-
Polyphenols exhibit diverse pharmacological activities that significantly contribute to the prevention of degenerative diseases, especially cardiovascular diseases and cancer[1,2]. In addition, polyphenols, such as punicalagin, ellagic acid, and kaempferol, have a good inhibitory effect on α-glucosidase, and have potential efficacy in the treatment of diabetes, especially type 2 diabetes, through the PI3K/Akt pathway[3,4]. The polarities of polyphenols are determined by their polyhydroxyl structure. In general, most polyphenols exhibit high water solubility, particularly anthocyanin components which possess a highly soluble ionic structure[5]. Considering this property, incorporating polyphenols into the water as a beverage or through regular tea consumption represents a favorable strategy for enhancing polyphenol intake among individuals. Pomegranate is an important medicinal and edible homologous fruit[6]. Its by-product, pomegranate peel, is listed in the 2020 Edition of the Pharmacopoeia of the People's Republic of China as a traditional Chinese medicinal herb, which is rich in polyphenolic compounds and exhibits a variety of pharmacological effects, including astringent, antidiarrhoeal, haemostatic, and anthelmintic properties[7,8]. The process of obtaining polyphenols from pomegranate peel, the by-product of pomegranate, offers potential benefits in terms of cost reduction, secondary utilization of the peel, and environmental sustainability[9]. In conclusion, the addition of pure natural polyphenols from pomegranate peel to tea drinking is not only beneficial to promote health, but also can reduce costs due to the abundance of pomegranate peel resources[10,11].
Deep eutectic solvents (DESs) refer to binary or ternary low-melting mixtures composed of hydrogen bond acceptors, such as quaternary ammonium salts, and hydrogen bond donors, including amides, carboxylic acids, and polyols[12]. Conventional extraction methods often involve expensive reagents and toxic solvents that pose risks to human health and environmental pollution[13]. As a novel green solvent, the use of DES can avoid organic solvent contamination in polyphenol extraction processes and meet the standards of green pollution-free practices[14]. In recent years, DES has gained increasing attention in the extraction of active plant components and has been widely applied in the extraction and separation of plant polyphenols, flavonoids, and other compounds due to its advantages including high extraction efficiency and good recovery yields. Moreover, due to its unique inclusiveness, DES can be combined with various extraction techniques such as microwave-assisted and ultrasound-assisted extractions to further enhance its efficiency. Qin et al. found that when using ultrasound-assisted low-melting solvents for polyphenol extraction from Ligustrum robustum leaves powder became rough with numerous gaps indicating thorough extraction of polyphenolic substances from the herbal material resulting in a higher yield at 101.46 ± 2.96 mg gallic acid equivalent (GAE)/g DW[15].
In this study, DES-based ultrasound-assisted extraction (DES-UAE) with glycerol/urea 1:2, mol/mol, 30% water content obtained the highest total phenolic content (TPC) from pomegranate peel. The information of components in pomegranate peel was analyzed using a UPLC-Q-TOF-MS/MS instrument. After obtaining information on specific components in the pomegranate peel, further metabolic pathway analysis of these compounds was conducted to explore their possible synthesis pathways in the pomegranate peel, which is conducive to understanding the biosynthesis mechanism of the pomegranate peel. The ability of polyphenols in pomegranate peel to inhibit diabetes-related proteins was further explored and compared with acarbose, which was visually represented in the form of molecular docking. Understanding the synthetic pathway of these components in plants helps to determine the source of these raw materials and ensures the reliability and safety of raw materials. For example, the polyphenols in the pomegranate peel can be used to make drinks, not only to meet the needs of the beverage formula but also as antioxidants. The second is to help assess the safety of these ingredients in beverages and ensure that products with these ingredients comply with relevant regulations and standards. Finally, it is beneficial to optimize the production process, improve production efficiency, ensure the stability and consistency of product quality, and improve market competitiveness. In addition, the polyphenols in pomegranate peel, especially Punicalagin, have a positive effect on the treatment of diabetes, and the development of pomegranate peel polyphenols into hypoglycemic drugs has a good application prospect.
-
Huaiyuan pomegranate from Anhui Province (China), purchased online, is characterized by yellow skin with red tinges and nearly colorless seeds, which have the same luster as crystal. After the pomegranate skin was dried (shade place), the measured water content was 17.4%, which was crushed (280 μm) and stored at room temperature.
Analytical grade reagents, including Folin-Ciocalteu reagent (Biotechnology grade, 2 M), Na2CO3 (≥ 99.9%), glycerol (99%), urea (99%), and gallic acid (GAE, 98%) were purchased from Macklin (Shanghai, China). Chromatographic grade reagents of acetonitrile (≥ 99.9%) and formic acid (≥ 88%) for UPLC-Q-TOF-MS/MS analysis were provided by Merck (Germany).
Preparation of DES
-
DES (glycerol/urea) was prepared according to the molar ratio of glycerol to urea 1:2 with a water content of 30%, and the mixture was placed in a hot bath. Stir continuously to obtain a clarified liquid and stored at room temperature.
Extraction procedure of DES-UAE
-
The DES-UAE method was used to extract polyphenols from pomegranate peel. The specific operations were as follows: 0.2 g of pomegranate peel powder was mixed with a certain amount of DES in a conical flask, the ultrasonic input power was set to 500 W and the frequency was set to 40 kHz, then TPC was extracted in an ultrasonic bath (XM-500UVF).
TPC determination
-
The TPC in pomegranate peel was determined by the Folin phenol method. The specific operation was as follows: Folin-Ciocalteu reagent was diluted 20 times with deionized water, and 1800 μL was mixed with 40 μL of the centrifuge sample. Reaction for 5 min at room temperature away from light, followed by the reaction with 1,200 μL of 7.5% Na2CO3 solution in the dark for 90 min at room temperature. After the reaction, the absorbance value was measured at 765 nm, and the TPC was expressed as GAE mg/g DW.
UPLC-Q-TOF-MS/MS analysis
-
To study the types and contents of polyphenols in pomegranate peel, the components were analyzed using a UPLC-Q-TOF-MS/MS instrument. The data acquisition mode was set to MSE continue full scan mode, the scanning range was m/z 50−1,200, and the scanning time was 0.2 s. Collision energies are used in MSE, 6 eV, and 15−45 eV for the low and high energy channels. HCOONa was used for mass spectrometer correction, and leucine enkephrine (positive ion mode m/z 556.2771, negative ion mode m/z 554.2615) was used for real-time mass correction. The ion source parameters for mass spectrometry were as follows: capillary voltage 3.0 and 2.5 kV in positive and negative ion modes, sample cone and offset voltage 40 and 80 V, ion source temperature 120 °C, desolvation temperature 500 °C, and desolvation flow rate 1,000 L/h. Acetonitrile (A) and 0.1% formic acid-water (B) were used as the mobile phases. The column temperature was 35 °C and the sample chamber temperature was 10 °C. The flow rate was 0.3 mL/min. The injection volume was 2 μL. Gradient elution: 0−2 min, 5% A; 2−32 min, 5%−100% A. Mass spectrometry data of polyphenols in pomegranate peel obtained by UPLC-Q-TOF-MS/MS were identified by the UNIFI platform.
Molecular docking
-
The molecular docking procedures of the identified compounds against diabetes-related enzymes including α-glucosidase, endothelial nitric oxide synthase (eNOS), angiotensin-converting enzyme (ACE) and aldose reductase (AR) were performed with AutoDock Vina to investigate the binding mode of ligands with the receptors. The crystal structures of α-glucosidase (PDB ID; 5NN8), eNOS (PDB ID; 1M9R), ACE (PDB ID; 1O8A), and AR (PDB ID; 2ACS) were obtained from the protein data bank. Before docking, the ligands and receptors were dehydrated, hydrogenated, charged, and saved in PDBQT format. In docking calculation, the receptor was considered semi-flexible while the ligand was flexible, and the grid matrix of the docking region was scaled to guarantee the full coverage of the protein molecule. The grids were set to (112, 92, 110), (90, 84, 104), (80, 90, 76), and (56, 56, 58), and the docking centers were (5.472, −30.250, 83.096), (14.726, 5.474, 38.000), (37.720, 34.803, 39.035) and (16.618, 29.512, 66.722) for α-glucosidase, eNOS, ACE, and AR, respectively. The best score with the suitable energetically favored conformations was applied for further analysis. The interactions of the identified compounds with amino acid residues of α-glucosidase, eNOS, ACE, and AR were analyzed and represented using ChimeraX.
Statistical analysis
-
The data collected in this study were recorded and calculated with Microsoft Excel. All experiments and measurements were conducted in triplicate.
-
The utilization of DES for the extraction of polyphenols from pomegranate peel exhibits enhanced permeability compared to conventional solvents, leading to a higher yield of polyphenols[16,17]. The cell wall of plant materials was disrupted through ultrasonic cavitation using the DES-assisted extraction method employed in this study, thereby facilitating the accelerated release and diffusion of polyphenols from cells into solvents[18]. In this study, glycerol/urea (1:2 mol/mol, 30% water content, extraction time 20 min, extraction temperature 30 °C, liquid/solid ratio 70 mL/g) was used as the extraction solvent and the maximum TPC obtained was 81.983 GAE mg/g DW. To reflect the high efficiency of the extraction method adopted in this study, the results of this study were compared with those of other studies. As shown in Table 1, the extraction results of this study were superior to those of Bertolo et al.[16], and Vargas-Serna et al.[19]. However, it was lower than Rajha et al.[20], and Kyriakidou et al.[21], which may be because this study used ultrasound-assisted extraction technology, while Rajha et al. used infrared-assisted extraction technology. The two methods had different influences on plant tissues, leading to the difference in results. The reason why the glycerol/urea system can obtain the highest extraction yield may be related to the structure of glycerol. Glycerol molecules contain multiple hydroxyl groups, which can form hydrogen bonds with polyphenols to promote the dissolution of polyphenols[22]. Alañón et al. highlighted the distinctive advantages of polyalcohol-based DESs in the extraction of phenolic acids and other phenolic compounds from plants[23]. At the same time, compared with sugar, glycerol is not as viscous as sugar, which is also conducive to the extraction of polyphenols[24].
Table 1. Comparison of the results of extracting polyphenols from pomegranate peel with different DES.
DES Extraction condition Quantity of extractives Operation steps Ref. Choline chloride : Lactic acid (65%:35%) 25 min of extraction at 45 °C,
20 g (CC-LAC)/1 g4.14 mg EAG mL−1 Pomegranate peels were frozen and lyophilized. [16] Choline chloride : Glycerol
(1:2, mol/mol)Extraction time 87 s, and a
solvent/solid ratio of 60.5 mL/g7.98 mg eq of gallic acid/g
of dry weightPomegranate peels were cut and dried in refractance window (RW) equipment at 80 °C for 4 h. [19] Malic acid : Glucose : Glycerol (1:1:1, mol/mol), IR 50 °C, 90 min 152 mg/g DM Pomegranate peel were dried in an oven at 50 °C for 48 h. [20] Choline chloride : Glycerol
(1:11, mol/mol)Liquid/solid ratio 47 mL g−1,
time 70 min, and 30% (v/v)
water concentration272.98 mg of gallic acid equivalents per g of dry matter Pomegranate peels were dried for 48 h at 40 °C. [21] Metabolome establishment
-
In this study, the UPLC-Q-TOF-MS/MS instrument was used to analyze the polyphenols in pomegranate peel. The system has the characteristics of high resolution and fast analysis speed and can realize the efficient separation and structural analysis of the components. Figure 1 was a total ion chromatogram, revealing the rich and diverse composition of the pomegranate peel. The higher the peak in the figure, the higher the abundance of the compound. A total of 62 compounds were identified, but this study focused on polyphenols in pomegranate peel. Therefore, non-polyphenolic substances such as fatty acids and steroids were removed, and 53 polyphenolic compounds remained, as shown in Table 2. Of these, a total of 10 compounds were identified as ellagic acids and their derivatives, while the remaining 32 compounds belonged to the flavonoid class, including 12 flavonols, six anthocyanins, five dihydroflavones, two dihydrochalcones, two flavanols, and one each of flavonol, dihydroflavonol, and chalcone. Man et al. used the UHPLC-QTOF-MS method to analyze nine varieties of pomegranate peel and a total of 64 compounds were annotated, including 13 flavonols, four flavanones and six flavan-3-ols compounds[25]. Punicalin, galloyl-O-punicalin, chebulagic acid, thymol, olivetonide, rutin, avicularin, hyperoside, phlorhizin, genistein, plantaginin, taxifolin were not detected in this study. Zheng et al. also used the UPLC-Q TOF MS technique to study the components in pomegranate peel and obtained a total of 25 components[26]. The key monomer compound of ellagic acid and its derivatives in the biosynthesis process is gallic acid. However, due to the drying treatment of pomegranate peel at 90 °C in this study, gallic acid itself was unstable, so it was decomposed during the drying process and not detected in the mass spectrum. Fresh pomegranate peel was freeze-dried and pulverized by Man et al. for qualitative analysis using UHPLC-QTOF-MS, resulting in the identification of 21 phenolic compounds, including gallic acid. These findings highlight the significant impact of drying temperature on the presence of gallic acid[27,28].
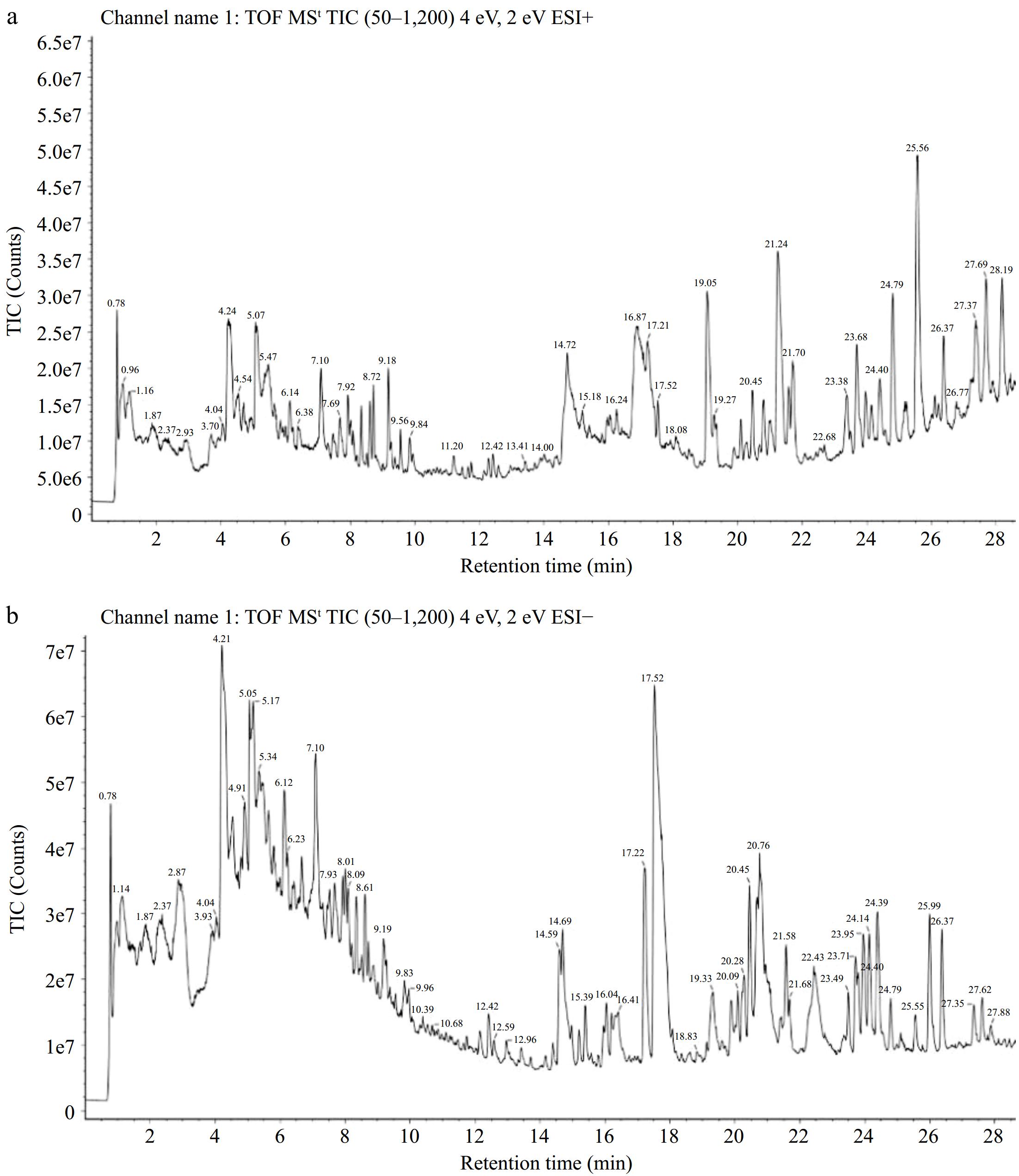
Figure 1.
Total ion chromatogram of the pomegranate peel extract was obtained using UPLC-Q-TOF-MS/MS in both (a) positive and (b) negative modes.
Table 2. Identification of the chemical compositions from pomegranate peel using UPLC-Q-TOF-MS/MS.
No. tR (min) Molecular formula Measured (m/z) Actual (m/z) Adducts Identification Error (ppm) 1 8.00 C41H28O26 935.0814 936.0869 [M-H]− Casuarinin 2.0 2 8.17 C28H32O16 623.1600 624.1690 [M-H]− 6-Methoxykaempferol 3-O-rutinoside −2.8 3 8.20 C41H28O26 935.0811 936.0869 [M-H]− Casuarictin 1.5 4 8.21 C34H24O22 785.0843 784.0759 [M+H]+ Pedunculagin 1.4 5 8.43 C27H30O16 655.1498 610.1534 [M+HCOO]− Kaempferol 3,7-diglucoside −2.8 6 8.52 C20H18O11 433.0776 434.0849 [M-H]− Guajavarin −0.1 7 8.65 C27H32O14 579.1731 580.1792 [M-H]−, [M+HCOO]− Naringin 2.1 8 8.80 C21H20O11 449.1081 448.1006 [M+H]+ Astragalin 0.6 9 8.81 C21H20O12 465.1030 464.0955 [M+H]+ Isoquercitrin 0.6 10 8.87 C21H20O10 433.1135 432.1057 [M+H]+ Apigenin 7-glucoside 1.4 11 8.90 C21H21ClO12 545.0696 500.0722 [M+HCOO]− Delphinidin-3-O-glucoside chloride −1.4 12 9.04 C15H12O6 287.0561 288.0634 [M-H]− Dihydrokaempferol 0.1 13 9.15 C28H34O15 609.1827 610.1898 [M-H]− Hesperidin 0.3 14 9.32 C7H6O3 139.0390 138.0317 [M+H]+ 4-Hydroxybenzoic acid 0.2 15 9.46 C34H24O22 783.0705 784.0759 [M-H]− Terflavin B 2.3 16 9.49 C21H20O10 431.0988 432.1057 [M-H]− Afzelin 1.0 17 9.50 C15H10O6 287.0546 286.0477 [M+H]+ Fisetin −1.3 18 9.71 C15H14O6 291.0865 290.0790 [M+H]+ (+/−)-Catechin 0.8 19 9.89 C21H24O10 435.1299 436.1370 [M-H]−, [M+HCOO]− Phlorizin 0.5 20 9.93 C20H18O10 417.0834 418.0900 [M-H]− Kaempferol 3-α-L-arabinopyranoside 1.7 21 10.17 C15H12O6 289.0703 288.0634 [M+H]+ Eriodictyol −1.3 22 10.30 C15H14O6 291.0977 290.0896 [M+H]+ (+)-Epicatechin 2.7 23 10.35 C22H26O11 484.1831 466.1475 [M+H]+ Agnuside 3.6 24 10.82 C30H26O13 593.1298 594.1373 [M-H]− Tiliroside −0.4 25 10.98 C13H8O8 315.0127 292.0219 [M+Na]+ Brevifolincarboxylic acid 5.0 26 11.14 C27H31ClO16 647.1391 646.1301 [M+H]+ Cyanidin-3,5-diglucoside 2.7 27 11.21 C10H10O4 217.0470 194.0579 [M+Na]+ Ferulic Acid −0.5 28 11.24 C16H12O7 315.0508 316.0583 [M-H]− Isorhamnetin −0.7 29 11.31 C34H26O22 787.0997 786.0916 [M+H]+ Tellimagrandin I 1.0 30 11.63 C26H30O13 551.1776 550.1686 [M+H]+ Neolicuroside 3.1 31 11.74 C15H12O5 273.0755 272.0685 [M+H]+ Naringenin −1.0 32 11.89 C15H14O5 273.0775 274.0841 [M-H]− Phloretin 2.5 33 11.93 C27H31ClO15 675.1323 630.1352 [M+HCOO]− Pelargonidin-3,5-Di-O-glucoside −1.6 34 12.15 C15H10O6 287.0549 286.0477 [M+H]+ Kaempferol −0.4 35 12.35 C48H28O30 1083.0610 1084.0665 [M-H]− Punicalagin 1.6 36 12.39 C17H14O8 391.0661 346.0689 [M+HCOO]− Syringetin −2.5 37 12.87 C30H26O12 577.1362 578.1424 [M-H]− Procyanidin B1 1.8 38 13.06 C14H6O8 300.9995 302.0063 [M-H]− Ellagic acid 1.8 39 13.15 C20H22O8 391.1371 390.1315 [M+H]+ trans−Resveratrol 4'-O-β-D-glucuronide −4.3 40 14.34 C21H20O6 369.1325 368.1260 [M+H]+ Curcumin −1.9 41 14.55 C28H24O5 439.1535 440.1624 [M-H]− 3,4-Dibenzyl-gallic acid Benzyl Ester −3.7 42 14.77 C15H11ClO5 329.0181 306.0295 [M+Na]+ Pelargonidin −1.8 43 15.78 C20H20O14 483.0785 484.0853 [M-H]− Gallic acid 3-O-(6'-O-galloyl)-β-D-glucopyranoside 0.9 44 16.71 C30H26O13 593.1303 594.1373 [M-H]− Prodelphinidin C 0.5 45 18.02 C21H22O9 419.1342 418.1264 [M+H]+ Liquiritin 1.4 46 19.89 C27H24O18 635.0874 636.0963 [M-H]− Corilagin −2.5 47 20.28 C41H28O27 951.0752 952.0818 [M-H]− Granatin B 0.7 48 21.54 C13H16O10 331.0675 332.0744 [M-H]− Glucogallin 1.2 49 21.72 C20H16O12 447.0564 448.0642 [M-H]− Ellagic acid 3-O-α-L-rhamnopyranoside −1.2 50 22.50 C21H21ClO10 467.0733 468.0823 [M-H]− Pelargonidin-3-O-glucoside −3.7 51 22.79 C9H8O4 181.0492 180.0423 [M+H]+ Caffeic acid −1.6 52 23.78 C9H8O3 165.0548 164.0473 [M+H]+ p−Coumaric acid 1.4 53 27.37 C21H21ClO11 483.0705 484.0772 [M-H]− Cyanidin 3-O-glucoside 1.2 Analysis of ellagic acid biosynthetic pathway
-
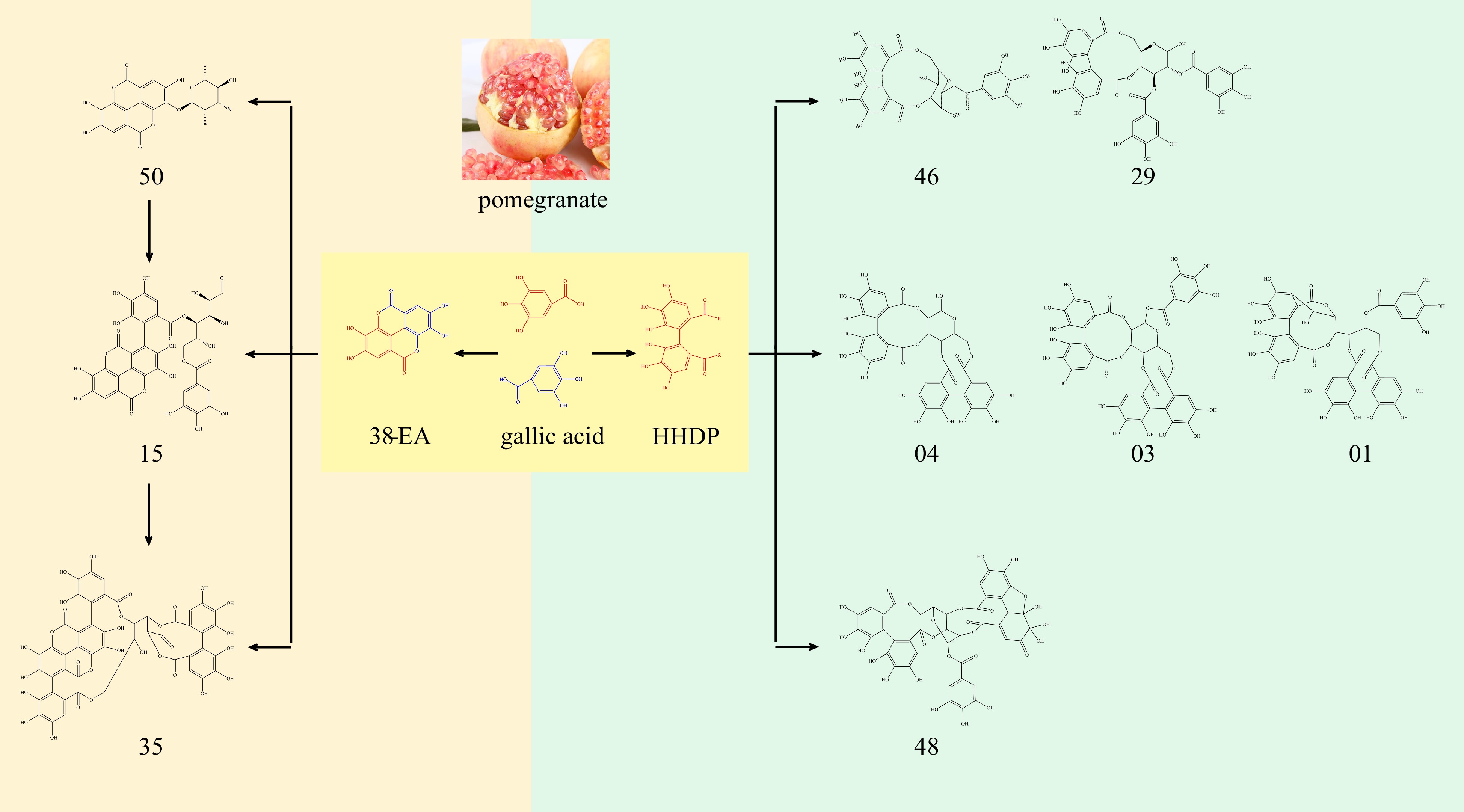
Gallic acid, the monomer that forms ellagic acid and its derivatives, has a variety of pharmacological activities, such as inhibiting thrombin and platelet aggregation, thus acting as a natural thrombin inhibitor[26]. Ellagic acid and its derivatives are one of the main components in pomegranate peel, they have rich pharmacological activities, such as anti-cancer, and antioxidant, especially in the scavenging of free radicals in the body has a strong advantage[7,29]. According to research, pomegranate peel extract had a better DPPH scavenging ability, to avoid oxidative stress caused by disease and ageing[30]. The study conducted by Fu et al. demonstrated the efficacy of ellagic acid in treating androgen-induced hair loss, thereby presenting a novel therapeutic option for individuals experiencing hair loss while also expanding the scope of ellagic acid's applications[31]. As shown in Fig. 2, two molecules of gallic acid are connected by an ester bond to form ellagic acid (No. 38) in Table 2, and ellagic acid is connected with 1 molecule of rhamnose to form glycosides, which is ellagic acid 3-O-α-L-rhamnopyranoside (No. 49). The benzene ring of ellagic acid is connected with one molecule of gallic acid, and the structure of glucose was connected with 1 molecule of gallic acid, forming terflavin B (No. 15). If one gallic acid was connected to each of the two benzene rings of ellagic acid, and then compound punicalagin (No. 35) was formed by connecting one molecule of glucose with one molecule of hexahydroxydiphenoyl (HHDP). Punicalagin is a characteristic compound in pomegranate peel, which is not only high in pomegranate peel but also has pharmacological activities such as nerve protection and diabetes treatment[32,33]. If two molecules of gallic acid are joined together in opposite ways from ellagic acid, the compound HHDP can be obtained. A glucose was connected to the HHDP to form a lactone ring with 10 carbons, and then a gallic acid was connected to the glucose to form compound corilagin (No. 46), and if two gallic acids were connected to glucose, compound tellimagrandin I (No. 29) is formed. On condition that a 6-carbon lactone ring was formed between HHDP with glucose and the glucose was attached to two molecules of gallic acid is pedunculagin (No. 4), or the gallic acid of three molecules was compound casuarictin (No. 3). After entering the body, ellagitannin in pomegranate peel is metabolized by intestinal flora into urolithin A and other substances, among which urolithin A has anti-cancer and anti-aging properties[34,35]. Pomegranate peel is a kind of medicinal material with the same origin as food and medicine. Ellagic acid contained in pomegranate peel has good solubility in water, and because of its rich pharmacological activity, it can play a good role in health care when added as tea in water. Moreover, the way to extract the active ingredients in the pomegranate peel can completely avoid the astringent taste caused by directly using the pomegranate peel, then increasing people's desire to drink.
Analysis of flavonoid biosynthesis pathway
-
The biosynthetic pathway of gallic acid and anthocyanin, the most hydrophilic flavonoid, was intricately linked to the shikimic acid pathway. The 3-dehydroshikimic acid, generated through the shikimic acid pathway, undergoes subsequent conversion to gallic acid facilitated by the coenzyme NADP and shikimate dehydrogenase. The biosynthetic pathway of anthocyanins differs from that of gallic acid. Firstly, the conversion of 3-dehydroshikimic acid to shikimic acid occurs through the co-interaction of NADPH and shikimate dehydrogenase, facilitating the subsequent production of anthocyanins via the phenylalanine and phenylpropane pathways[36]. Flavonoids and their derivatives constitute a major constituent of pomegranate peel, representing significant polyphenolic compounds. They are commonly present in the daily human diet and exhibit diverse pharmacological activities, encompassing potent antioxidant, antibacterial, anti-inflammatory, and other properties. These attributes confer significant advantages in scavenging free radicals within the body, mitigating oxidative stress reactions, and reducing the susceptibility to associated diseases[37]. The antioxidant effect has a vital function in averting cardiovascular disease, cancer, and neurodegenerative diseases[38,39]. Relevant research has demonstrated the potential application of flavonoid polyphenols in the domain of dermatological treatment[40]. Experimental evidence has substantiated that pomegranate peel extract exhibits antioxidative, anti-inflammatory, and anti-apoptotic properties in murine models[41]. The simultaneous discovery of the significant advantages in solubility, resistance to decomposition, and high stability exhibited by polyphenols further enhances their potential for application in various fields[42].
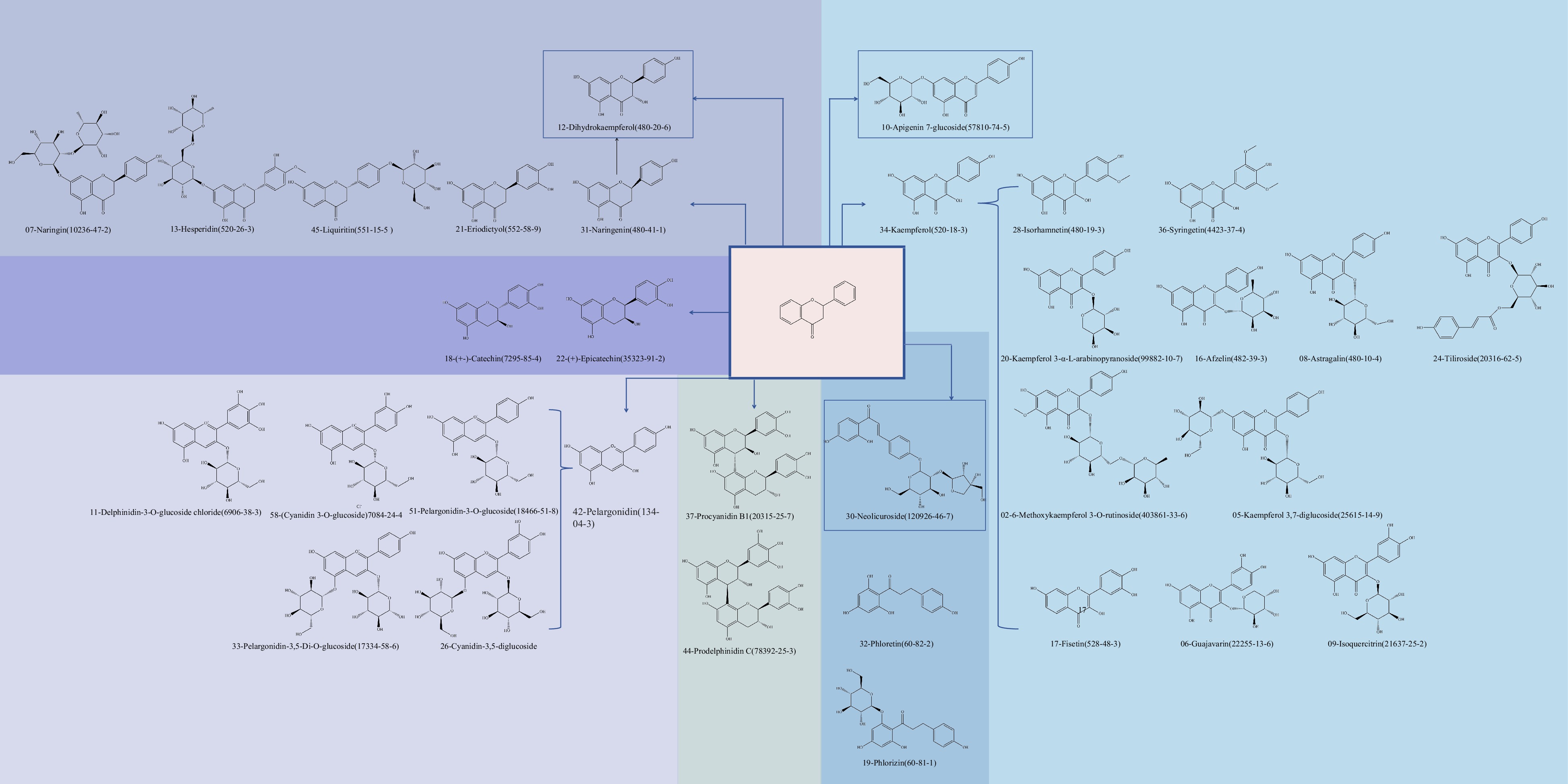
The extraction of flavonoid polyphenols from pomegranates led to the identification of nine distinct classes of compounds derived from the 2-phenylchromogen parent nucleus. The only flavonoid identified in this study is apigenin 7-glucoside (No. 10), as illustrated in Fig. 3. Flavonol compounds, particularly kaempferol (No. 34) and its derivatives, are found in abundance with a total of 12 different types. These flavonol compounds undergo methylation or glycosylation reactions to generate various derivatives. For example, there are only two compounds that undergo only 3'-hydroxymethylation, such as isorhamnetin (No. 28), and four compounds that undergo 3'-hydroxyglucosylation, such as kaempferol 3-α-L-arabinopyranoside (No. 20), which all contain one glycoside, with the only difference being the type of glycoside. And two compounds contain two glycosides. In addition, compound fisetin (No. 17) and its two derivatives are methylated at positions 3 and 5. Naringenin (No. 31) is postulated as the precursor of dihydroflavone, while the addition of hydroxyl, methoxy, or one or two sugar units leads to the formation of No. 21, No. 45, No. 13 and No. 07. Simultaneously, dihydrokaempferol can be synthesized through the introduction of a hydroxyl group at the third position of No. 31. Pomegranates contain two distinct flavanols, namely (+)-catechin (No. 18) and (+)-epicatechin (No. 22), which exist as optical isomers. Of the anthocyanin compounds, pelargonidin (No. 42) was the starting material, and the other five compounds have undergone 1 to 2 methylation and glycosylation reactions. The biflavonoid compounds include procyanidin B1 (No. 37) and prodelphinidin C (No. 44). In addition, this research identified chalcones and their reduced dihydrochalcones, such as neolicuroside (No. 30) and No. 32 and No. 19. The flavonoids present in pomegranate peel are highly suitable for tea preparation due to their exceptional water solubility and thermal stability. These compounds exhibit potent antioxidant activity, effectively scavenging free radicals within the body, thereby mitigating oxidative stress response, safeguarding cells against oxidative damage, and combating chronic diseases. Consequently, utilizing pomegranate peel as a raw material for tea confers significant health benefits to the human body[43].
Molecular docking results analysis
-
The molecular docking analysis of the α-glucosidase, eNOS, ACE and AR inhibitory potentials of the identified compounds above was performed using AutoDock Vina. The identified compounds showed binding affinities ranging from −6.1 to −12.2 kcal/mol against α-glucosidase, as displayed in Fig. 4. Of the 53 identified compounds, as well as acarbose (No. 54), a positive control used to treat diabetes, the highest binding affinity against α-glucosidase was recorded as −12.2 kcal/mol for Punicalagin (No. 35). As shown in Fig. 5 and Table 3, 10 hydrogen bonds were formed between Punicalagin (No. 35) and α-glucosidase, while acarbose only formed eight hydrogen bonds with α-glucosidase. From the results of molecular docking, Punicalagin had a better ability to inhibit α-glucosidase than acarbose. Yi et al. also carried out molecular docking experiments of punicalagin and α-glucosidase, and obtained the result of intermolecular energy of −7.99 kcal/mol[32]. The treatment of diabetes by acarbose relied on its structure similar to oligosaccharides, reversible binding with α-glucosidase, inhibiting the activity of α-glucosidase and achieving hypoglycemic effect. The molecular weight of Punicalagin was higher than that of acarbose, and the inhibition mechanism of α-glucosidase by Punicalagin was different from that of acarbose. The inhibition of α-glucosidase by Punicalagin might be because the polyphenol structure of Punicalagin contained a large number of hydroxyl groups, and the hydrogen bond formed between Punicalagin and the enzyme impeded the function of the enzyme, thus to achieve the role of hypoglycemia.
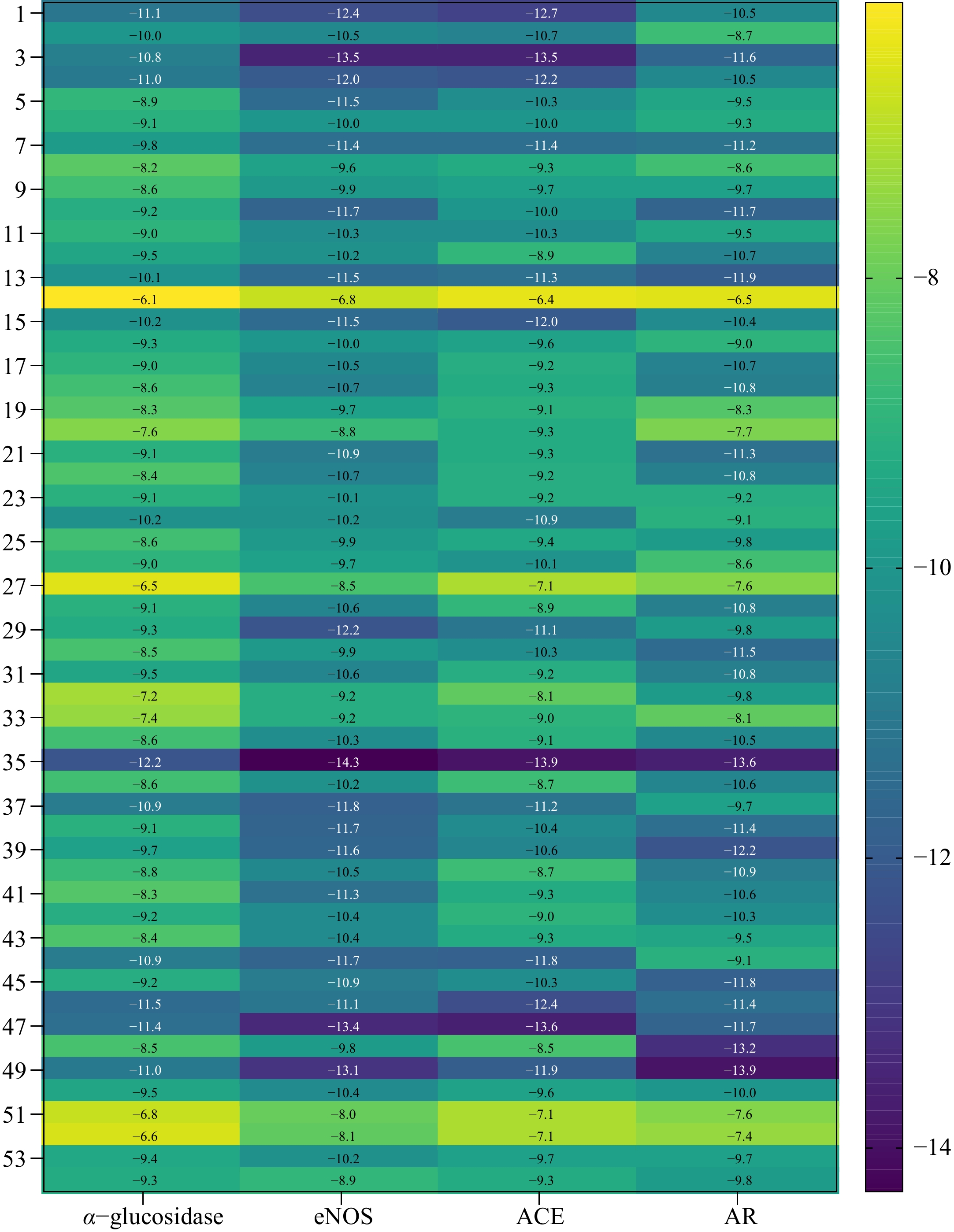
Figure 4.
Heatmap of the binding energy of the identified compounds and acarbose to α-glucosidase, eNOS, ACE, and AR.
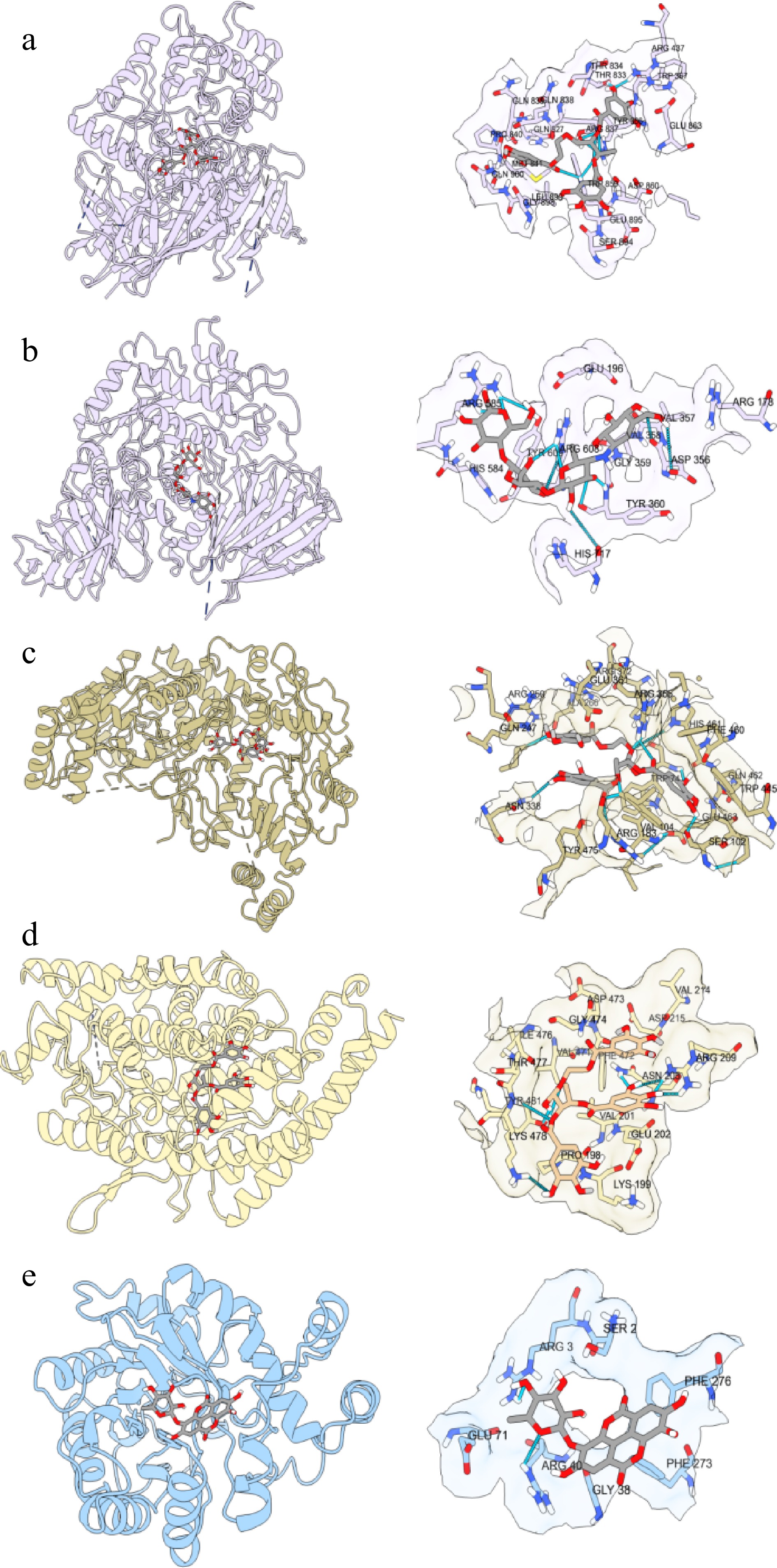
Figure 5.
(a), (b) Molecular interactions of the identified compounds and acarbose against α-glucosidase, (c) eNOS, (d) ACE, and (e) AR. (a) Punicalagin-α-glucosidase; (b) acarbose-α-glucosidase; (c) Punicalagin-eNOS; (d) Punicalagin-ACE; (e) Ellagic acid 3-O-α-L-rhamnopyranoside-AR.
Table 3. Molecular docking results of Punicalagin (No. 35), acarbose and α-glucosidase.
α-glucosidase Punicalagin
(No. 35)Acarbose No. of
hydrogen bondsBond length (Å) ARG437 O18 1 2.076 THR833 O18 1 1.927 ARG837 O3 2 3.572, 2.133 O4 2 2.679, 3.244 O5 2 2.204, 2.843 TRP859 O12 1 3.338 O3 1 3.456 TYR360 O5 1 1.848 ARG585 O8 2 3.354, 2.516 O17 1 3.719 ARG608 O2 2 3.582, 2.741 O7 1 3.285 O14 1 3.150 The associated proteins that cause diabetes complications are eNOS, ACE, and AR. The interactions of the 53 identified compounds and acarbose with eNOS, ACE, and AR were also analyzed, and it was calculated that the highest binding affinities with −14.3, −13.9, and −13.9 kcal/mol for Punicalagin, Punicalagin, and Ellagic acid 3-O-α-L-rhamnopyranoside (No. 49). The key mechanism of pomegranate peel polyphenols in the treatment of diabetes was to reduce oxidative stress and lipid peroxidation, and to combine with the generated reactive oxygen species, to achieve the role of preventing complications[44]. Punicalagin, a natural compound derived from pomegranate peel had the characteristics of being more effective and safer, not only playing a role in lowering blood sugar, but also having a good therapeutic effect on complications of diabetes, such as liver damage[45,46].
-
In this study, the outer layer of the pomegranate, which was derived from the fruit itself, was selected as the object of study and ultrasonic-assisted extraction technology with glycerol/urea (1:2, mol/mol, 30% water content) was adopted to extract the peel. This method not only improved extraction efficiency, but also reduced energy consumption and environmental pollution. A total of 53 polyphenols were obtained from pomegranate peel extracted by UPLC-Q-TOF-MS/MS, including 10 ellagic acids and their derivatives and 32 flavonoids (such as flavonoids, dihydroflavonoids, flavonols, anthocyanins, etc.). Ellagic acid and flavonoids, as well as their derivatives, all had good anti-inflammatory and antioxidant properties, which meant that they have great potential to prevent chronic diseases, such as cancer, cardiovascular, and cerebrovascular diseases, etc. According to the heatmap and molecular docking results, Punicalagin (No. 35) in pomegranate peel had a good inhibitory effect on α-glucosidase with a binding affinity of −12.2 kcal/mol and 10 hydrogen bonds formed. The polyphenols in pomegranate peel had a good therapeutic effect on diabetes and its complications, and the effective prevention and treatment of these diseases could improve quality of life and health status, and the development of pomegranate peel had a good application prospect in functional food or beverage. In addition, polyphenols also had good water solubility, and the pomegranate peel compounds added to the water as a tea for daily drinking were not only beneficial to individuals' well-being but also conducive to enhancing corporate profitability and improved the utilization of resources. Furthermore, this approach was of paramount importance as it effectively mitigated the environmental pollution caused by pomegranate peel. If the pomegranate peel is discarded at will, the phenolic substances in it will have a certain impact on water and soil. Extracting useful compounds from pomegranate peel could both solve environmental problems and create economic value, in line with the strategy of sustainable development. Therefore, this study provided an effective way for the comprehensive utilization of pomegranate peel, which has good practical significance and application prospects.
-
The authors confirm contribution to the paper as follows: conceptualization: Liu J, Cui Q; data curation: Wen L, Liu J, Cui Q; formal analysis: Lu X, Ye Z; funding acquisition: Liu J, Cui Q; investigation: Lu X, Ye Z, Jiang L, Wen L, Liu J, Cui Q; methodology: Lu X, Jiang L, Liu J, Cui Q; project administration: Cui Q; resources: Ye Z, Jiang L; supervision: Liu J, Cui Q; visualization: Lu X, Jiang L; writing-original draft: Lu X; writing-review & editing: Liu J, Cui Q. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
This work was supported by Zhejiang Provincial Natural Science Foundation of China under Grant Nos LQ24H280009 and LQ22H280007, National Natural Science Foundation of China (82204552), Research Project of Zhejiang Chinese Medical University (2022JKZKTS10), National College Students' Innovation and Entrepreneurship Training Program (S202310344074), Zhejiang Xin-miao Talents Program (2023R410043).
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Xiaoxian Lu, Zi Ye
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Lu X, Ye Z, Jiang L, Wen L, Liu J, et al. 2024. UPLC-Q-TOF-MS/MS analysis of pomegranate peel polyphenols and preliminary study on the biosynthesis of ellagic acid and flavonoids. Beverage Plant Research 4: e028 doi: 10.48130/bpr-0024-0031
UPLC-Q-TOF-MS/MS analysis of pomegranate peel polyphenols and preliminary study on the biosynthesis of ellagic acid and flavonoids
- Received: 01 June 2024
- Revised: 07 July 2024
- Accepted: 22 July 2024
- Published online: 06 August 2024
Abstract: Polyphenols are natural substances that are consumed in the daily diet and are found abundantly in a diverse range of fruits and vegetables. Deep eutectic solvent is a new kind of green solvent, which is widely used in the extraction and separation of polyphenols because it is non-toxic and pollution-free, and meets the requirements of environmental protection. This research used ultrasonic-assisted deep eutectic solvent (glycerol/urea 1:2, mol/mol, 30% water content) to extract polyphenols from pomegranate peel with improved extraction efficiency. The extracts were analyzed and identified by UPLC-Q-TOF-MS/MS, and 53 compounds were obtained, of which 10 were ellagic acid and its derivatives, and 32 were flavonoids. These polyphenols exhibit a wide range of pharmacological properties, including, but not limited to anti-inflammatory and antioxidant effects. Long-term intake of these polyphenols can effectively prevent chronic diseases, such as cardiovascular and cerebrovascular diseases, chronic tumors, etc. Polyphenols generally have good water solubility, especially anthocyanin compounds with cationic structures that have the best water solubility. Therefore, the incorporation of polyphenols into water to prepare tea and its regular consumption confers significant health benefits. Compared with acarbose, Punicalagin had a better inhibitory ability on α-glucosidase with a binding affinity of −12.2 kcal/mol and 10 hydrogen bonds were formed, indicating that Punicalagin had a potential anti-diabetic function. Pomegranate peel, a by-product of the pomegranate fruit, is typically discarded as waste in daily operations. However, exploring secondary applications for pomegranate peel not only contributes to cost reduction for enterprises but also promotes environmental sustainability and facilitates sustainable development. The extraction technology of pomegranate peel polyphenols also provides a new idea and method for the deep processing of agricultural products. This work will help to understand the biosynthetic pathways of pomegranate peel polyphenols, to facilitate the development of beverages containing pomegranate peel polyphenols.
-
Key words:
- Pomegranate peel /
- Polyphenols /
- Deep eutectic solvents /
- UPLC-Q-TOF-MS/MS /
- Ellagic acid /
- Flavonoids