-
Given the growing influence of climate change on global food production, cultivating crops that are climate-resilient to cope with abiotic stress is a crucial task in agriculture. Abiotic stressors that negatively impact crop yields include salinity, drought, high temperatures, and nutrient shortages. According to recent reports, the production of ten major crops, including barley, rice, sorghum, wheat, sugarcane, and maize, has decreased globally by 1%[1]. Severe droughts and flash floods are brought on by drastic changes in rainfall patterns, while the steady rise in temperature melts ice caps, raising the level of sea water. Due to the intrusion of salt water into agricultural lands brought on by the rise in sea levels, salt depositions occur. Scientists and farmers are working on creating climate-resilient crops using a variety of techniques that combine conventional breeding, genetic engineering, improved agricultural practices, and cutting-edge technology in order to address these issues and guarantee food security.
-
The main abiotic stresses brought on by shifting climatic patterns are severe droughts, flash floods, temperature increases, extremely low temperatures, and salt water intrusion into agricultural areas. The change in rainfall patterns has a significant impact on agricultural areas that rely heavily on rainfall for irrigation and water sources, drying up the land and rendering it unusable for cultivation.
Drought stress
-
Drought is one of the most important water-related stresses that severely affect plant's growth and development. Drought is often defined as the absence of rainfall, specifically in arid and semi-arid agricultural areas. There are several factors triggering drought stress, such as high temperature, high light intensity and wind. These factors cause and increased evaporation rate from both the soil and the plant itself. Drought stress is experienced, when the soil is depleted with the sufficient amount of moisture needed by the plants. In terms of plant's physiology, water stress occurs when water loss is greater than water uptake.
One of the most significant water-related stresses that negatively impacts a plant's ability to grow and develop is drought. Lack of rainfall is a common definition of drought, especially in arid and semi-arid agricultural regions. Drought stress is brought on by a number of factors, including wind, high temperatures, and intense light. These elements lead to a faster rate of evaporation from the plant and the soil. When the soil no longer contains an adequate amount of moisture required by the plants, drought stress occurs. When water loss exceeds water intake, water stress is experienced by plants physiologically. The species of the plant, the crop stage at which drought stress occurred, the growing environment, and other factors all affect how symptoms of drought stress appear in plants. Certain plant species display more severe symptoms than others because they are more susceptible to the effects of drought. The reproductive stage of rice is particularly vulnerable to drought, which results in pollen non-viability and non-fertilization. Grain filling and ultimately grain yield are negatively impacted by this. The stages of wheat that are most susceptible to water deficit are jointing, tillering, and pollen dehiscence[2]. At these stages, any reduction in the water supply results in significant yield losses[3]. Plant growth, nutrient and water relations, phenology, respiration, photosynthesis, and assimilate partitioning are all impacted by drought stress[4].
Plant growth is significantly impeded by drought as it directly impacts cellular division, elongation, and differentiation. Drought has these effects because it causes a reduction in photosynthesis energy, enzymatic and biochemical process errors, and loss of cell turgor. Turgor loss suppresses cell growth and expansion, which is crucial for plant growth, development, and establishment in the early stages. Plant morphology, anatomical features, and phenotype are impacted by drought stress. Stress from water changes a leaf's ultrastructure and anatomy. When plants are stressed by drought, their leaf area, canopy, and stomata count all decrease. Plants under stress also exhibit cutinized leaf surfaces and thicker cell walls[5]. In contrast to cereals, where drought stress triggers the formation of tube leaves, succulents and xenophytes exhibit the development and expansion of large vessels as well as the submersion of stomata. To ensure efficient photosynthesis, two essential parameters are reduced in a water deficit: leaf area and stomatal opening. This results in a reduction in the efficiency of photosynthetic activities. Reduced size is the primary result of drought stress on plants. One of the biggest factors influencing plant size reduction and biomass production is a low photosynthetic rate[6]. Under water stress conditions, changes in carbon metabolism occurs due to diminished photosynthesis and active respiration. Plant's growth rate is determined by CO2 assimilation and the respiration ratio. Plants under drought conditions tends to use relatively greater amount of energy resources to uptake water from the soil, especially under severe conditions[7,8]. Drought negatively affects the Kreb's Cycle and the synthesis of adenine triphosphate (ATP), leading in the reduction of respiration rate. Lower respiration results in too much containment of heat inside the plant system, causing enzymatic and biochemical processes to fail. When plants are exposed to drought stress, they produce reactive oxygen species (ROS) inflicting damage to cellular components[9, 10].
Drought interferes with assimilate partitioning because most of them are translocated to the roots, just like it does with other biochemical processes. The main purpose of this assimilate root translocation is to enhance soil moisture uptake[11]. The rate at which a plant photosynthesizes and the amount of sucrose present in its leaves both affect how assimilates are transported from source to sink[12]. Drought inhibits photosynthesis and lowers the sucrose content of leaves, which in turn lowers the rate at which assimilates are exported from source to sink[13]. Drought also negatively impacts assimilate distribution under moisture stress by limiting the sink's capacity to utilize the incoming assimilates effectively[14]. A complex network of genes contributes to plant responses to drought stress at the molecular level. Stressful stimuli, like drought, cause changes in gene coding and patterns in plants. Plants exposed to drought experience changes in many of their gene expression patterns. Expressions linked to late responses, such as water transport, osmotic balance, oxidative stress, and the damage-repair processes, are expressed after primary changes involving early responses, such as signal transduction, transcription, and translation factors[15]. Abscisic acid (ABA) signal transduction has a close relationship with both drought sensing and signal transduction. ABA activates genes induced by drought and plays a major role in plant responses to it[16].
Waterlogging
-
The simple definition of waterlogging is an area with an excessive amount of water, either temporarily or permanently saturating the soil. In anaerobic environments, waterlogging inhibits plant growth and productivity, which results in plant death. Approximately 12% of cultivated soil is impacted by excess water, making waterlogging a significant issue in agricultural areas worldwide. Under waterlogging conditions, yield loss is estimated to be between 39% and 40%[17]. Because soil flooding lowers the endogenous levels of nutrients in the plant system, it has a negative effect on plant growth. Reduced potassium/sodium (K+/Na+) uptake and slowed K+ transport to the shoot system are the results of low oxygen levels in the root zone. Limited uptake of nitrogen (N) and the ensuing redistribution of nitrogen within the shoot cause early leaf senescence and stunted growth of shoots in flooded plants[18,19]. Furthermore, insufficient micro and macronutrients cause the photosystem II (PS) to function poorly, which makes the process ineffective. It is widely accepted that nutrient deficiencies strongly correspond with the negative consequences of waterlogging. Plants that experience water logging switch to anaerobic respiration because it is more difficult for them to respire aerobically. The rhizosphere's reduced oxygen content produces hypoxic and anoxic conditions that change the cells' redox state[20−22]. The intermediate electron carriers in the electron transport chain occur as a result of oxygen depletion.
Plants respond to soil flooding initially by inhibiting stomatal aperture and reducing stomatal conductance. Low O2 also reduces hydraulic conductivity, which has an impact on the roots' permeability. Due to reduced stomatal aperture, lower leaf chlorophyll contents, early leaf senescence, and a smaller leaf area, oxygen deficiency typically causes a rapid reduction in photosynthetic rate[23,24]. Waterlogging has an impact on photosynthetic product translocation from 'source' (leaf) to 'sink' (root), much like drought stress does. An essential flood adaptation is the preservation of photosynthetic activity and the build-up of soluble sugar in the roots. Regardless of whether they are tolerant or intolerant, one of the main reactions of plants to anoxic conditions is reduction of root respiration. The respiratory capacity of bitter melon was found to be approximately 28% lower than that of non-flooded melon. Survival in flooded conditions may depend on reduced root respiration and the roots' capacity to scavenge any oxygen present around the rhizosphere[22,25]. Roots in a flooded environment experience hypoxia, which inhibits metabolic activity and reduces ATP production. Reduced ATP limits the energy available for root growth, which in turn slows down vegetative growth[22].
High temperature
-
Excess solar radiation from global warming results in a sharp rise in temperature. These occurrences are frequently among the most restricting elements influencing plant development and yield. Elevated temperatures can result in significant harm to plants, including sunburned leaves, twigs, and branches; abscission and senescence of leaves; inhibition of root and shoot growth; discoloration and damage to fruit; and decreased yield[26−28]. One of the main factors contributing to a notable decline in yield and dry matter production in practically all crops, including wheat, barley, rice, and maize, has been identified as heat stress. Elevated temperatures modify the physiological mechanisms and developmental growth patterns[29]. These reactions may vary depending on the phenological stage. Extended heat stress during seed development can result in a reduction of seed viability by delaying germination or causing vigor loss[30].
Coleoptile growth in corn stops at 40 °C and reduces again at 45 °C[31]. High temperatures reduce the net assimilation rate, shoot dry mass, and relative growth rate in plants; leaf expansion is only slightly impacted[32,33]. Plants die too soon as a result of the reduction in the length of the first internode, which has a significant effect on shoot growth[34]. Plants cultivated in high temperatures showed shorter internodes, more tillering, earlier senescence, and a decrease in total biomass in sugarcane[35]. Heat stress has an impact on anthesis and grain filling in a variety of temperate cereal crops. It lengthens the grain filling period and slows down kernel growth, which can cause losses in wheat kernel density and weight of up to 7%[26]. The starch, protein, and oil contents of maize kernels were among the plant products whose quality was greatly diminished by heat stress[36,37]. Given that there were fewer grains per panicle at maturity as the temperature rose, it seemed that both grain weight and grain number in rice were susceptible to heat stress[38]. Common beans[39] and groundnuts[40] lose yield when exposed to heat in temperate and tropical climates.
High temperatures cause anatomical changes such as decreased cell size, stomata closure and reduced water loss, increased densities of stomata and trichomatous, and larger xylem vessels in both the root and the shoot[41]. Photosynthetic processes are significantly altered as a result of substantial subcellular modifications in chloroplasts. Research findings indicate that certain effects of high temperatures on photosynthetic membranes lead to the swelling or loss of grana stacking. Grape plant mesophyll cells exhibited a number of physiological changes in response to heat stress, including swollen stroma lamellae, clumped vacuole contents, disrupted cristae, and empty mitochondria[42]. Antenna-depleted photosystem-II (PSII) is formed as a result of these alterations, which lowers photosynthetic and respiratory activity[42]. In general, it is clear that elevated temperatures have a significant impact on anatomical structures, affecting them not just at the cellular and tissue levels but also at the sub-cellular level. Low plant growth and productivity may be the consequence of all these modifications combined with high temperature stress[43].
Saline stress
-
Salinity is one of the main agricultural hazards that results in enormous yearly losses exceeding USD
${\$} $ -
Since plants are multicellular, sessile creatures that need to withstand environmental stressors in order to adapt and live, they have evolved a variety of stress-tolerance mechanisms. Generally speaking, there are two primary mechanisms that allow plants to tolerate environmental stresses: avoidance and tolerance. A complex interplay of physiological, morphological, phonological, biochemical, and molecular responses characterizes plant adaptation to water stresses.
Escape mechanism
-
Different escape mechanisms have evolved by plants to help them deal with abiotic stresses and improve their chances of surviving and procreating. These mechanisms are ways for plants to lessen or avoid the harmful effects of unfavorable environmental circumstances. Because plants can change their phenology, they can potentially escape from water stress. One common way that plants escape is by shortening their life cycle, growing seasonally, or allowing them to multiply before the environment becomes unfavorable[2,9,14]. A shorter life cycle can cause an early onset of the reproductive stage, which can result in water deficit escape[2,14,19]. Plant phenology is largely determined by the plant's genotype and growing environment. Plants that flower and mature early thus have an advantage over those that mature later[2,9,14]. Early flowering allows plants to begin reproductive growth before the terminal stress by shortening their vegetative stage[53]. This guarantees the production of progeny for the propagation of the next generation. Plants' most effective escape strategy is early maturity, but this has a detrimental effect on overall production[54]. Lower yield potential results from shorter photosynthesis and nutrient accumulation times needed to achieve higher yields[55]. Delay in germination, in which seeds wait for ideal environmental conditions before bursting into life, is another means of escape. The ability of seeds to withstand adverse conditions is known as dormancy.
Avoidance mechanism
-
Plants experience morphological and anatomical changes as a result of environmental pressures. These modifications protect the plants' systems from disturbance and enhance their capacity to withstand stressful conditions. There are many different anatomical changes that plants have experienced at the organ and organizational levels. Variations have been observed in root, xylem, and leaf morphology in response to environmental pressures. Morphological changes can include slower internode growth, larger leaves with more surface area, altered branching patterns, and faster growth of shoots and roots in response to various stresses. The main reasons for alteration at the anatomical level in plant organs like roots are modifications to the conditions for cell differentiation and decreased cell elongation, which result in a variety of anatomical characteristics. Plants typically regulate their stomatal transpiration and maintain water uptake from the soil through an extensive and effective root system in order to minimize water loss. Effective root systems have thick, deep roots that allow plants to scavenge water from a significant depth of soil and a considerable distance from the plant[4,56,57]. Cuticle and trichomes are two examples of plant structures that support the preservation of a high tissue water potential in plants. Nevertheless, because of the energy required to create them, these mechanisms result in a decrease in plant yield. Thus, in order to supply energy for maintaining a relatively high water potential, plants with avoidance mechanisms typically have smaller sizes[4,58,59].
Tolerance mechanism
-
According to several sources[4,59], plant tolerance is the capacity of a plant to continue growing and storing nutrients while continuing its vegetative stage. Physiological and biochemical modifications are a part of these processes. Mechanisms involved in plant tolerance to drought include the accumulation of compatible solute and osmotic adaptation, the induction of an antioxidant system, modifications to metabolic pathways, an increase in the root/shoot ratio, and stomata closure[60]. A build-up of active compounds, such as sugars and amino acids, helps plants under water stress modify their osmotic conditions and lower their osmotic potential. With the help of osmotic adjustment, plants can survive in situations where there is a water shortage by effectively absorbing water and maintaining cell turgor pressure. The resilience of their cell membrane is another feature of plants that use tolerance as a mechanism for adaptation. Electrolytes and water cannot escape from cells when the cell membrane is stable. Plants with a cuticle layer have been shown to sustain leaf turgor under stress for longer[61,62]. In order to minimize water loss and maintain energy production, plants may modify their photosynthetic pathways in response to stress. Different mechanisms of rice tolerance are shown in Fig. 1 for different flooding scenarios, Fig. 2 for drought stress, and Fig. 3 for salt stress tolerance.
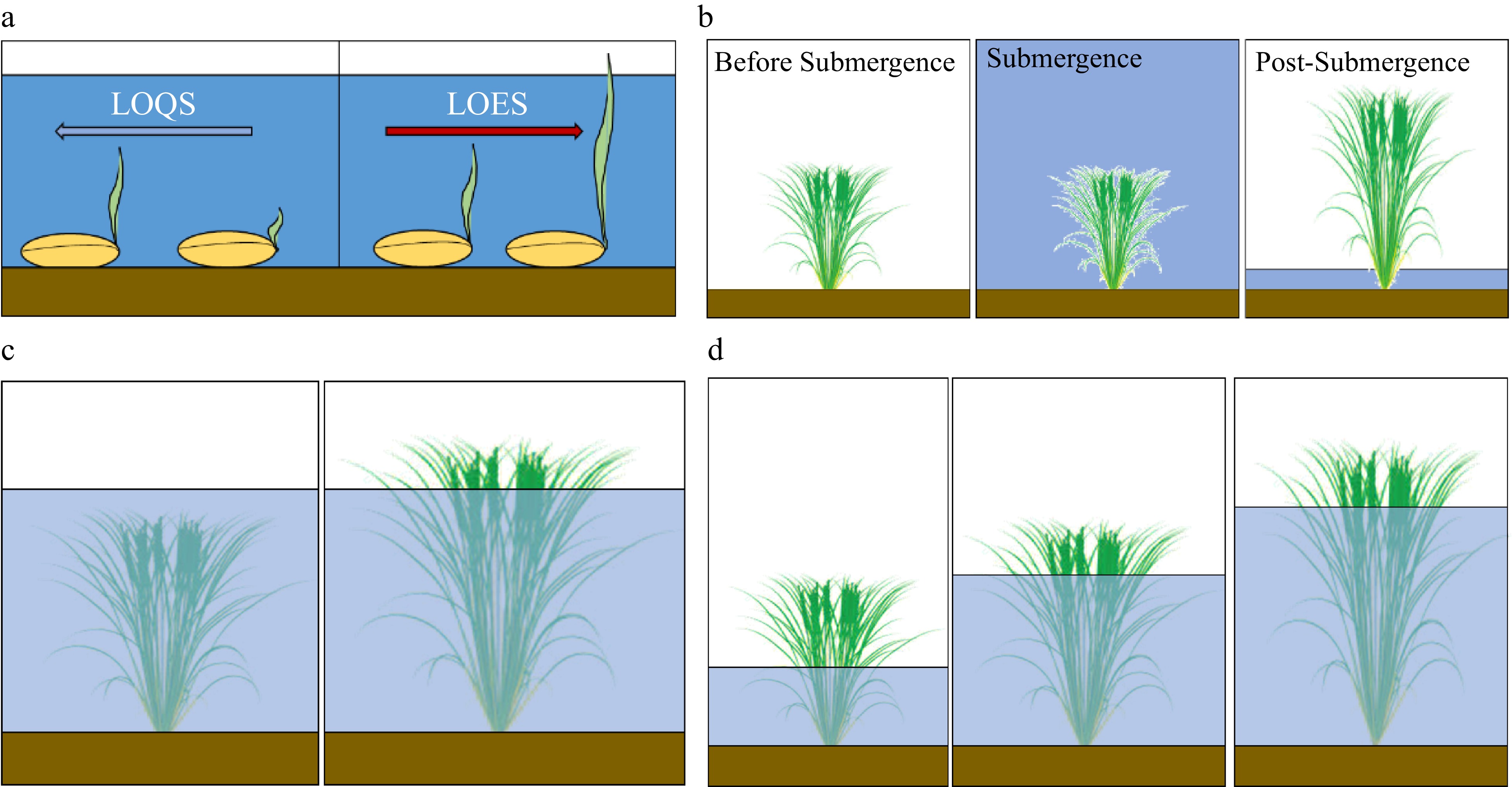
Figure 1.
Complete submergence: Rice coping mechanism. (a) Flash flooding at germination. Low O2 escape (LOES) and low O2 quiescence (LOQS) strategies[117]. Oxygen is one of the important factors that affects germination. Under submergence, the root formation is inhibited. Sensitive cultivars exhibit LOQS strategy, that inhibits coleoptile elongation. Tolerant varieties exhibit faster coleoptile elongation. (b) Flash flooding (< 2 weeks). Under complete submergence, shoot elongation of tolerant varieties is inhibited (quiescence), conserving carbohydrate reserves and allowing survival under water. After water subsided, growth and rejuvenation is resumped[118]. (c) Severe flash flooding (< 2 weeks). The response to flooding in rice is a function of the SNORKEL (SK) locus, responsible for stem elongation[119]. The expression of these genes promotes elongation of the rice internodes, enabling plants to elongate. (d) Deepwater (slow rise stagnant flooding). Rice plants tolerant to deepwater flooding, where water depth is from 50 cm to around 4 m, show significant stem elongation as water levels rise. The deepwater-flood-tolerant rice becomes flattened, and generates new roots and tillers[120].

Figure 2.
Drought tolerance mechanism in sugarcane. A tropical crop having a C4 photosynthetic metabolism is sugarcane. Under moderate water stress, stomatal conductance (gs), transpiration rate (E), internal CO2 concentration (Ci), and photosynthetic rate decrease, primarily because of stomatal limitations. When sugarcane plants experience mild to moderate dryness, this is the most frequent initial adaptation, along with the suppression of stalk and leaf growth. But non-stomatal restrictions brought on by water stress have also been implicated in sugarcane photosynthesis suppression, whenever there is a lot of tension or stress[121].
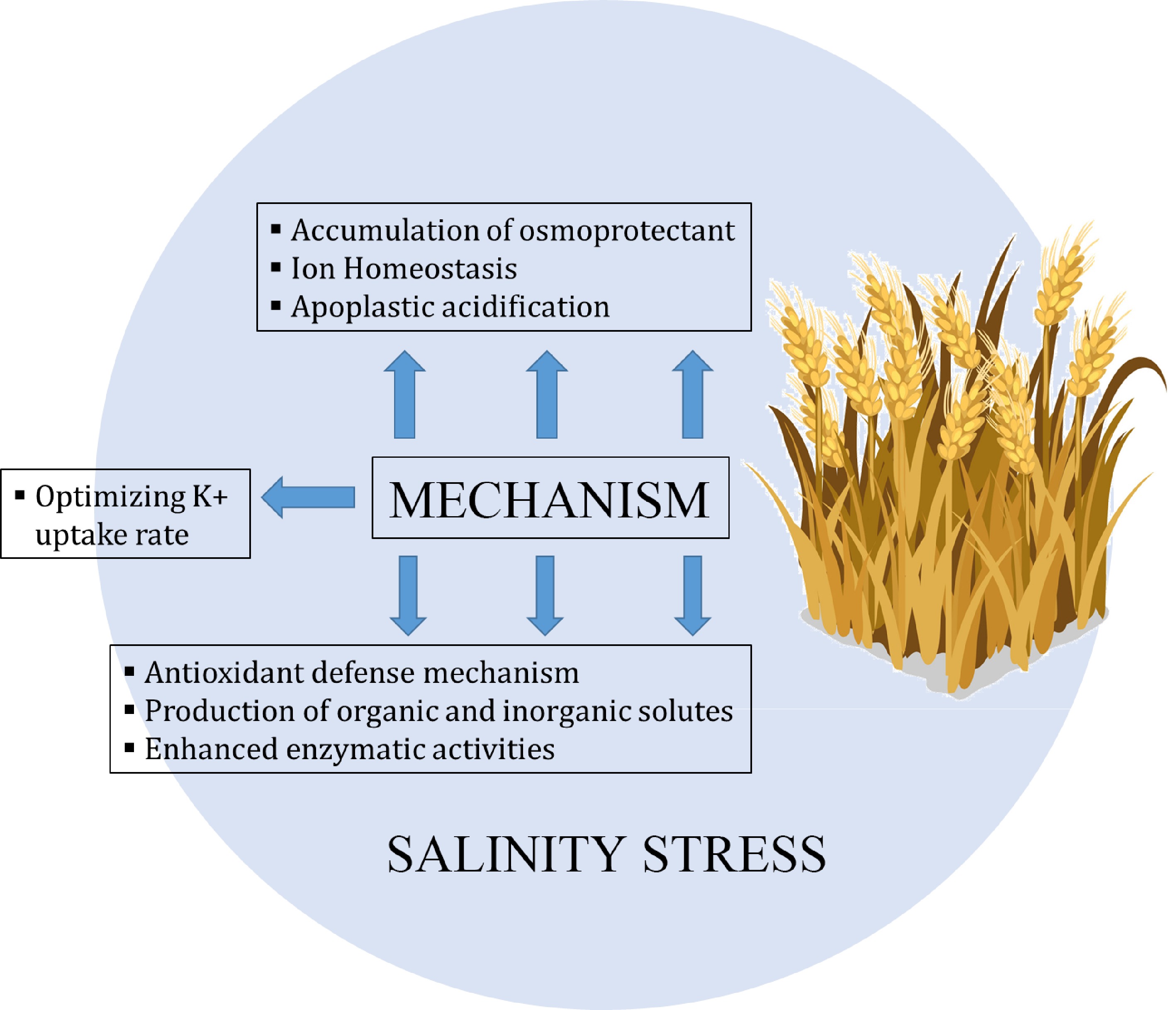
Figure 3.
Saline stress response mechanism in wheat[122]. The defensive system against anti-oxidant species is triggered by osmoregulation, which also controls the water interaction between plants. The molecular weight of osmoprotectants is low, they have no net charge, and they are hydrophilic in nature. The salt-tolerant cultivars of bean plants have lower protein levels and higher levels of proline and other amino acids than the salt-sensitive variants. In order to maintain a low Na+ ion concentration and increase the K+ concentration, ionic homeostasis is a crucial function that controls ion flux. The cytosolic activity of the different enzymes depends on the control of intracellular Na+ and K+ ions (homeostasis), which also regulates membrane potential and cell volume. Excessive salt buildup in the root zone of plants causes the development of reactive oxygen species (ROS), severe osmotic stress, and ion toxicity in plants. The breakdown of proteins and changes to DNA sequencing are both brought on by ROS in plants. The superoxide dismutase and catalase enzymes, among others, are activated by salt stress in plants to create an anti-oxidative mechanism.
Molecular mechanism
-
Plants have the ability to create molecular defenses against abiotic stressors through their genetic and sensory systems. Numerous compartments within cells, including the cell wall (CW), plasma membrane (PM), cytoplasm, mitochondria, chloroplasts, peroxisomes, endoplasmic reticulum, and nucleus, are susceptible to abiotic stress[63]. This may set off chemical reactions. The downstream regulatory proteins and secondary messengers, such as Ca2+, ROS, and protein kinases, receive the signals from these stress sensors. Abiotic stress also sets off a range of reactions that entail multiple levels of control, signal transduction, and stress sensing. Because of this, plants have evolved defense mechanisms to change how they develop so they can endure and procreate in harsh environments[64]. Gene expression results in the creation of distinct proteins in a variety of plant components, which shield the cell from damage and activate a vast array of genes essential for abiotic resistance mechanisms in plants. Many kinds of proteins are produced, such as chaperones and late embryogenesis abundant proteins (LEA proteins), which are crucial to the development of tolerance. Stress-associated genes are all simultaneously focused on the stress response[65]. Three distinct stages of regulation govern genes related to stress in plants: transcriptional, posttranscriptional, and posttranslational[66,67].
-
Plant breeders have tested various breeding techniques to improve abiotic stress tolerance in crop plants[59,68−71]. The production of hybrid, mutants and transgenic plants are some of the well-known methods for this purpose[58,70]. The wide range of drought related genes in the plant genome has opened amazing opportunities for crop improvement.
Conventional breeding approach
-
Abiotic stress-tolerant plants have been produced through conventional breeding methods in a number of attempts[59,72,73]. With this technique, plants that have a history of withstanding abiotic stress are chosen, crossed, and their genes are exchanged to create offspring with a different genetic make-up. The production of stress-tolerant plants can be accelerated significantly through the application of plant breeding techniques[4,39]. It is thought that using wild relatives or cultivars can extract tolerance genes from their varied genetic makeup. Compared to their domesticated descendants, these wild relatives of crop plants exhibit more resilient tolerance[74]. It has been possible to produce crops that are both highly productive and resistant to stress through conventional breeding. In order to create new breeds of abiotic-tolerant crops that are resistant to drought, salt, cold, and submersion, breeders have taken advantage of genetic variation in crops at the intraspecific, interspecific, and intergeneric levels. The Philippine Rice Research Institute[75], India[76], and Pakistan[77] farmers have been able to produce rice varieties that are drought-tolerant thanks to the efforts of the International and Philippine Rice Research Institutes[78,79]. From 2011 to 2021, a total of 35 rice varieties resistant to drought, 38 rice varieties resistant to salt, and two rice varieties resistant to heat were developed in the Philippines (Table 1). Nevertheless, the low variation in gene pools and the source of the tolerant genes limit conventional breeding.
Table 1. Rice mutant varieties developed and released in the Philippines, 1971 to 2020.
No. Mutant cariety ID No. Year Character improvement details 1 BPI-121-407 1202 1971 Early maturity, very short stem, stiff-strawed, high tillering, resistance to diseases and moderate resistance to bacterial leaf blight 2 PARC 1 1203 1970 Narrow and long grains with less chalky areas 3 PARC 2 1204 1973 Early maturity (5−10 d), narrow and long grains with less chalky areas and good eating quality 4 BPI Ri-10 1205 1983 Early maturity, semi-dwarf, resistance to pest, high yield, non-seasonal (for both dry and wet seasons) and good eating quality 5 PSB Rc78 2393 1999 Better yield potential, 8 d shorter in maturity and 5 cm shorter in height as compared to the original variety, good quality 6 Milagrosa Mutant 2394 1974 High yield and resistance to diseases 7 Azmil Mutant 2395 1976 High yield, drought resistance and susceptible to blight 8 Bengawan Mutant 2396 1984 High yield, short plant stature and early maturity 9 PR22902 3368 1994 High yielding and early maturing 10 NSIC Rc 346 4467 2013 Agronomic traits: maturity, plant type, grain and panicle traits 11 NSIC Rc 272 4468 2011 Agronomic traits: maturity, plant type, grain and panicle traits 12 NSIC 2019 Rc 572 4903 2020 Agronomic traits: maturity, plant type, grain and panicle traits, grain quality Source: International Atomic Energy Agency (IAEA), Mutant Variety Database (MVD), 2022. Induced mutation approach
-
Wide genetic variation in key traits is necessary for crop improvement through plant breeding. Frequently, the natural gene pool lacks this desired variation. Although spontaneous mutation is a natural process that broadens genetic variation, it occurs very infrequently and cannot be utilized in plant breeding to create new, improved varieties. Therefore, physical or chemical mutagens are used to induce genetic variability in order to increase the rate of mutations[80]. In order to create lines that are resistant to disease and abiotic stress, mutation techniques like somaclonal variation and in vitro mutagenesis have been extensively employed. In rice breeding, the most widely used techniques are tissue culture, chemical, or physical mutagens that induce mutation[81]. Population variability brought about by mutations makes it possible to choose the best mutation or set of mutations[82]. In the context of climate change, tolerance traits to stresses, such as drought, saline, submersion, and high temperature, are identified more quickly and with less resources thanks to this tool[83]. In rice breeding, induced mutation via physical mutagen has been widely employed to produce mutant populations with a broad range of variation required for effective selection. Rice lines with improved traits have been successfully developed through mutation breeding[84]. There are currently 861 muts registered in the International Atomic Energy Agency's Mutation Variety Database[85].
Genetic engineering
-
The identification of restriction enzymes and ligases was made possible by the discovery that bacterial plasmids could replicate independently of the bacterial chromosome, among other discoveries[86]. This allowed for the covalent insertion and linking of genetic material from a completely different species into the plant genome. The method for creating transgenic plants with abiotic stress tolerance was made possible by the introduction of genes into plants through the use of the soil bacterium Agrobacterium tumefasciens. Using microscopic DNA-coated particles, the method mechanically penetrates plant cells or allows a segment of DNA to enter naturally[87]. The expression of particular and related stress genes serves as the foundation for the application of contemporary molecular biology techniques to genetically modify stress-tolerant crops and to explain the response mechanisms to abiotic stress tolerance. Genetic engineering has therefore shown to be a quicker route to creating plants that can withstand abiotic stress.
Three categories of genes are involved in the expression of genes related to stress response: genes that encode proteins, proteins whose functions are still unknown, and regulatory proteins[88]. The first attempts to create transgenics (primarily tobacco) with increased resistance to abiotic stress involved 'single action genes' (Fig. 4)—genes that alter a single metabolite to confer increased resistance to drought or salt stress[89]. In order to improve tolerance to abiotic stresses, genetic engineering is currently concentrated on increasing compatible solutes at the molecular level[90−92]. These solute advancements have improved resistance to a variety of abiotic stresses, including cold, salt, and drought[93].
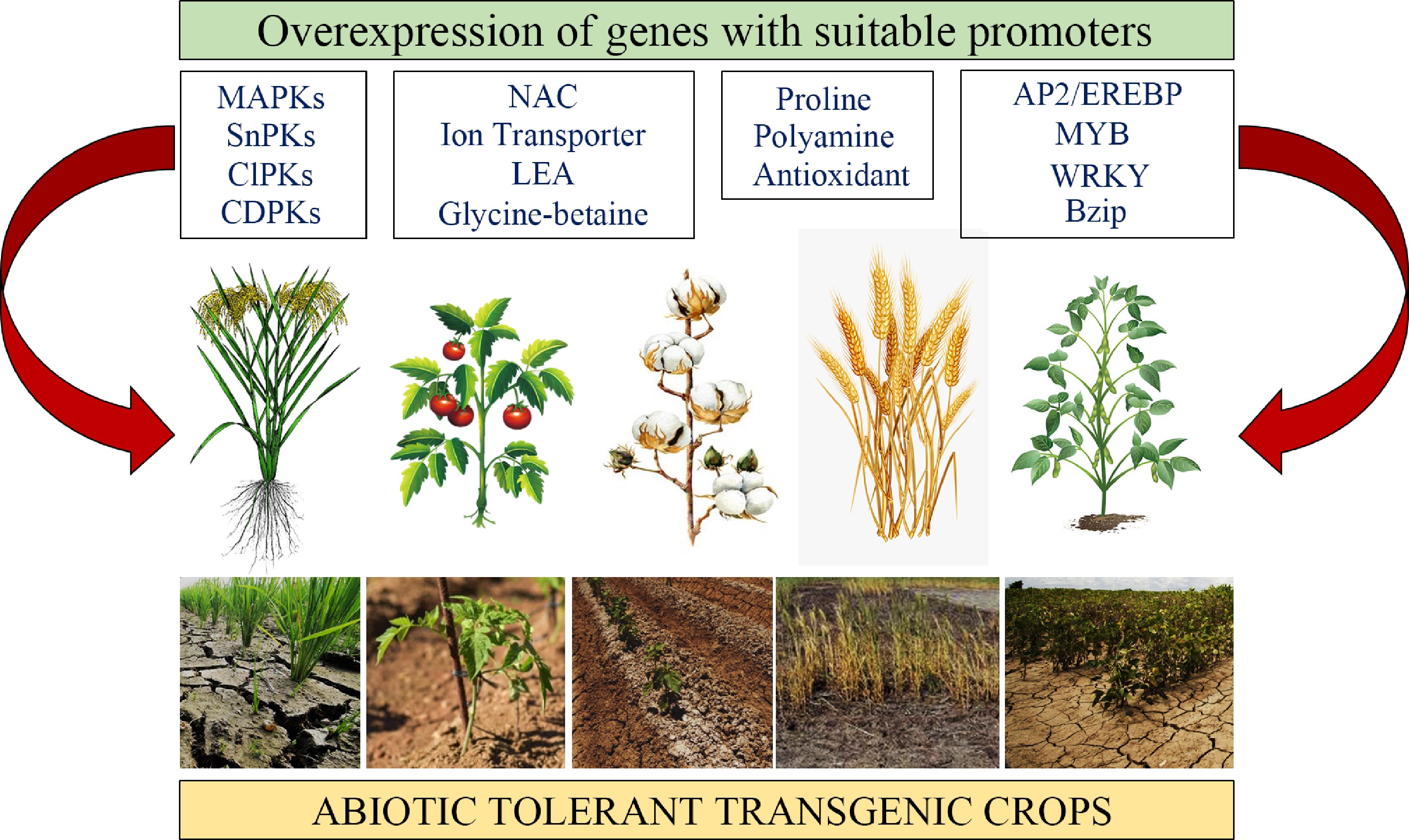
Figure 4.
Transgenic approach. Transgenic strategies by overexpressing different groups of genes, including regulatory and functional genes with suitable promoters (stress inducible) to improve abiotic tolerance in crops[91].
Molecular marker
-
The application of closely linked DNA markers to agronomically significant genes (gene 'tagging') as molecular tools for marker-assisted selection (MAS) in crop improvement is known as 'molecular breeding', or the use of molecular markers in plant breeding. In contrast to the more traditional plant breeding approach, MAS uses the presence or absence of a marker to support or replace phenotypic selection in a way that may be more effective, efficient, dependable, and economical.
Plant breeders typically work with hundreds to thousands of plants in populations that are kept apart, and a crucial part of the breeding program is selecting plants that have the gene combinations needed for desired characteristics[94]. Tight linkage between the markers and target genes or quantitative trait loci (QTL) enhances the efficacy and efficiency of selection through MAS. MAS offers the following benefits[95]: (1) time savings by replacing time-consuming and technically complex field trials with molecular tests; (2) removing the unreliable phenotypic evaluation associated with field trials due to environmental effects; (3) genotype selection at the seedling stage; (4) gene 'pyramiding', or combining multiple genes at once; (5) preventing the transfer of undesirable or deleterious genes ('linkage drag', which is particularly relevant when the introgression of genes from wild species is involved); (6) selection for traits with low heritability; (7) testing for specific traits where phenotypic evaluation is not practical.
Abiotic stress tolerance and yield are two examples of agriculturally significant traits that are quantitative in nature and are regulated by multiple genes; these traits are also referred to as polygenic, multifactorial, and complex traits[95]. QTLs are the areas of genomes that contain the genes linked to a specific quantitative trait. It is not possible to identify using only traditional phenotypic evaluation. Selection for QTLs was made possible by the significant advancement in the characterization of quantitative traits brought about by the development of DNA or molecular markers in the 1980s[95]. Marker-assisted backcrossing (MAB), a type of MAS, attempts to prevent donor introgressions throughout the rest of the genome while transferring a particular allele at the target locus from a donor line to a recipient line[96]. Utilizing molecular markers to facilitate genetic dissection of progeny at each generation speeds up the selection process, increasing genetic gain per unit of time[97,98]. The following are the main benefits of MAB[96]: (1) quick breeding of new genotypes with advantageous traits; (2) efficient background selection for the recurrent parent genome; (3) minimization of linkage drag surrounding the locus being introgressed; and (4) efficient foreground selection for the target locus. Furthermore, it states that 'the number of backcrossers, population size, and availability of flanking and/or closely linked markers for the target locus all affect how effective MAB is'[99].
The most studied rice genotype FR13A (FR for flood resistant), a photoperiod landrace from Orissa, India, is highly submergence tolerant, but agronomically undesirable, with low yield and long awns, poor cooking quality and lacking aroma[100]. This cultivar can survive complete submergence for up to two weeks, while most varieties are intolerant of over four days of submergence[101]. Most of the tolerance is controlled by a major sub1 QTL (Fig. 5) with large effect, mapped on rice chromosome 9, and accounts for 70% of the phenotypic variation for survival under submergence. The QTL on chromosome 9 (sub1 QTL) is a primary determinant of submergence tolerance[102]. There are three identified ethylene-response-factor-like (ERF) genes in the Sub1 region, viz., Sub1A, Sub1B and Sub1C[103]. Sub1A is the gene conferring submergence tolerance in rice and it has two alleles, Sub1A-1 (tolerance-specific) and Sub1A-2 (intolerance-specific), with Sub1A-1 allele as the primary determinant of submergence tolerance[104]. The relative locations of Sub1 QTL region and the robust foreground Sub1 markers, SC3 (RM8300) and ART5 in chromosome 9 are indicated in Fig. 3. A successful introgression of sub1 QTL in chromosome 9 was previously reported for a Thai Jasmine rice, KDML 105, widely grown in the rainfed lowland regions of Thailand, but very intolerant to submergence stress[105]. KDML 105 was crossed with FR13A, IR67819F2-CA-61 and IR49830-7-1-2-2, the source of SubQTL. The introgression of the SubQTL was facilitated by four backcrosses and genotyping of 467 BC4F2 with seven markers (RM285, R1164, 126GIR, 180DIR, RBO783, RM219, RM105) for foreground selection of major QTL on chromosome 9, two markers (RM221, RM240) for minor QTL on chromosome 2, and two markers (OSR4, RM11) for minor QTL on chromosome 7 and with 47 SSLP (simple sequence length polymorphism) markers (four markers spanning each chromosome) for whole-genome scanning of the top nine most submergence-tolerant BC4F2 lines with favorable genetic background (background selection).
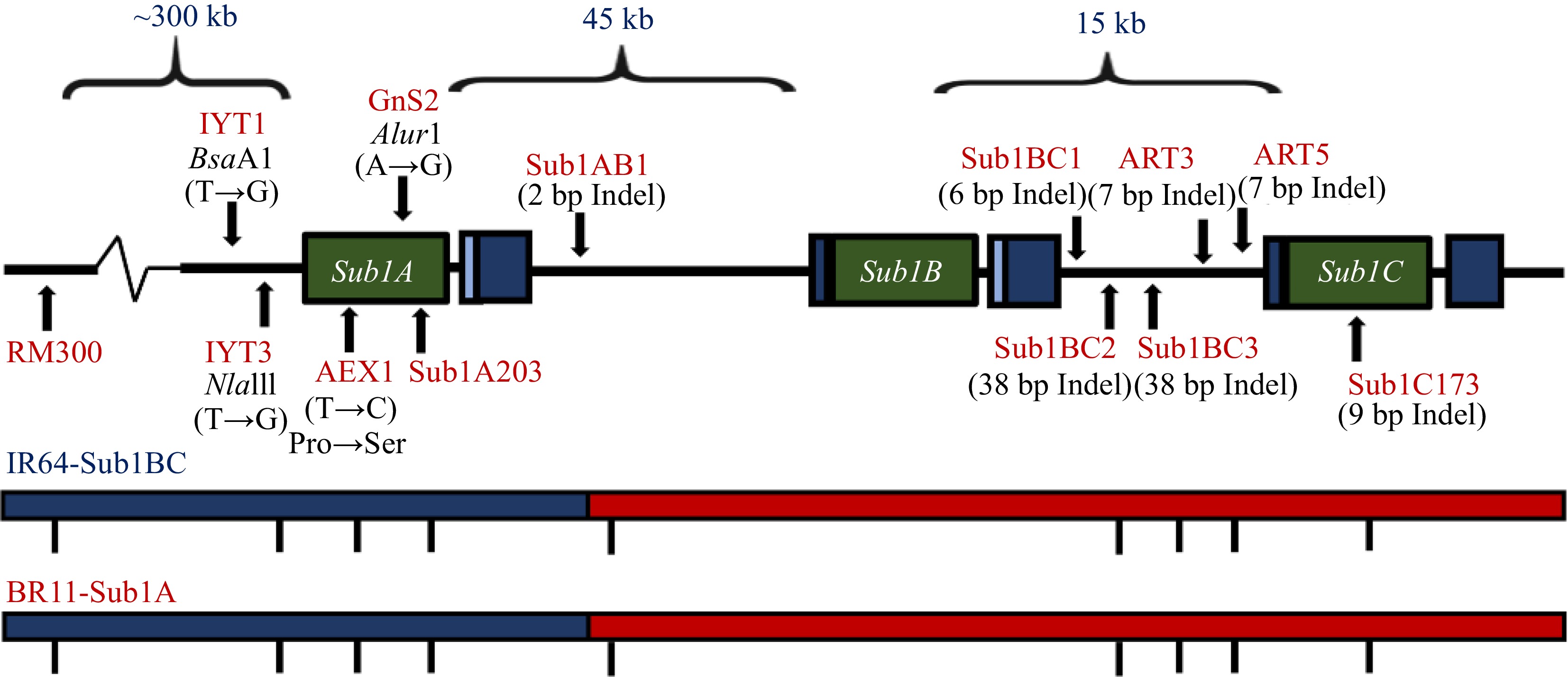
Figure 5.
The Sub1 QTL conferring submeregnce tolerance. Relative location of Sub1 QTL region and SC3 (RM8300) and ART5 foreground markers in chromosome 9 of the rice genome, conferring tolerance to complete submergence[104].
Genome editing
-
With the use of nucleases like transcriptional activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9, genome editing, a recent technological advancement, offers a way to introduce targeted mutation, insertion/deletion (indel), and precise sequence modification in a wide range of organisms[106−108]. The targeted genomic locus experiences double-strand breaks due to these sequence-specific nucleases, which can be repaired by either the major intracellular repair pathway, homology-directed repair (HDR) or non-homologous end joining (NHEJ). While HDR inserts targeted point mutations or the insertion of desired sequences through recombination, NHEJ introduces indels into the genome[109]. The CRISPR-Cas9 system's simplicity gave rise to options for genome editing in a range of biological contexts.
The CRISPR-Cas9 system is a rapidly developing technique for genetic modification that allows target genes to be inserted into the plant genome without requiring the involvement of any other species (Fig. 6). A single guide RNA (sgRNA) directs the cleavage mediated by the Cas9 nuclease. By base pairing, the sgRNA identifies the target DNA[53, 54]. The short guide RNAs (sgRNAs) have a length of 20 to 22 nucleotides and can be readily assembled into oligonucleotides. Cas9 nuclease is able to target any DNA by altering the guide sequence. Its modular design and capacity to target small sgRNA confer further benefits. These sgRNAs ensure that the target DNA sequence is edited for a specific trait by having a high specificity and few off-target effects. Because of these benefits, the CRISPR-Cas9 system is amenable to multiplexing, which allows mutations to be inserted into several genes or genomic loci simultaneously. A wide range of applications in both basic and applied plant biology research have been made possible by the CRISPR-Cas9 system's high efficiency and target assurance in genome modifications. The application of the CRISPR-Cas9 system to genetically modify plants to withstand abiotic stress has not yet been documented. With the aforementioned benefits, however, the CRISPR-Cas9 system can offer an enormous platform for studying the functions of genes and genomes as well as enhancing a plant's resistance to abiotic stressors.
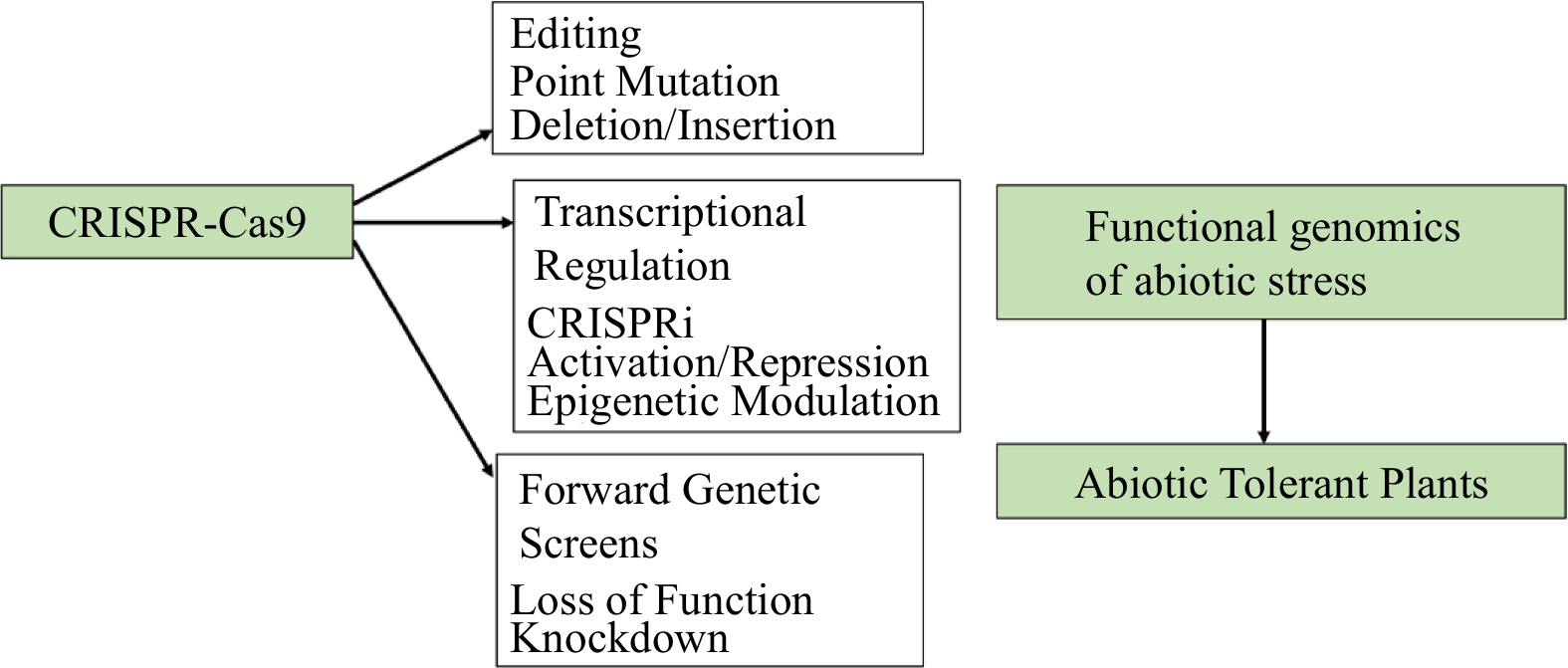
Figure 6.
The CRISPR-Cas9 system. The CRISPR-Cas9 system in the context of functional genomics for abiotic stress tolerance. CRISPR-Cas9 system uses point mutations, insertions or deletions, transcriptional regulation through CRISPR interference, activation, repression or epigenetic modulation, or through generation of loss-of-function, knock-down or activation mutants[123].
-
The growing number of extreme and intense weather events brought about by the ongoing alteration of climate patterns has an impact on plant growth, development, and yield. Adverse conditions leading to low crop production in farmers fields are not desirable. Climate change will only make the stress conditions that agriculture ecosystems are already vulnerable to worsen, such as drought, flooding, salinity, and high temperatures. In order to ensure food security for everyone on the planet, a substantial amount of harvested land that is vulnerable to these extreme conditions is currently needed. To counteract the negative effects of unfavorable climate change-related conditions, plants need to undergo genetic engineering to increase their resistance to abiotic stresses.
In comparison to the effects of single stresses alone, the effects of combined stresses on plants are completely different. When heat and drought are combined, photosynthetic rates are reduced more than when either stress is present alone[110,111]. A variety of stresses can affect a plant's ability to regulate its stomata, use nutrients and water efficiently, reproduce, and react to pests and diseases[112]. When subjected to single stressors, plants exposed to combined stressors respond differently. Because stimulation, signal transduction, and regulatory networks differ when multiple stresses occur at the same time, conflicting, additive, or unrelated responses are activated[113,114]. Because various stressors necessitate distinct adaptation mechanisms, plants may combine molecular, physiological, and biochemical response mechanisms, or they may prioritize one type of mechanism over another[115,116].
The development of acceptable and adoptable varieties in these environments that are tolerant of multiple stresses has been the focus of current plant breeding programs. Enhancing physiological efficiencies while simultaneously being exposed to abiotic stresses is one of the major goals of these programs.
-
The author confirms sole responsibility for the following: article research and review, and manuscript preparation.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study
-
The author acknowledges the following individuals and institution: Dr. Nenita V. Desamero, Jonathan M. Concepcion, Rj D. Buluran, Josielyn C. Bagarra, and the Department of Agriculture-Philippine Rice Research Institute, Central Experiement Station and Branch Stations.
-
The author declares that there is no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Cabusora CC. 2024. Developing climate-resilient crops: adaptation to abiotic stress-affected areas. Technology in Agronomy 4: e005 doi: 10.48130/tia-0024-0002
Developing climate-resilient crops: adaptation to abiotic stress-affected areas
- Received: 20 July 2023
- Revised: 28 January 2024
- Accepted: 31 January 2024
- Published online: 07 March 2024
Abstract: Abiotic stresses, caused by climate change pose a huge threat to agriculture. In particular, climate change related drought stress will have large negative impact on crop growth, development and eventually production. As the changes in the weather patterns have a direct impact on farmers' capability to grow crops, the urgency of improving farmers' adaptive capacity should be addressed to minimize the potential negative impacts of climate change. Availability of adaptation technologies that would reduce crop production losses is of utmost importance in attaining climate change resilient crops. One potential adaptive measure is the use of crop varieties resilient to climate change related stresses. Various breeding technologies have been used to develop new durable crops, if not, enhanced or improve the ability of crops to survive under adverse environmental conditions, brought about by the changes in climate. One of the most sustainable strategies to mitigate these effects to agriculture is the development of climate resilient crops. Crops that can thrive under extreme weather conditions as effects of the changing climates. Conventional breeding may not be enough to develop new breeds of crops with better durability to abiotic stresses such as drought, salinity, submergence, high and low temperatures. Thus, other strategies as stand-alone or in combination with conventional breeding, are explored to enhance genetic variability for improving tolerance to abiotic stresses. These are the biotechnological approaches including marker assisted breeding, mutation breeding, genetic engineering and genome editing. These technologies offer a better future for developing climate change resilient crops.
-
Key words:
- Climate change /
- Abiotic stress /
- Resilient /
- Breeding /
- Biotechnology












