-
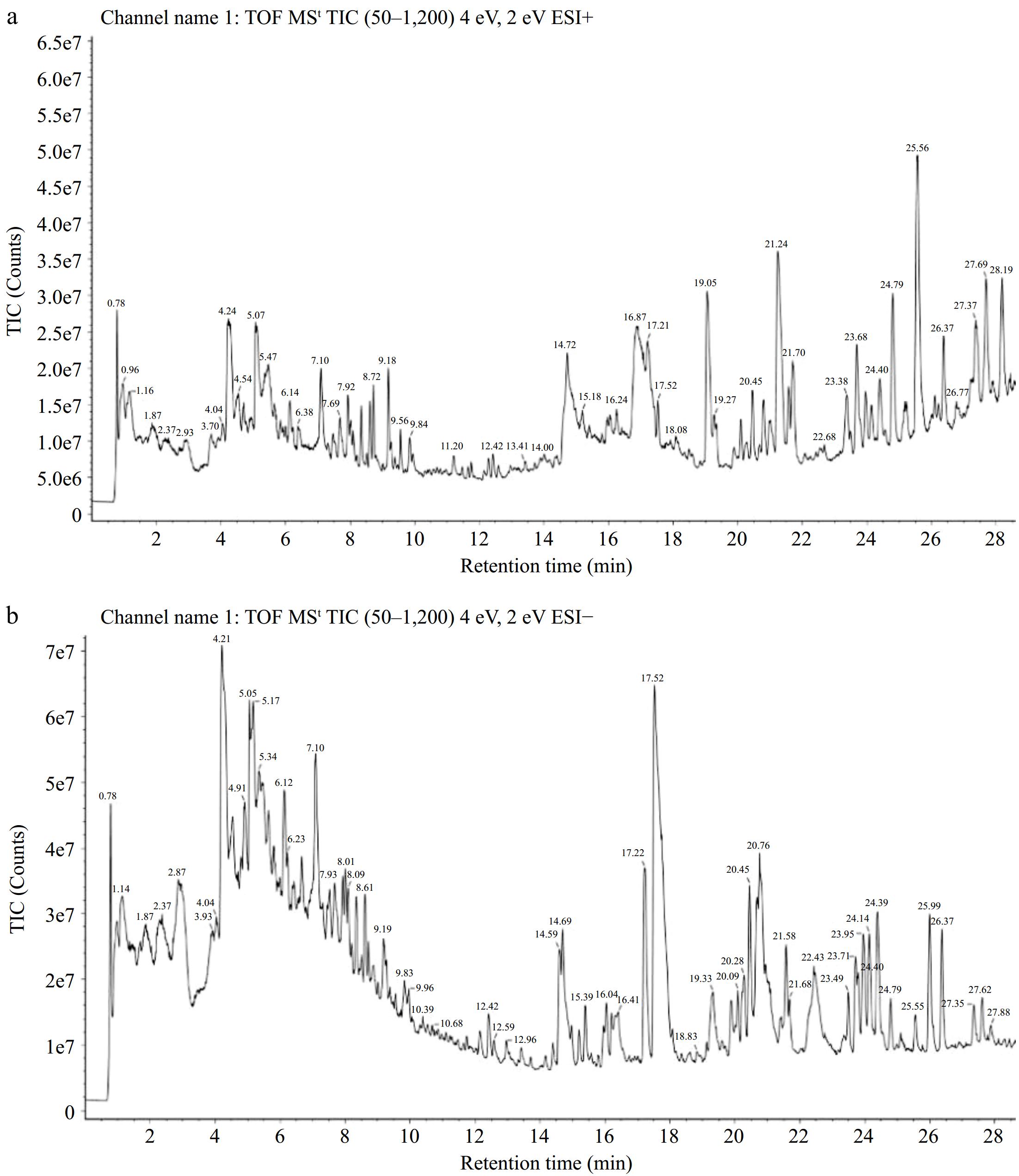
Figure 1.
Total ion chromatogram of the pomegranate peel extract was obtained using UPLC-Q-TOF-MS/MS in both (a) positive and (b) negative modes.
-
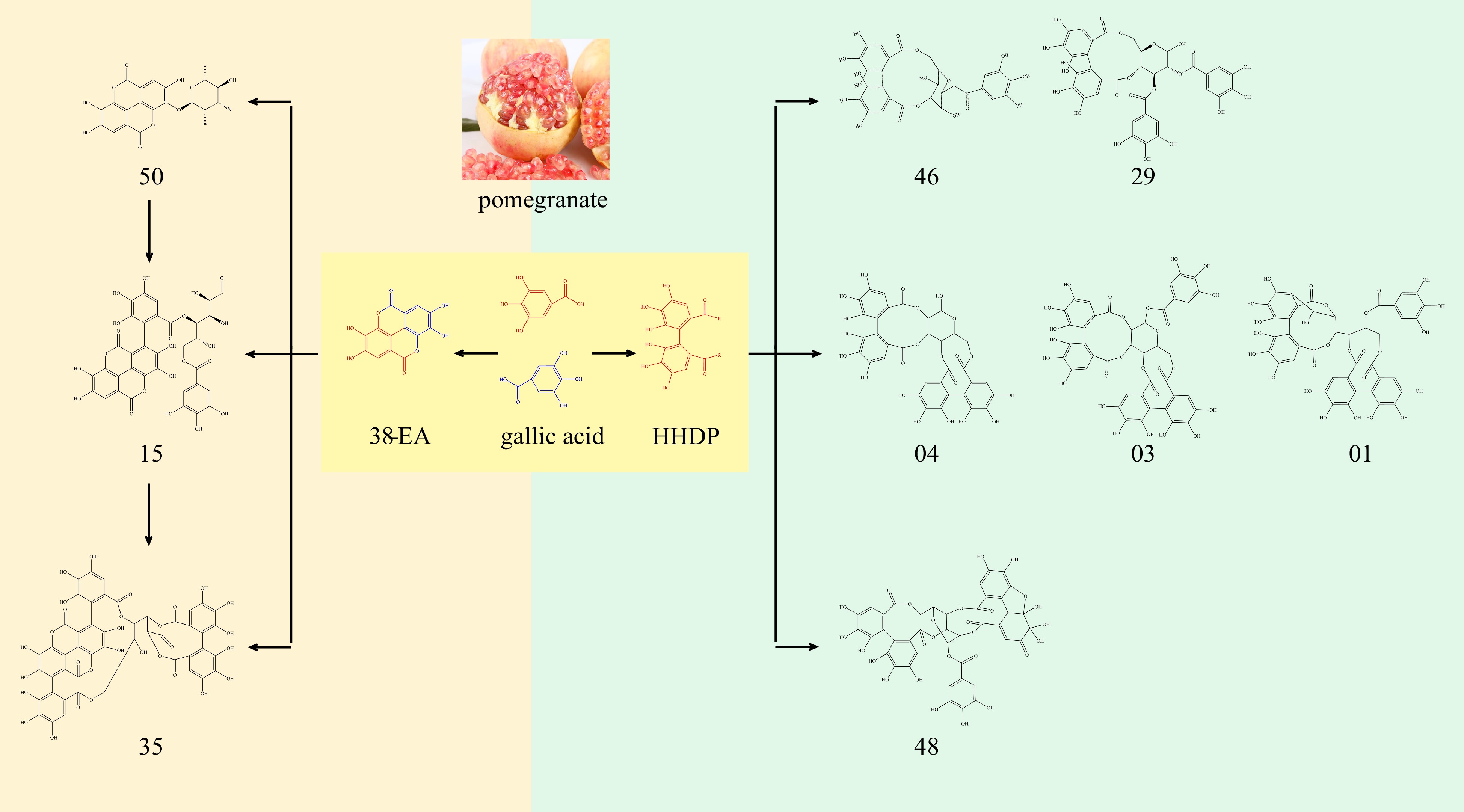
Figure 2.
Synthesis of ellagic acid and its derivatives.
-
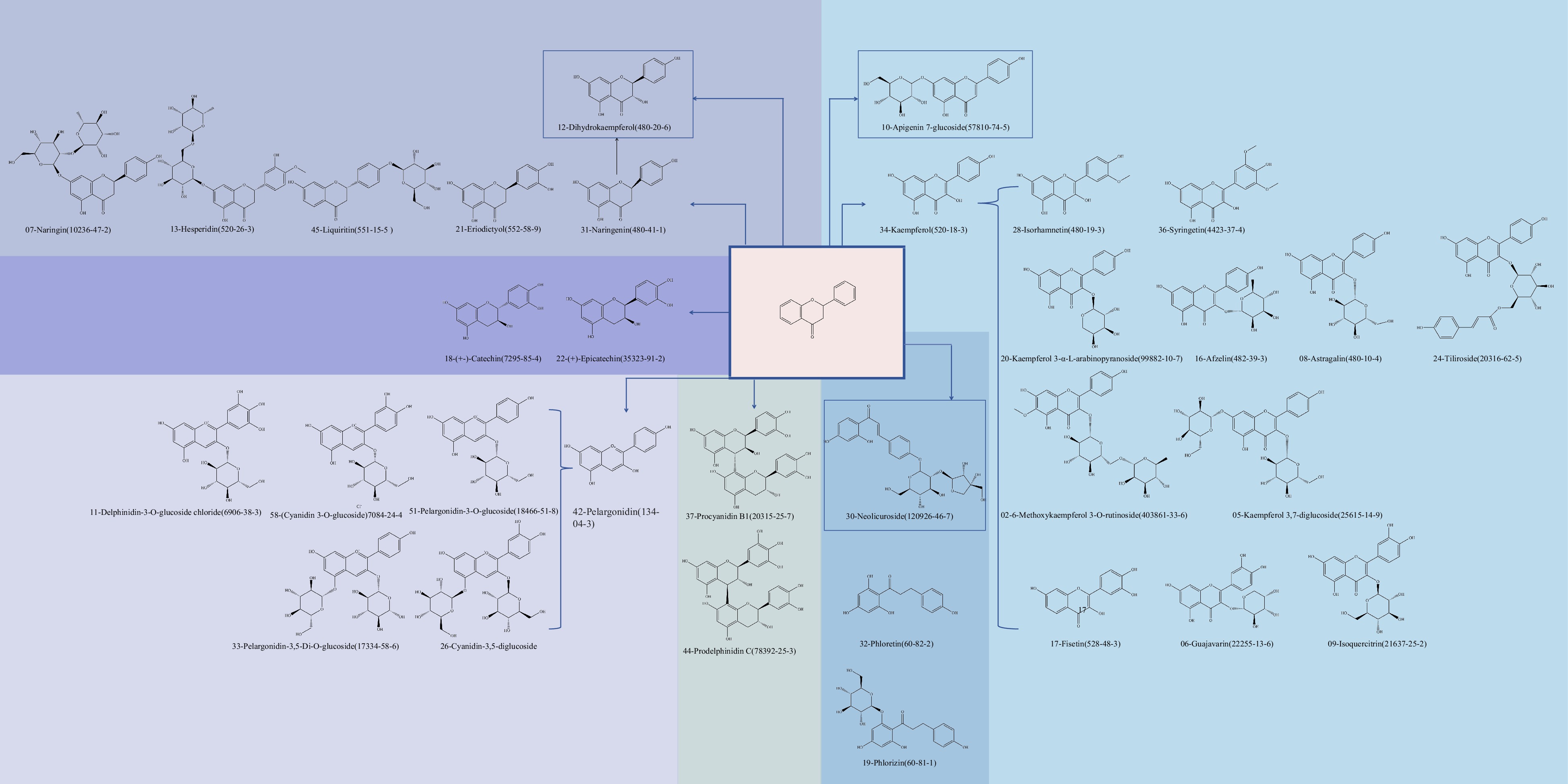
Figure 3.
Synthesis of flavonoid and its derivatives.
-
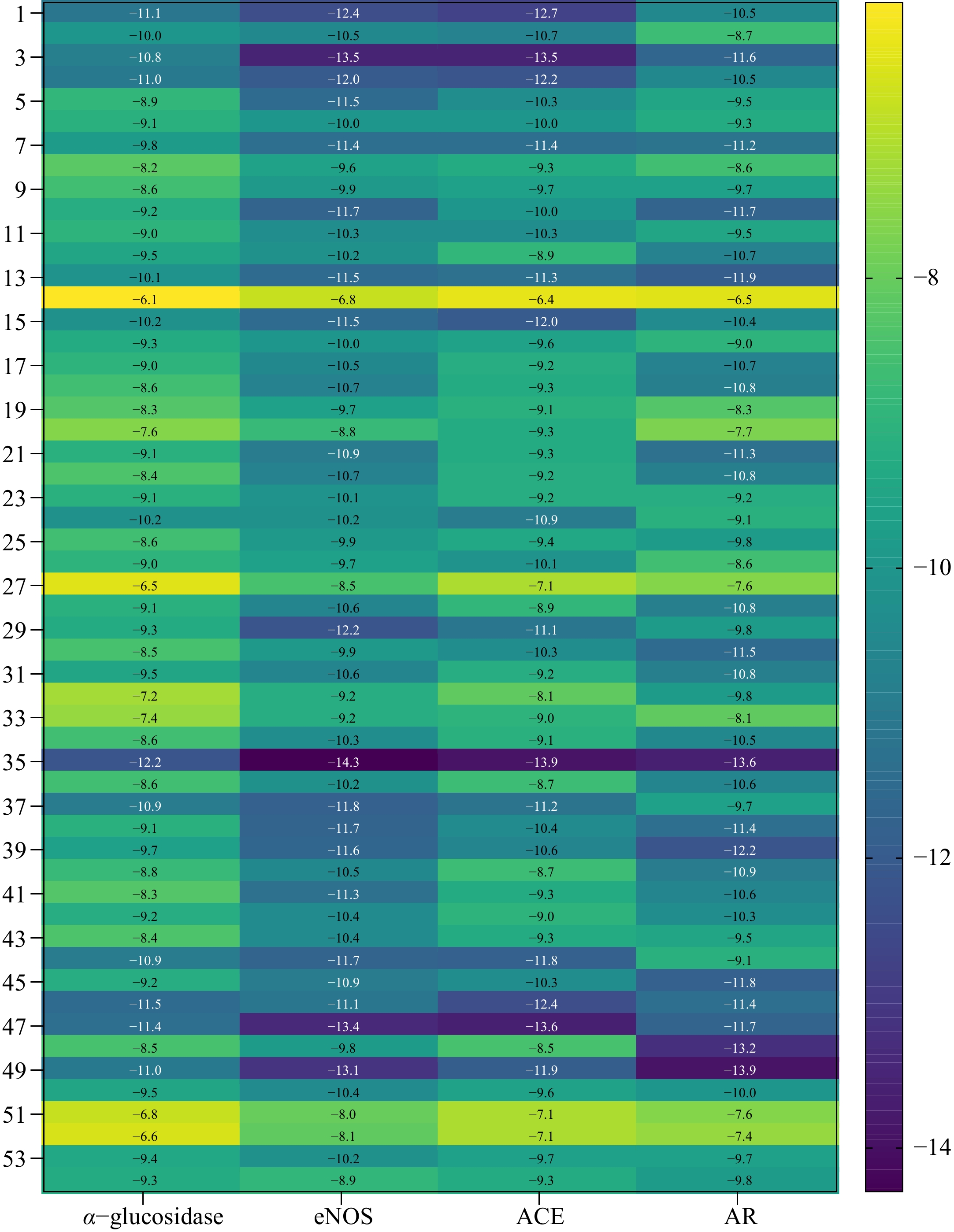
Figure 4.
Heatmap of the binding energy of the identified compounds and acarbose to α-glucosidase, eNOS, ACE, and AR.
-
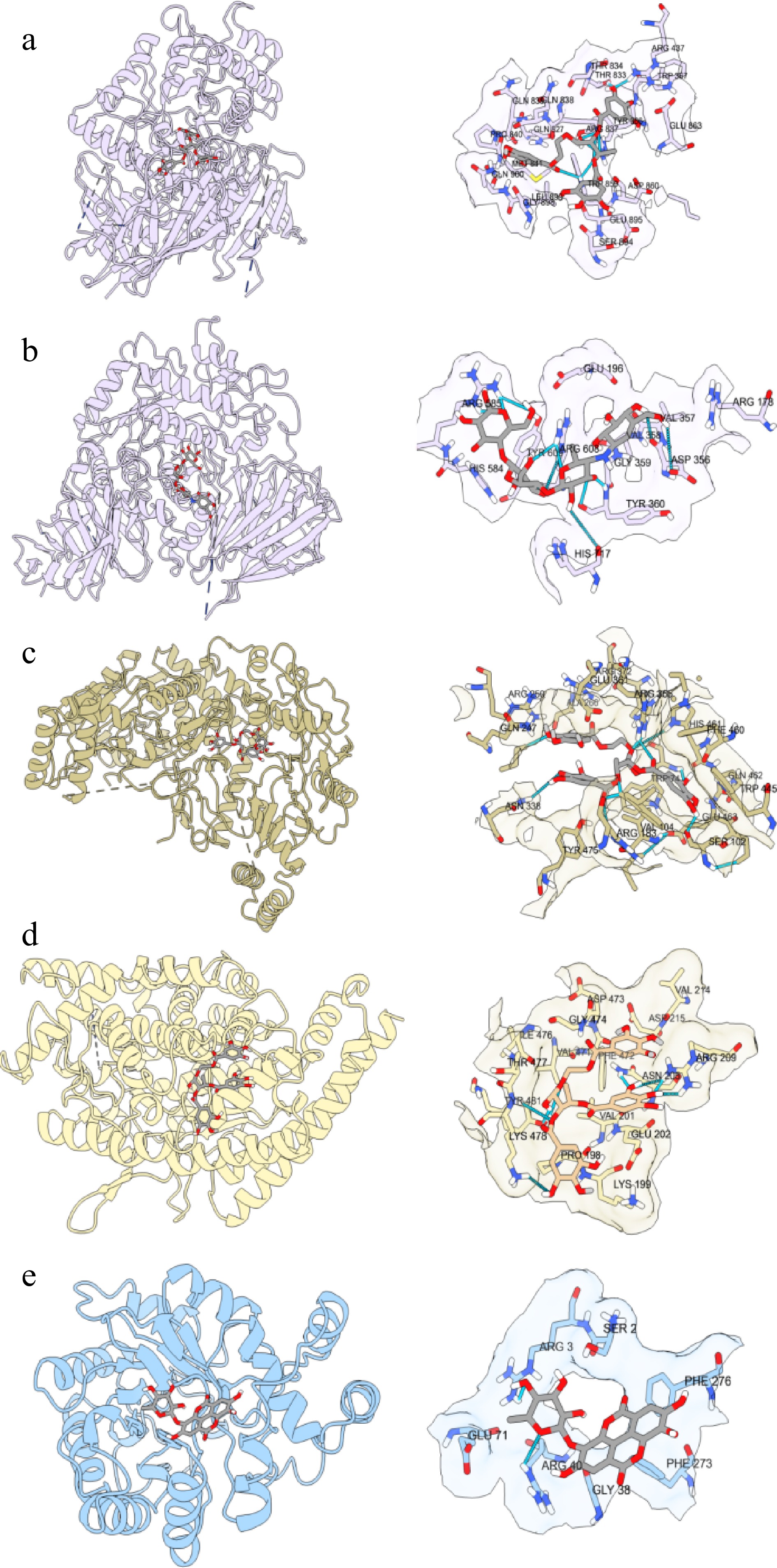
Figure 5.
(a), (b) Molecular interactions of the identified compounds and acarbose against α-glucosidase, (c) eNOS, (d) ACE, and (e) AR. (a) Punicalagin-α-glucosidase; (b) acarbose-α-glucosidase; (c) Punicalagin-eNOS; (d) Punicalagin-ACE; (e) Ellagic acid 3-O-α-L-rhamnopyranoside-AR.
-
DES Extraction condition Quantity of extractives Operation steps Ref. Choline chloride : Lactic acid (65%:35%) 25 min of extraction at 45 °C,
20 g (CC-LAC)/1 g4.14 mg EAG mL−1 Pomegranate peels were frozen and lyophilized. [16] Choline chloride : Glycerol
(1:2, mol/mol)Extraction time 87 s, and a
solvent/solid ratio of 60.5 mL/g7.98 mg eq of gallic acid/g
of dry weightPomegranate peels were cut and dried in refractance window (RW) equipment at 80 °C for 4 h. [19] Malic acid : Glucose : Glycerol (1:1:1, mol/mol), IR 50 °C, 90 min 152 mg/g DM Pomegranate peel were dried in an oven at 50 °C for 48 h. [20] Choline chloride : Glycerol
(1:11, mol/mol)Liquid/solid ratio 47 mL g−1,
time 70 min, and 30% (v/v)
water concentration272.98 mg of gallic acid equivalents per g of dry matter Pomegranate peels were dried for 48 h at 40 °C. [21] Table 1.
Comparison of the results of extracting polyphenols from pomegranate peel with different DES.
-
No. tR (min) Molecular formula Measured (m/z) Actual (m/z) Adducts Identification Error (ppm) 1 8.00 C41H28O26 935.0814 936.0869 [M-H]− Casuarinin 2.0 2 8.17 C28H32O16 623.1600 624.1690 [M-H]− 6-Methoxykaempferol 3-O-rutinoside −2.8 3 8.20 C41H28O26 935.0811 936.0869 [M-H]− Casuarictin 1.5 4 8.21 C34H24O22 785.0843 784.0759 [M+H]+ Pedunculagin 1.4 5 8.43 C27H30O16 655.1498 610.1534 [M+HCOO]− Kaempferol 3,7-diglucoside −2.8 6 8.52 C20H18O11 433.0776 434.0849 [M-H]− Guajavarin −0.1 7 8.65 C27H32O14 579.1731 580.1792 [M-H]−, [M+HCOO]− Naringin 2.1 8 8.80 C21H20O11 449.1081 448.1006 [M+H]+ Astragalin 0.6 9 8.81 C21H20O12 465.1030 464.0955 [M+H]+ Isoquercitrin 0.6 10 8.87 C21H20O10 433.1135 432.1057 [M+H]+ Apigenin 7-glucoside 1.4 11 8.90 C21H21ClO12 545.0696 500.0722 [M+HCOO]− Delphinidin-3-O-glucoside chloride −1.4 12 9.04 C15H12O6 287.0561 288.0634 [M-H]− Dihydrokaempferol 0.1 13 9.15 C28H34O15 609.1827 610.1898 [M-H]− Hesperidin 0.3 14 9.32 C7H6O3 139.0390 138.0317 [M+H]+ 4-Hydroxybenzoic acid 0.2 15 9.46 C34H24O22 783.0705 784.0759 [M-H]− Terflavin B 2.3 16 9.49 C21H20O10 431.0988 432.1057 [M-H]− Afzelin 1.0 17 9.50 C15H10O6 287.0546 286.0477 [M+H]+ Fisetin −1.3 18 9.71 C15H14O6 291.0865 290.0790 [M+H]+ (+/−)-Catechin 0.8 19 9.89 C21H24O10 435.1299 436.1370 [M-H]−, [M+HCOO]− Phlorizin 0.5 20 9.93 C20H18O10 417.0834 418.0900 [M-H]− Kaempferol 3-α-L-arabinopyranoside 1.7 21 10.17 C15H12O6 289.0703 288.0634 [M+H]+ Eriodictyol −1.3 22 10.30 C15H14O6 291.0977 290.0896 [M+H]+ (+)-Epicatechin 2.7 23 10.35 C22H26O11 484.1831 466.1475 [M+H]+ Agnuside 3.6 24 10.82 C30H26O13 593.1298 594.1373 [M-H]− Tiliroside −0.4 25 10.98 C13H8O8 315.0127 292.0219 [M+Na]+ Brevifolincarboxylic acid 5.0 26 11.14 C27H31ClO16 647.1391 646.1301 [M+H]+ Cyanidin-3,5-diglucoside 2.7 27 11.21 C10H10O4 217.0470 194.0579 [M+Na]+ Ferulic Acid −0.5 28 11.24 C16H12O7 315.0508 316.0583 [M-H]− Isorhamnetin −0.7 29 11.31 C34H26O22 787.0997 786.0916 [M+H]+ Tellimagrandin I 1.0 30 11.63 C26H30O13 551.1776 550.1686 [M+H]+ Neolicuroside 3.1 31 11.74 C15H12O5 273.0755 272.0685 [M+H]+ Naringenin −1.0 32 11.89 C15H14O5 273.0775 274.0841 [M-H]− Phloretin 2.5 33 11.93 C27H31ClO15 675.1323 630.1352 [M+HCOO]− Pelargonidin-3,5-Di-O-glucoside −1.6 34 12.15 C15H10O6 287.0549 286.0477 [M+H]+ Kaempferol −0.4 35 12.35 C48H28O30 1083.0610 1084.0665 [M-H]− Punicalagin 1.6 36 12.39 C17H14O8 391.0661 346.0689 [M+HCOO]− Syringetin −2.5 37 12.87 C30H26O12 577.1362 578.1424 [M-H]− Procyanidin B1 1.8 38 13.06 C14H6O8 300.9995 302.0063 [M-H]− Ellagic acid 1.8 39 13.15 C20H22O8 391.1371 390.1315 [M+H]+ trans−Resveratrol 4'-O-β-D-glucuronide −4.3 40 14.34 C21H20O6 369.1325 368.1260 [M+H]+ Curcumin −1.9 41 14.55 C28H24O5 439.1535 440.1624 [M-H]− 3,4-Dibenzyl-gallic acid Benzyl Ester −3.7 42 14.77 C15H11ClO5 329.0181 306.0295 [M+Na]+ Pelargonidin −1.8 43 15.78 C20H20O14 483.0785 484.0853 [M-H]− Gallic acid 3-O-(6'-O-galloyl)-β-D-glucopyranoside 0.9 44 16.71 C30H26O13 593.1303 594.1373 [M-H]− Prodelphinidin C 0.5 45 18.02 C21H22O9 419.1342 418.1264 [M+H]+ Liquiritin 1.4 46 19.89 C27H24O18 635.0874 636.0963 [M-H]− Corilagin −2.5 47 20.28 C41H28O27 951.0752 952.0818 [M-H]− Granatin B 0.7 48 21.54 C13H16O10 331.0675 332.0744 [M-H]− Glucogallin 1.2 49 21.72 C20H16O12 447.0564 448.0642 [M-H]− Ellagic acid 3-O-α-L-rhamnopyranoside −1.2 50 22.50 C21H21ClO10 467.0733 468.0823 [M-H]− Pelargonidin-3-O-glucoside −3.7 51 22.79 C9H8O4 181.0492 180.0423 [M+H]+ Caffeic acid −1.6 52 23.78 C9H8O3 165.0548 164.0473 [M+H]+ p−Coumaric acid 1.4 53 27.37 C21H21ClO11 483.0705 484.0772 [M-H]− Cyanidin 3-O-glucoside 1.2 Table 2.
Identification of the chemical compositions from pomegranate peel using UPLC-Q-TOF-MS/MS.
-
α-glucosidase Punicalagin
(No. 35)Acarbose No. of
hydrogen bondsBond length (Å) ARG437 O18 1 2.076 THR833 O18 1 1.927 ARG837 O3 2 3.572, 2.133 O4 2 2.679, 3.244 O5 2 2.204, 2.843 TRP859 O12 1 3.338 O3 1 3.456 TYR360 O5 1 1.848 ARG585 O8 2 3.354, 2.516 O17 1 3.719 ARG608 O2 2 3.582, 2.741 O7 1 3.285 O14 1 3.150 Table 3.
Molecular docking results of Punicalagin (No. 35), acarbose and α-glucosidase.
Figures
(5)
Tables
(3)