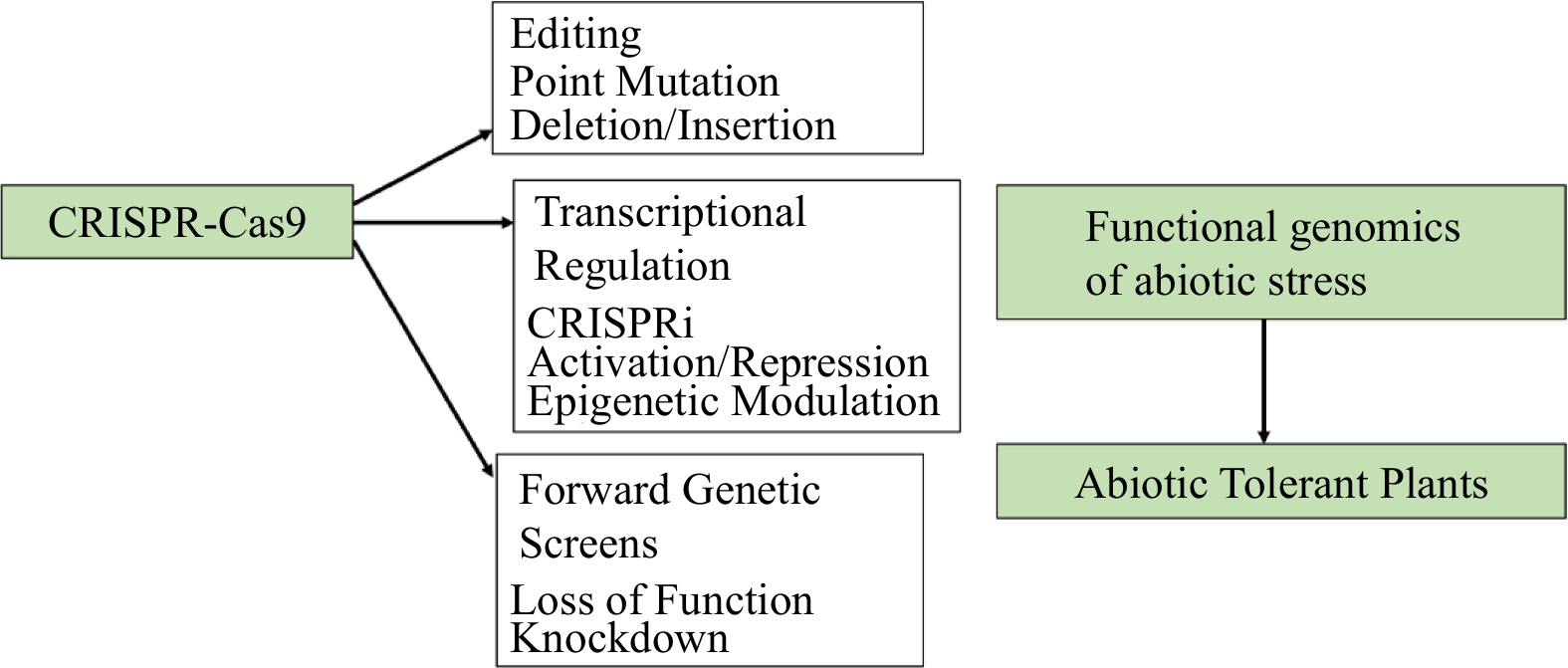
Figure 6.
The CRISPR-Cas9 system. The CRISPR-Cas9 system in the context of functional genomics for abiotic stress tolerance. CRISPR-Cas9 system uses point mutations, insertions or deletions, transcriptional regulation through CRISPR interference, activation, repression or epigenetic modulation, or through generation of loss-of-function, knock-down or activation mutants[123].
-
No. Mutant cariety ID No. Year Character improvement details 1 BPI-121-407 1202 1971 Early maturity, very short stem, stiff-strawed, high tillering, resistance to diseases and moderate resistance to bacterial leaf blight 2 PARC 1 1203 1970 Narrow and long grains with less chalky areas 3 PARC 2 1204 1973 Early maturity (5−10 d), narrow and long grains with less chalky areas and good eating quality 4 BPI Ri-10 1205 1983 Early maturity, semi-dwarf, resistance to pest, high yield, non-seasonal (for both dry and wet seasons) and good eating quality 5 PSB Rc78 2393 1999 Better yield potential, 8 d shorter in maturity and 5 cm shorter in height as compared to the original variety, good quality 6 Milagrosa Mutant 2394 1974 High yield and resistance to diseases 7 Azmil Mutant 2395 1976 High yield, drought resistance and susceptible to blight 8 Bengawan Mutant 2396 1984 High yield, short plant stature and early maturity 9 PR22902 3368 1994 High yielding and early maturing 10 NSIC Rc 346 4467 2013 Agronomic traits: maturity, plant type, grain and panicle traits 11 NSIC Rc 272 4468 2011 Agronomic traits: maturity, plant type, grain and panicle traits 12 NSIC 2019 Rc 572 4903 2020 Agronomic traits: maturity, plant type, grain and panicle traits, grain quality Source: International Atomic Energy Agency (IAEA), Mutant Variety Database (MVD), 2022. Table 1.
Rice mutant varieties developed and released in the Philippines, 1971 to 2020.
Figures
(6)
Tables
(1)