-
Figure 1.
Schematic representation of glycoprotein sources and derivatives influencing the growth of A. muciniphila. Porcine stomach mucin, bovine milk, and egg white/egg yolk serve as sources for glycoproteins and glycans, which are potentially critical for the growth of A. muciniphila within the gut environment. The inset shows a microscopic view of A. muciniphila colonizing the gut mucosa.
-
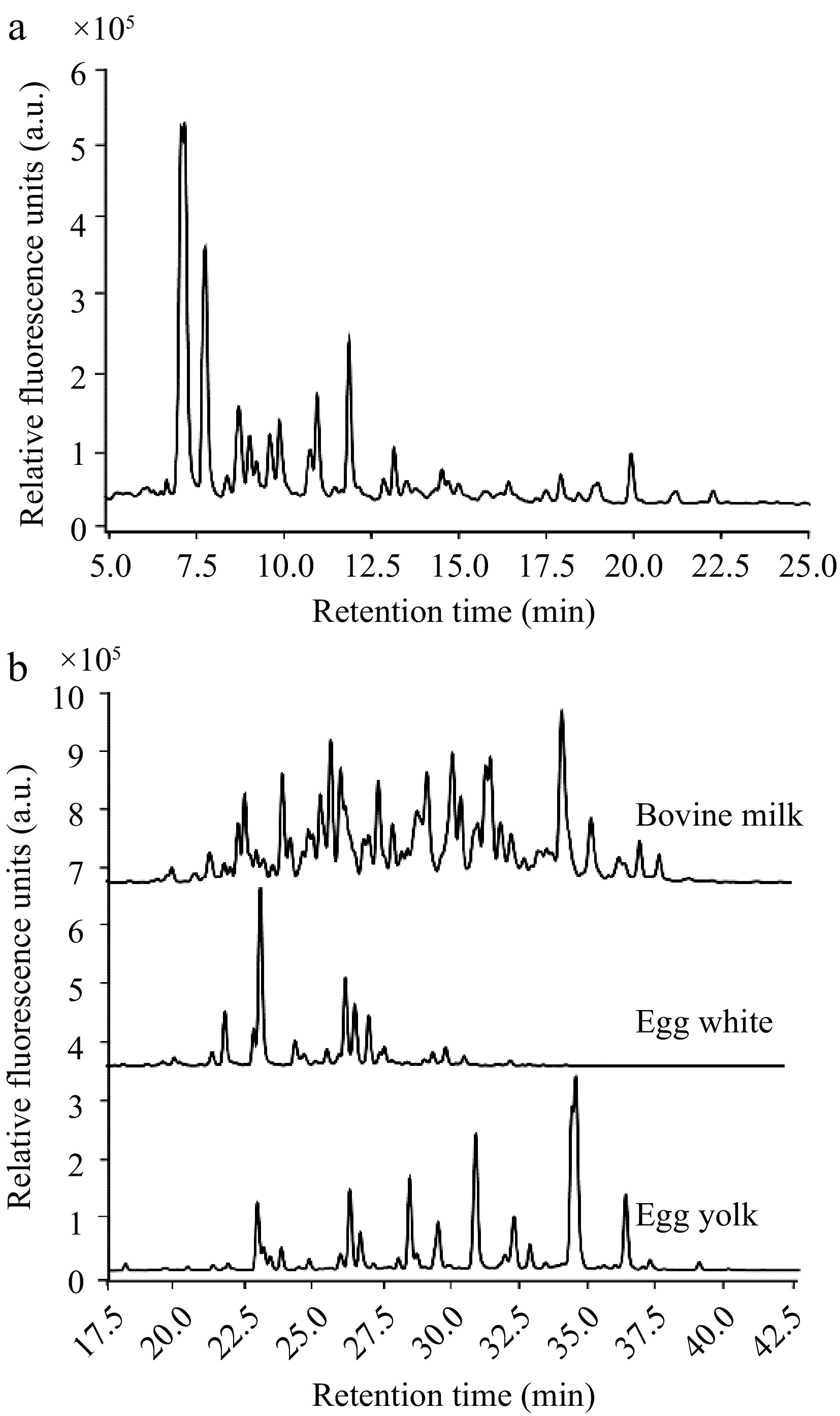
Figure 2.
HPLC elution profiles of the glycan types investigated. (a) Range of mucin O-glycans by their retention times. (b) Comparison of the retention profiles of N-glycans from bovine milk, egg white, and egg yolk, reinforcing the diversity in N-glycan structures across different food sources.
-
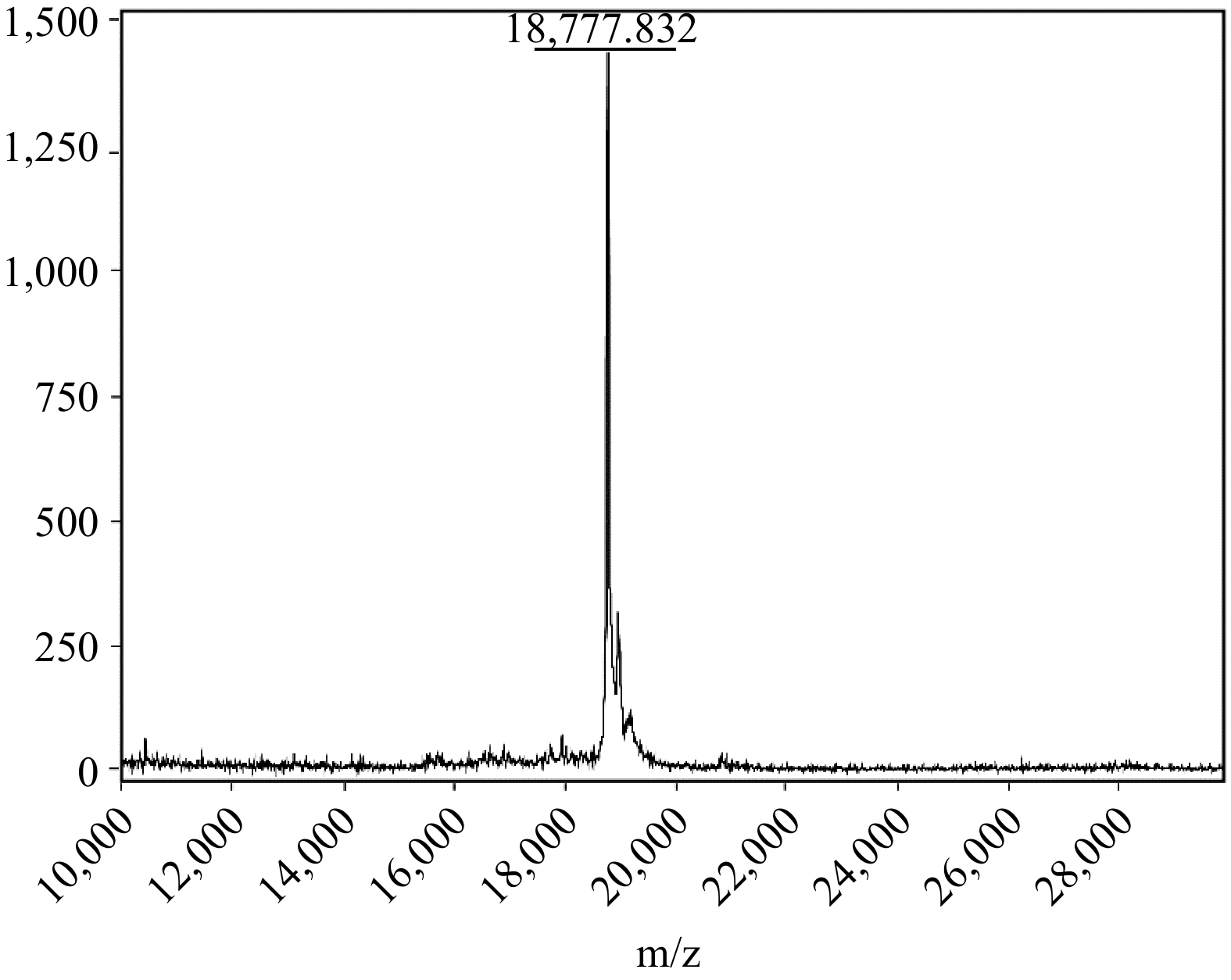
Figure 3.
MALDI-ToF-MS analysis of the recombinant SUMO-MUC5AC protein.
-
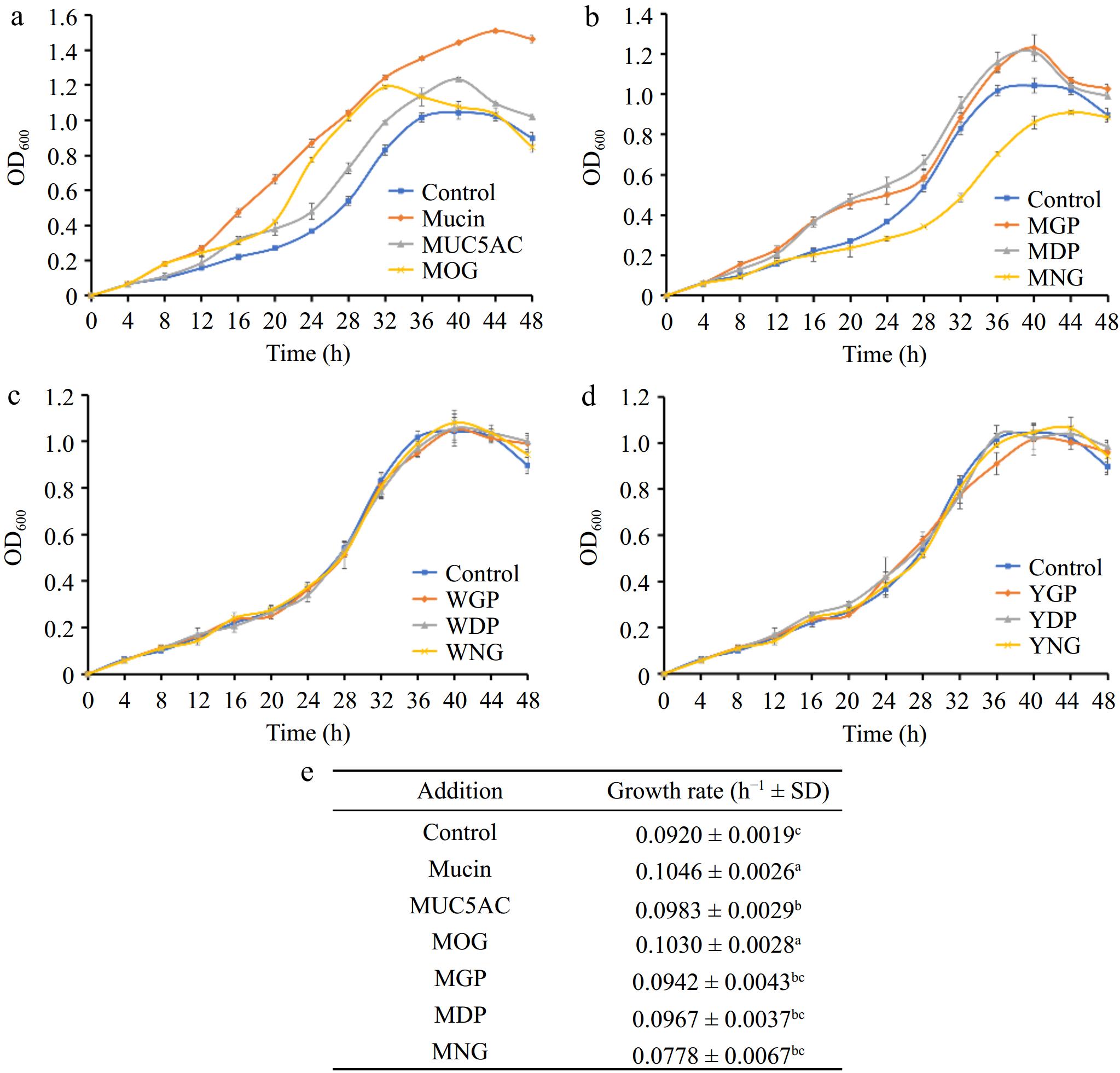
Figure 4.
(a) Comparative effects of mucin O-glycosylation, (b) bovine milk N-glycosylation, (c) egg white N-glycosylation, and (d) egg yolk N-glycosylation on the growth of A. muciniphila, (e) growth rates. A. muciniphila's responses to various glycan and protein substrates are denoted by differing letter groupings, which treatments yielded significant differences in bacterial growth (p < 0.05). (MOG: mucin O-glycan, MUC5AC: nonglycosylated mucin, MGP: bovine milk glycoprotein, MDP: bovine milk deglycosylated protein, MNG: bovine milk N-glycan, WGP: egg white glycoprotein, WDP: egg white deglycosylated protein, WNG: egg white N-glycan, YGP: egg yolk glycoprotein, YDP: egg yolk deglycosylated protein, YNG: egg yolk N-glycan).
-
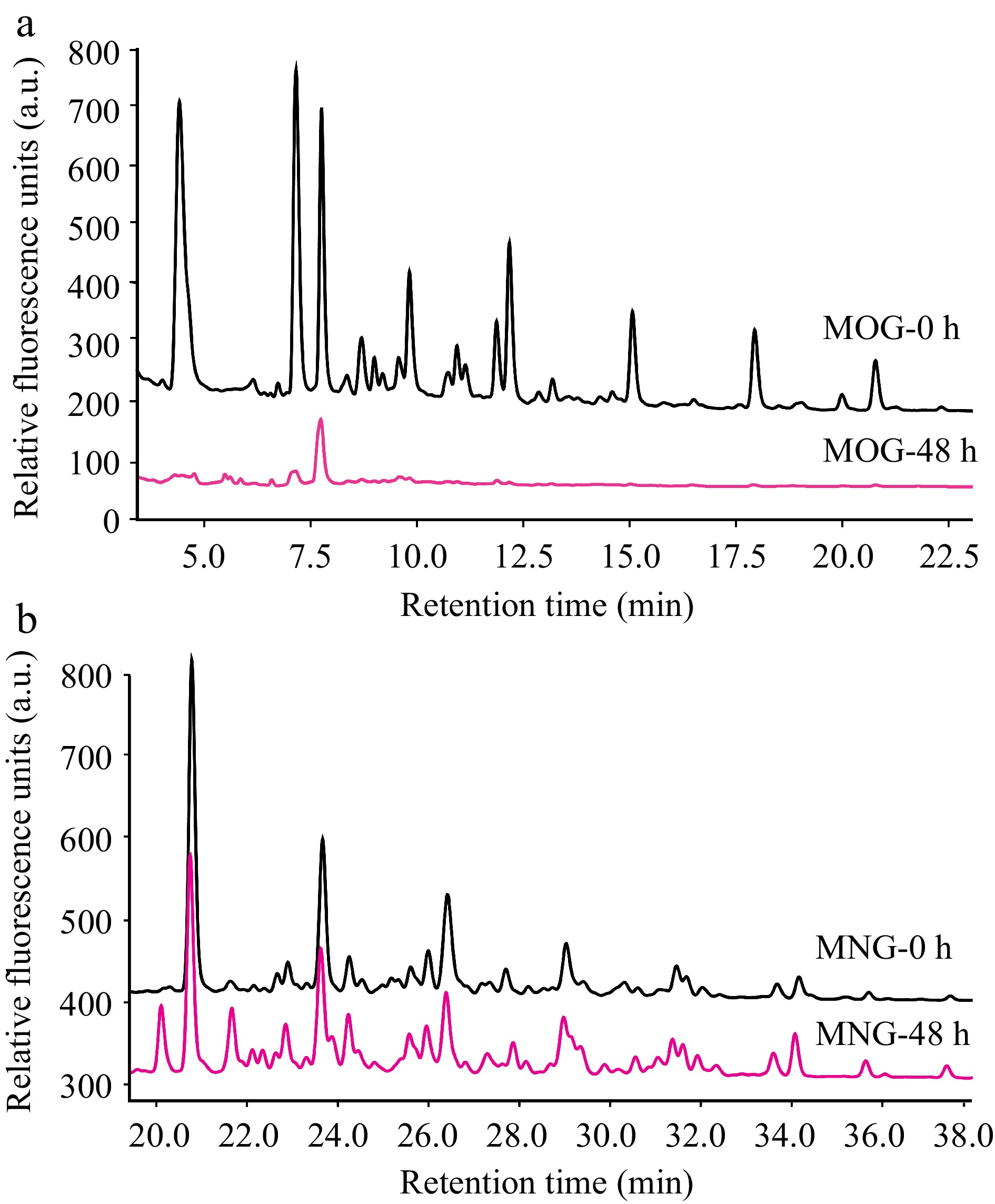
Figure 5.
HPLC profiles that detail A. muciniphila's utilization patterns for (a) mucin O-glycan and (b) bovine milk N-glycan throughout the fermentation process. The depicted chromatograms offer a visual representation of the degree to which A. muciniphila can hydrolyze and assimilate these contrasting glycan types, thus providing insights into the bacterium's substrate specificity and potential nutritional preferences within the intestinal milieu. (MOG: mucin O-glycan, MNG: bovine milk N-glycan).
-
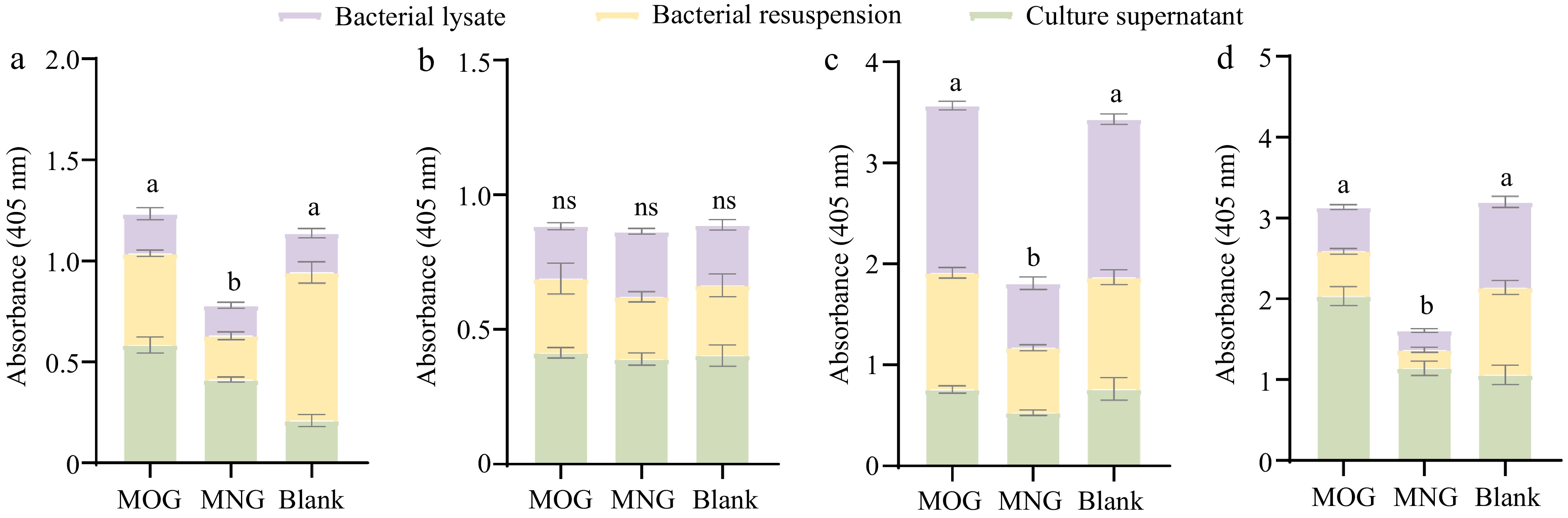
Figure 6.
Differential activity profiles of A. muciniphila's exoglycosidases when assayed against four synthetic substrates: (a) pNP-β-GlcNAc, (b) pNP-α-Fuc, (c) pNP-β-Gal, and (d) X-Gal-α-Neu5Ac, where variations in enzyme activities are statistically significant (p < 0.05) as indicated by different letters. Comparisons between the various substrate groups reveal nuanced enzyme responses to the presence of glycan molecules, shedding light on A. muciniphila's adaptability in glycan utilization. (MOG: mucin O-glycan, MNG: bovine milk N-glycan).
-
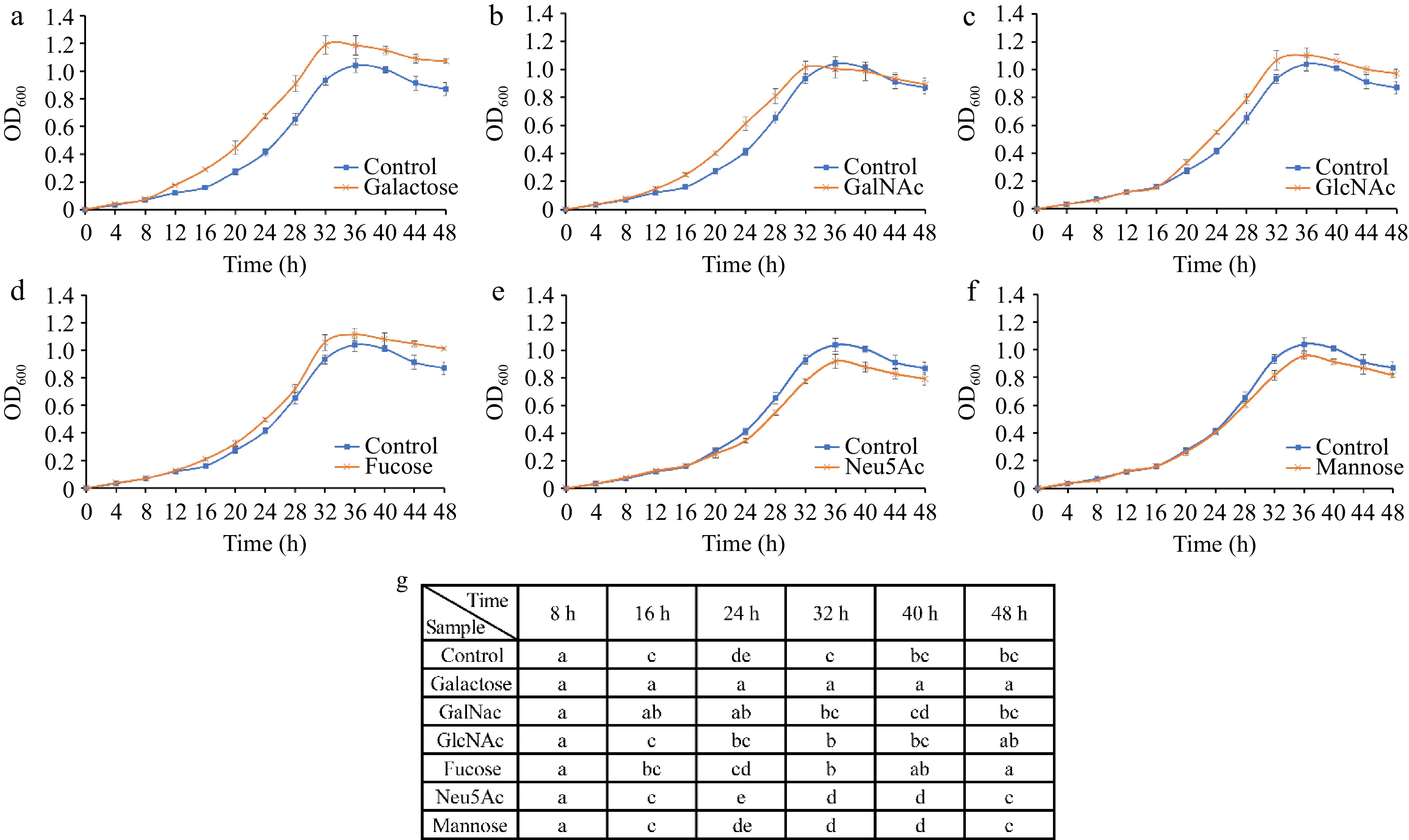
Figure 7.
Growth curves of A. muciniphila following supplementation with individual monosaccharides—(a) Galactose, (b) GalNAc, (c) GlcNAc, (d) Fucose, (e) Neu5Ac, and (f) mannose—over a time course. (g) Analyses the significant differences in growth observed across the various sugar treatments at each time point. The distinct lettering denotes statistically significant discrepancies between groups (p < 0.05).
-
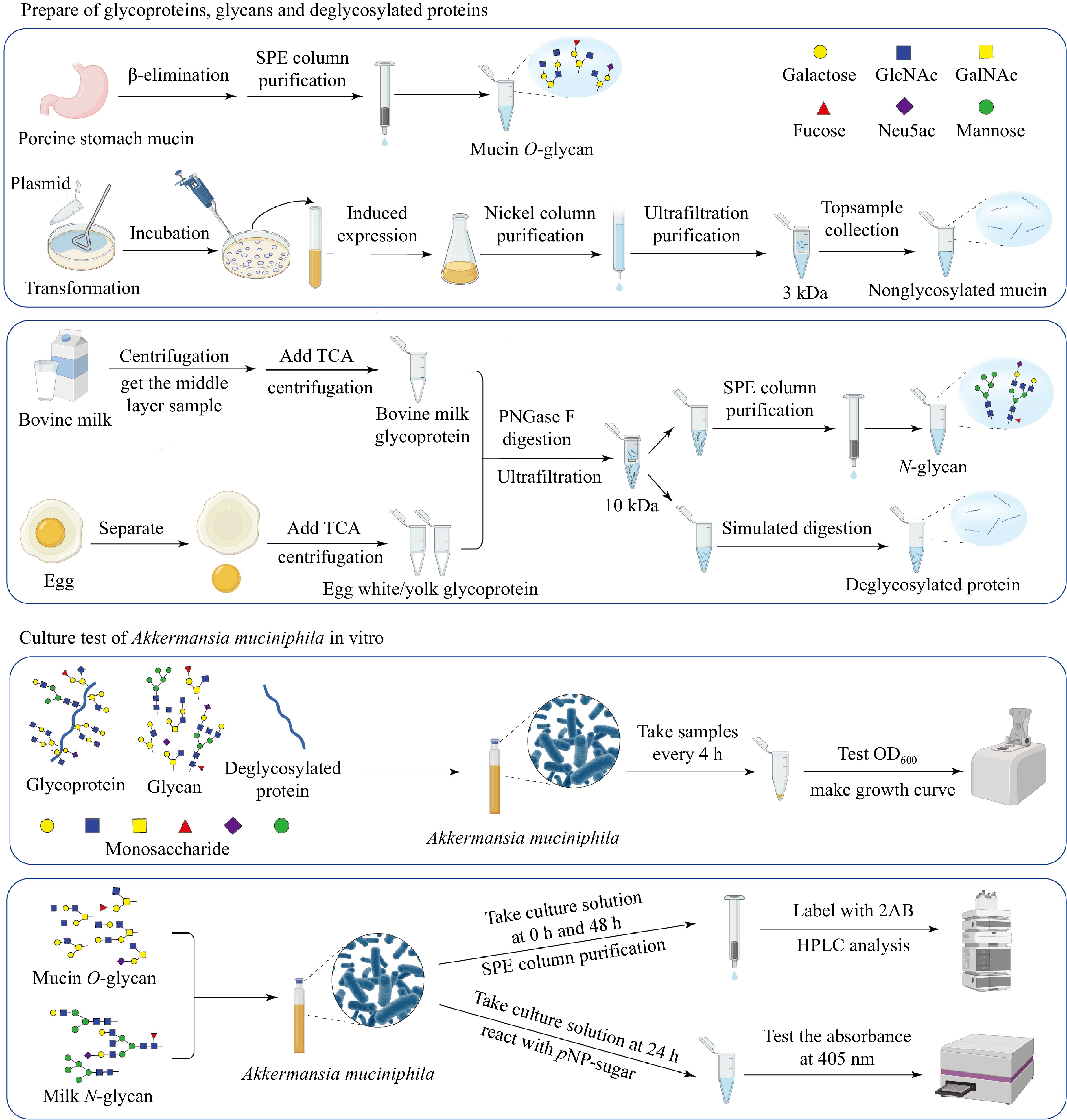
Figure 8.
Workflow illustrating the preparation of glycoproteins, glycans, and deglycosylated proteins from porcine stomach mucin, bovine milk, and egg, followed by the in vitro culture assays of A. muciniphila. The schematic demonstrates the processes, from substrate preparation through the purification steps, to the analysis of microbial growth and metabolic activity in response to different substrates.
Figures
(8)
Tables
(0)