-
Tea is celebrated for its diverse array of active components, such as tea polyphenols, amino acids, tea polysaccharides, and caffeine, all contributing to its varied health benefits[1]. The majority of tea leaves, over 60% of the annual yield, are harvested during the summer and fall[2]. These leaves typically have higher levels of tea polyphenols, anthocyanins, and purine alkaloids, but lower amino acid content compared to those harvested in the spring[3]. Tea can be classified into six distinct types based on processing techniques: green tea (unfermented), white tea (slight-fermented), yellow tea (slight-fermented), oolong tea (semi-fermented), black tea (fully fermented), dark tea (post-fermented by microorganisms), each with its unique flavor profile[4]. Enhancing the value-added utilization of tea resources is a key research priority in the tea industry.
The growing consumer interest in functional foods and beverages made from natural ingredients has fueled innovation in tea-based products[5]. The bioactive compounds and the flavor profiles of tea can be modified and enhanced through microbial fermentation[6]. Kombucha, a fermented tea beverage originating from the Bohai Sea region in China, later spread to the Soviet Union and Germany, and has since gained global popularity[7−9]. It is distinguished by its sweet and sour taste and unique flavor. The bacterial cellulose (BC) produced and floating on the surface of the brew is considered a valuable renewable resource[10−13].
The fermentation process involves complex interactions and uncertainties among the microorganisms present. This review utilizes sources from the Web of Science, ScienceDirect, Springer, China National Knowledge Infrastructure (CNKI), and other databases from the past five years. The production of kombucha, tea wine, and tea vinegar typically begins with sugar tea soup, where sugars serve as a carbon source and tea soup as a nitrogen source. A symbiotic culture of acetic acid bacteria and yeast (sometimes including a small amount of lactic acid bacteria) inoculated to kombucha fermentation, tea wine primarily fermented by yeasts, while tea vinegar requires initial alcoholic fermentation by yeast followed by inoculation with acetic acid bacteria[14−16]. This review delves into how microbial fermentation transforms tea components, creating distinctive flavor profiles in fermented tea beverages and altering organic acids, polyphenols, caffeine, and volatile components.
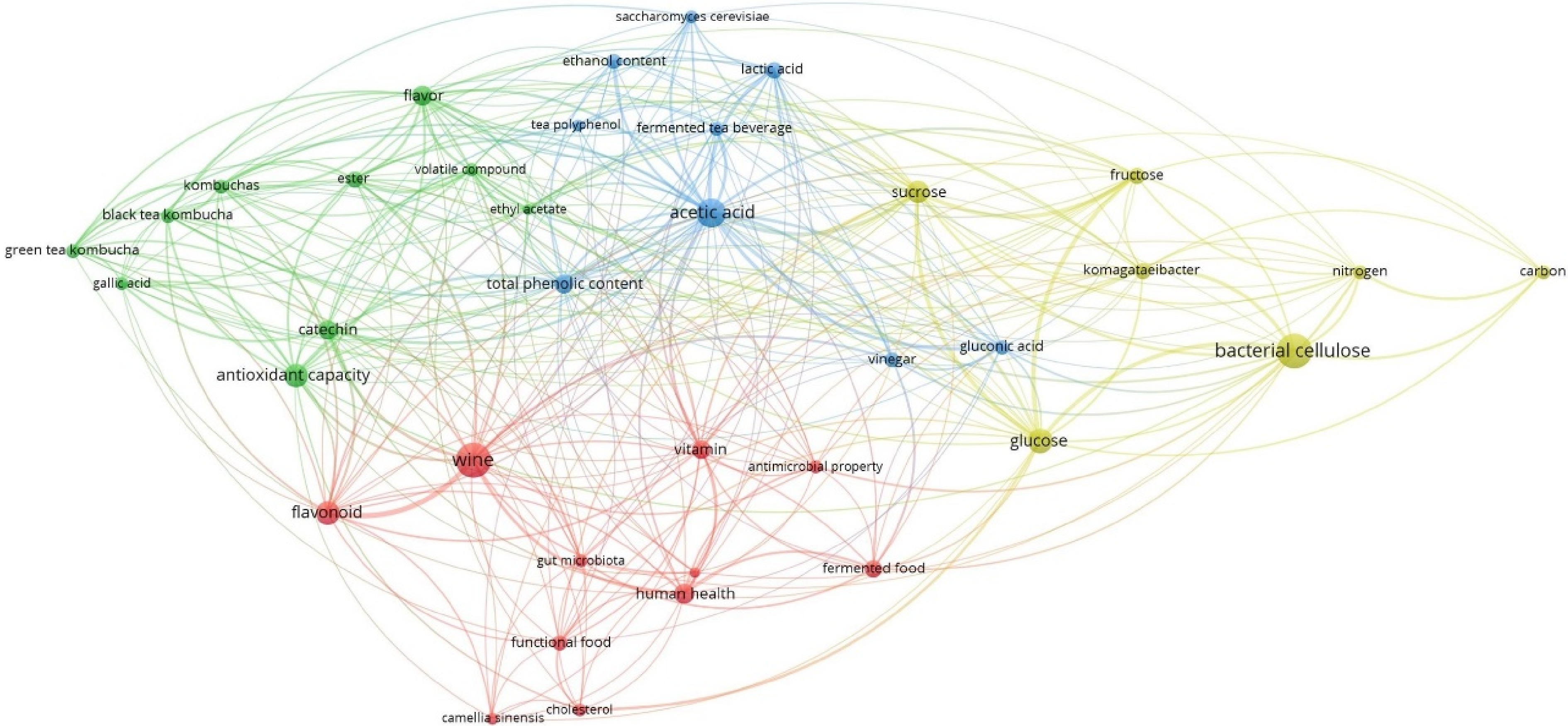
The analysis using VOSviewer — a software tool for constructing and visualizing bibliometric networks — identifies several key research areas in studies on kombucha, tea wine, and tea vinegar, including evaluation of functional food, bioactive compounds, microbial metabolism, and bacterial cellulose research (Fig. 1).
-
In the world of kombucha, yeast, and acetic acid bacteria (AAB) are the primary microorganisms found[8]. Common yeast species include Candida, Debaryomyces, Dekkera, Pichia, Saccharomyces, Saccharomycopsis, Schizosaccharomyces, Sporopachydermia, Zygosaccharomyces, and Zygotorulaspora[8,17,18]. For tea wine fermentation, yeasts such as Schizosaccharomyces pombe, Saccharomyces cerevisiae, and Jiuqu are employed[15,19], with the latter being exclusive to tea wine. In kombucha, the principal AAB species involved are Acetobacter, Gluconobacter, Gluconacetobacter, and Komagataeibacter[8,17,18,20]. Tea vinegar fermentation primarily utilizes Acetobacter aceti, Acetobacter pasteurianus, and other Acetobacter species[21,22]. Yeast fermentation is noted for producing ethanol and carbon dioxide, as well as smaller amounts of glycerol, higher alcohols, and esters[14]. The metabolic processes of Acetobacter species are known for their 'oxidative' fermentation, involving the incomplete oxidation of substrates by primary dehydrogenases in the respiratory chain. This process oxidizes ethanol, carbohydrates, and sugar alcohols (also known as polyols or polyhydric alcohols) into their corresponding organic acids, aldehydes, or ketones, aiding in energy production[23,24].
-
During the fermentation of tea beverages, yeast initiates the process by breaking down glucose and fructose[25]. These sugars are then converted into pyruvate through glycolysis, ultimately entering the Tricarboxylic Acid (TCA) cycle to produce various metabolites, including organic acids[26]. AAB play a crucial role by oxidizing glucose into gluconic and glucuronic acids and converting ethanol into acetic acid[27,28]. As fermentation advances, the sugar concentration decreases, while the accumulation of organic acids leads to a reduction in pH, typically stabilizing around 3.0[29−31].
In the specific context of tea wine fermentation, the predominant organic acids are malic and succinic acids, with traces of acetic, lactic, and gluconic acids also present[31,32]. Tea vinegar, in contrast, contains not only the organic acids found in tea wine but also oxalic, citric, tartaric, and ascorbic acids, among others[22], demonstrating a volatile acidity of about 4%[21]. These organic acids, common to tea wine and tea vinegar, are also found in kombucha. However, kombucha is distinguished by its primary organic acids: acetic, gluconic, glucuronic, D-saccharic acid 1,4-lactone (DSL), ascorbic, succinic, malic, lactic, tartaric, and citric acids[29,30,33−35].
The alcohol content in these fermented tea beverages varies with the microorganisms involved. Yeasts are capable of converting glucose and fructose into ethanol[14]. During tea wine fermentation, ethanol levels increase[36], with alcohol content ranging from 8% to 16% vol, depending on specific fermentation conditions[32,37−39]. For tea vinegar, the alcohol concentration declines as ethanol is first oxidized to acetaldehyde by alcohol dehydrogenase (ADH) on the acetic acid bacteria's plasma membrane surface, then further oxidized to acetic acid by aldehyde dehydrogenase (ALDH)[40]. This process culminates in the complete oxidation of ethanol, where acetate is converted into CO2 and water[24]. In kombucha fermentation, ethanol levels initially rise before decreasing[29]. The acetic acid produced by AAB can enhance yeast's ethanol production, stimulating its invertase and fermentation activity[41]. As kombucha is classified as a non-alcoholic beverage, its alcohol concentration is kept below 0.5% (v/v)[42].
-
Research involving Saccharomyces cerevisiae (S. cerevisiae) highlights the crucial role of mannoprotein N-glycosyl phosphorylation in the adsorption of polyphenols by yeast and its cell walls, with a particular affinity for adsorbing polymeric and oligomeric tannins and derived pigments[43]. Studies on green tea polyphenols (GTP) have shown their protective effects on S. cerevisiae cells against ethanol-induced damage, enhancing cell wall permeability through up-regulation of the active gene for the guanosine diphosphate mannose transporter and promoting mannose protein formation. Additionally, GTP significantly enhances genes related to proline metabolism and biosynthesis, thereby increasing S. cerevisiae's tolerance to ethanol. As powerful antioxidants, GTPs reduce the accumulation of ROS, with associated genes for ROS production or mitochondrial electron transport being down-regulated[44]. Yeast cultures actively uptake ethanol, which is then oxidized to acetaldehyde and acetyl-CoA, providing a primary source of high-energy electrons for NADH and NADPH[45]. Research on tea wine has shown that catechins interact with ethanol to form hydrogen bonds, creating molecular clusters that transition the direct electron-transfer process of catechin to proton-coupled electron transfer, lowering the energy barrier of the redox reaction and enhancing antioxidative capacity[46].
Fermentation generally increases the total polyphenol content in kombucha compared to unfermented tea soup, and enhances the bioavailability of these polyphenols[29,47,48]. A wide range of phenolic compounds, including flavonoids, phenolic acids, other polyphenols, lignans, and stilbenes, have been identified in kombucha[30,49]. These complex phenolic compounds may degrade in kombucha's low pH environment and through enzymes (e.g., β-glucosidase, esterase) produced by bacteria and yeasts[49]. The increase of epigallocatechin and gallic acid in kombucha indicates the hydrolysis of gallate groups attached to polyphenols via ester bonds, such as epigallocatechin gallate[50,51]. The concentration of ester catechins decreases in kombucha fermented from green tea, black tea, and Pu'er raw tea, while the total catechins content increases in green tea kombucha[33,52].
In contrast, the polyphenol content decreases in black tea wine compared to unfermented tea[36,37]. The content of various catechins, including (−)epigallocatechin gallate (EGCG), (+)-catechin (C), (−)catechin gallate (CG), (−)-epicatechin gallate (ECG), (−)gallocatechin gallate (GCG) increases in Keemun black tea wine, with no significant change in gallic acid content[32]. The contents of (−)-epigallocatechin (EGC), EGCG, and gallic acid also decrease in Dancong tea wine[39]. Total catechin contents are significantly reduced in green tea wine[53]. The ester bonds of catechins like EGCG and ECG are reactive and prone to hydrolysis, releasing gallic acid, EGC, and EC. This hydrolysis can lead to further decomposition under yeast dioxygenase catalysis, producing simple phenols and B-ring fission metabolites of catechins[54].
In tea vinegar fermentation, the contents of tea polyphenols and flavonoids initially decrease and then stabilize[16,55]. When AAB strains are inoculated into a black tea infusion with ethanol to produce black tea vinegar, the contents of tea polyphenols and thearubigins decrease, while theabrownins increase[22]. The content of C and EC decreases, while the EGCG content increases, generally raising the total catechin content in tea vinegar[55].
Microbial strains exhibit distinct polyphenol transformation capabilities during the fermentation of tea soup to kombucha. Acetobacter pasteurianus, Zygosaccharomyces bailii, and Debaryomyce hansenii can increase the content of EGCG, a major component of tea polyphenols. Z. bailii and Acetobacter xylinum enhance the concentration of low molecular weight tea polyphenols. Synthetic microbial communities constructed with Z. bailii, L. plantarum, and D. hansenii significantly increase the EGCG content in tea broth, along with total phenols and flavonoid contents[56]. Microbiomes containing the enzyme shikimate dehydrogenase are responsible for gallic acid production. Compared to kombucha produced with Brettanomyces bruxellensis and Schizosaccharomyces pombe, which produces higher ethanol, kombucha without Brettanomyces bruxellensis shows lower ethanol and increased production of theobromine, rutin, and chlorogenic acid. The concentration of gallic acid and caffeic acid increases, while catechin and epicatechin decrease after kombucha fermentation[57].
The mid-fermentation stage, spanning days 5 to 10, plays a pivotal role in the biotransformation of phenolic compounds during the fermentation of black tea kombucha. During this period, flavonoids experience the most significant degradation, whereas the concentration of phenolic acids increased substantially[49]. Compared to non-fermented tea, the levels of gallic, chlorogenic, protocatechuic, p-coumaric, and ellagic acids, along with rutin, vitexin, and resveratrol, are significantly elevated in black tea kombucha, while ferulic acid and syringic acid exhibit a slight decrease[48].
The biotransformation of hydroxycinnamic acids, such as caffeic, ferulic, and p-coumaric acids, is facilitated by the decarboxylases or reductases of yeast[58]. In Saccharomyces cerevisiae, the presence of three cofactors — FADH2, S-adenosyl-l-methionine, and NADPH — promotes the conversion of p-coumaric acid into high levels of caffeic and ferulic acids[59]. Additionally, the ester bonds of chlorogenic acid can be hydrolyzed by the ester hydrolases of S. cerevisiae, leading to the formation of caffeic acid and quinic acid. Furthermore, p-coumaric acid can undergo decarboxylation by S. cerevisiae to form 4-vinyl phenol[19].
Caffeine
-
Caffeine, a member of the methylxanthine class, is a crucial component of tea and provides essential nitrogen for microbial metabolism[48,60,61]. Both bacteria and fungi have developed pathways for caffeine degradation, mainly through N-demethylation and C-8 oxidation processes[62]. The degradation sequence starts with the conversion of caffeine into theobromine and theophylline, which are then transformed into xanthine and eventually broken down into carbon dioxide and ammonia by yeast via the purine catabolic pathway[63]. The Pichia manshurica strain, a caffeine-degrading yeast isolated from kombucha, exhibits maximum caffeine dehydrogenase activity when utilizing glucose as a carbon source, with fructose being the second most effective[64].
In fermented tea beverages, such as kombucha, tea wine, and tea vinegar, the caffeine content undergoes a notable reduction during fermentation compared to their unfermented counterparts[48,50,65]. Kombucha, in particular, shows an increase in theobromine content as the caffeine content decreases[56,57]. The consumption of caffeine stabilizes to lower rates in the mid to late stages of tea wine fermentation[37]. In tea vinegar, the caffeine content is reduced by approximated 40%[55]. This general decrease in caffeine content across all fermented tea beverages underscores the significant impact of microbial activity on this stimulant during the fermentation process.
-
During the fermentation of kombucha, tea wine, and tea vinegar, the original volatile components found in tea broth — such as alcohols like linalool and geraniol, terpenes like squalene and limonene, as well as aldehydes and ketones — are significantly reduced or eliminated. Microorganisms transform terpenoids, including limonene and linalool into 4-pinacol type terpenoids[66]. In kombucha, the microbial reduction of dihydrolinalool and α-terpineol results in fluctuations in β-limonene content, which initially increases and then decreases[67]. The fermentation process in these three types of tea beverages alters the main volatile substances present in the tea broth and produces new volatile compounds, including alcohols, esters, and acids. These new compounds play a crucial role in developing distinct flavor profiles. The specific volatile compounds produced are influenced by the fermentation substrate, the microorganisms involved, and the fermentation conditions.
In tea wine, the primary volatile components are alcohols, esters, and aromatic compounds[19,31,32,37,53]. The volatile profile of kombucha is primarily composed of alcohols, acids, and esters[52,67−71]. Tea vinegar is characterized mainly by its content of acids and alcohols[72]. The transformation of aroma compounds during fermentation is affected by metabolic pathways related to amino acid metabolism, fatty acid synthesis, and terpene synthesis[53]. This complex interplay of biochemical reactions gives each beverage its unique and characteristic aroma.
Alcohols
-
Research has identified 2-phenylethanol, isoamyl alcohol, and isobutanol as the primary alcohols in fermented tea beverages like kombucha, tea wine, and tea vinegar[19,32,70,72]. Additional alcohols such as nerolidol, nerol, citronellol, n-pentanol, and 2-methyl-1-butanol are also present, with unique substances like citronellol, 1-nonyl alcohol, and benzyl alcohol found in summer and autumn tea vinegar, and Jinjunmei black tea wine[37,70,72]. A noted increase in tea concentration during the fermentation of apple tea wine significantly boosts the production of fusel alcohols[73]. The fermentation of tea wine by S. cerevisiae results in the depletion of amino acids such as isoleucine, leucine, and phenylalanine, correlating with the formation of their respective alcohols: isoamyl alcohol, and 2-phenylethanol[19]. These amino acids are derived not only from the tea broth but can also be synthesized from carbohydrates by yeast, where glucose undergoes glycolysis to form pyruvate, subsequently entering the amino acid biosynthesis pathway[74]. The Ehrlich pathway typically leads to the production of higher alcohols from the catabolism of some amino acids during fermentation[75].
Esters
-
During the fermentation of tea wine with various teas, esters such as ethyl caprylate, ethyl caproate, and ethyl decanoate have been identified[19,31,32,37]. Other distinctive esters detected include isoamyl caprylate, isoamyl acetate, diethyl succinate, 3-methylbutyl decanoate, ethyl sorbate, monoethyl succinate, lactic acid ethyl ester, and ethyl acetate[31,32,37]. In tea vinegar, notable esters include ethyl acetate and lactic acid ethyl ester[72]. Studies on kombucha fermentation have revealed compounds like 2-(1H-indol-3-yl) ethyl acetate, ethyl 2-phenylacetate, ethyl hexanoate, and methyl hexanoate[70]. Introducing Pichia kluyveri as a starter culture in kombucha fermentation enhances the rapid buildup of acetic acid and the production of acetate esters, particularly isoamyl acetate and 2-phenethyl acetate[76]. Ethyl esters, featuring an ethanol (alcohol group) and a medium-chain fatty acid (acid group), include compounds like ethyl hexanoate, ethyl octanoate, and ethyl decanoate. Acetate esters, formed from acetate (acid group) and an alcohol group (either ethanol or a complex alcohol from amino acid metabolism), include esters such as ethyl acetate and isoamyl acetate[77].
Acids
-
Acids such as octanoic acid and decanoic acid have been identified in tea wine, tea vinegar, and kombucha[19,70,72]. The fermentation of kombucha leads to the formation of a bacterial cellulose film on the solution's surface, which restricts the available oxygen and thus influences the presence of fatty acids, either by depleting or converting them[51,78]. During the fermentation period from the 7th to the 10th day, especially when using raw Pu-erh tea as the base, a notable reduction in alcohols and aldehydes are observed, while the concentration of acids increase. By the end of the fermentation process, around day 14, the volatile composition is predominantly characterized by acids and esters, indicating a significant transformation in the chemical profile of the tea over the fermentation period[52].
-
The Symbiotic Culture of Bacteria and Yeast (SCOBY) in kombucha produces a floating layer of bacterial cellulose (BC), a sustainable and renewable biomaterial that is garnering significant research interest[79,80]. BC is particularly valued for its potential applications in food packaging, where its antioxidant properties are highly beneficial[81,82]. Genera such as Komagataeibacter and Gluconacetobacter within the AAB group are recognized for their ability to produce large quantities of extracellular cellulose[83]. This production can also be achieved through the combined fermentation of strains such as Brettanomyces bruxellensis and Komagataeibacter spp., or through co-culture methods involving Saccharomyces cerevisiae and Komagataeibacter rhaeticus[84,85]. Moreover, AAB can synthesize water-soluble cellulose polysaccharides from substrates including glucose, fructose, ethanol, and glycerol. This process involves the formation of uridine diphosphate glucose (UDPGlc), which then polymerizes into the β-1,4-glucan chain characteristic of bacterial cellulose[86,87]. Tea, particularly green tea serves as a nitrogen source in this process and is noted for producing a higher yield of BC with enhanced hydrogen-donating capabilities[88]. Plant xanthines in black tea, such as theaflavins, thearubigins, and caffeine have also been shown to boost cellulose production[89]. Furthermore, incorporating phytochemicals such as carotenoids, alkaloids, tannins, flavonoids and anthocyanins into the BC matrix can endow the material with specific properties, notably enhancing its antioxidant and antimicrobial activities[89,90].
-
Different types of tea, including black, green, white, oolong, dark, and summer teas, serve as the primary raw materials in the production of fermented beverages such as kombucha, tea wine, and tea vinegar[15,21,22,30−33,35−39,55,72] (Table 1). Generally, fermentation enhances the phenolic content and antioxidant capacity of these drinks[58]. In the case of kombucha, the fermentation process notably affects polyphenols when using black tea, leading to a greater variety of phenolic compounds[51]. Specifically, phenolic compounds newly formed in black tea kombucha represent 42.72% of the total phenolics, compared to a mere 0.97% in green tea kombucha[30]. Moreover, green tea kombucha features elevated levels of epicatechin, catechin, and kaempferol relative to its black tea kombucha[91]. The abundant amino acids in green tea promote sugar consumption, boosting alcohol production and enhancing yeast fermentation. Consequently, wines derived from green tea display higher concentrations of total and specific catechins (EC, EGC, ECG, and EGCG) than those produced from black, oolong, and dark teas[31].
Table 1. Kombucha, tea wine, and tea vinegar prepared from different types of tea.
Beverage Fermentation conditions Substrate Results Ref. Kombucha 3% (w/v) SCOBY,
100 mL/L kombucha,
fermented at 25 °C for 10 d.Green tea pH = 3.2, total acid: 0.36% (w/v acetic acid), alcohol: 7.29 g/L,
total phenols: 0.70 mg GAE/mL, theaflavin: 0.028 (% w/v),
theobromine: 1.330 (% w/v).[30] Black tea pH = 3.5, total acid: 0.32% (w/v acetic acid), alcohol: 4.90 g/L,
total phenol: 1.09 mg GAE/mL, theaflavin: 0.151 (% w/v), theobromine: 1.998 (% w/v).Kombucha 30 g SCOBY, 100 mL kombucha, fermented at 27 °C for 14 d. Black tea Days 7 and 14 of fermentation: acetic acid: 3.18, 9.18 (mg/mL),
Alcohol: 4.69, 5.83(mg/mL).
Days 0, 7 and 14 of fermentation:
polyphenolics: 79.38, 64.81, 67.20 (mg/g DW),
flavonoids: 17.97, 14.46, 13.87 (mg/g DW), and
total catechins: 2.184 ,0.99, 0.464 (mg/g DW).[33] Green tea Days 7 and 14 of fermentation: acetic acid: 4.22, 7.65 (mg/mL),
alcohol: 2.81, 4.18 (mg/mL).
Days 0, 7 and 14 of fermentation:
polyphenolic compounds: 74.40, 100.33, 67.40 (mg/g DW),
flavonoids: 16.57, 18.49, 15.11 (mg/g DW),
total catechins: 18.253, 9.770, 11.844 (mg/g DW).Kombucha 10% SCOBY and kombucha,
28 ° C fermented for 1, 7, 14 d.Green tea Tea soup: pH = 5.54, alcohol: 0, acidity: 20.12 (mg acetic acid/L),
TFC: 254.1 (mg/L), TPC: 269.0 (mg/L).
At 1, 7, 14 d fermentation:
pH = 3.50, 2.61, 2.49, alcohol: 0.2%, 3.0%, 2.75%,
acidity: 610.34, 7,039.21, 9,147.4 (mg acetic acid/L),
TFC: 196.2, 146.8, 181.3 (mg/L), TPC: 277.6, 299.6, 320.1 (mg/L).[29] Black tea Tea soup: pH = 5.34, Alcohol: 0, acidity: 23.5 (mg acetic acid/L),
TFC: 231.7 (mg/L), TPC:183.1 (mg/L),
At 1, 7, 14 d fermentation: pH = 3.54, 2.62, 2.53,
Saccharose: 10.88, 9.5, 7.5 (Brix-g/100 mL),
alcohol: 0.3%, 3.25%, 2.0%,
acidity: 501.02, 7,039.08, 9,083.03 (mg acetic acid/L),
TFC: 149.1, 90.5, 126.7 (mg/L), TPC: 201.0, 219.5, 206.0 (mg/L).White tea Tea soup: pH = 6.53, alcohol: 0, acidity: 21.09 (mg acetic acid/L)
TFC: 209.3 (mg/L), TPC: 184.6 (mg/L);
At 1, 7, 14 d fermentation: pH = 3.56, 2.53, 2.37,
saccharose: 10.13, 10.13, 9.5 (°Brix-g/100 mL),
alcohol: 0.4%, 3.5%, 3.0%,
acidity: 620.13, 7,048.06, 9,132.20 (mg acetic acid/L)
TFC: 132.6 (mg/L), 83.8 (mg/L),111.6 (mg/L),
TPC: 200.8 (mg/L), 205.6 (mg/L), 228.1 (mg/L).Pu'er tea Tea soup: pH = 5.58, alcohol: 0, acidity: 20.42 (mg acetic acid/L),
TFC: 359.9 (mg/L), TPC: 229.5 (mg/L),
At 1, 7, 14 d fermentation: pH = 3.62, 2.38, and 2.32,
alcohol: 0.4%, 3.5%, 3.0%,
acidity: 600.09, 7,059.47, 9,071.02 (mg acetic acid/L),
TFC: 292.5, 198.1, 242.5 (mg/L), TPC: 219.8, 270.5, 271.9 (mg/L).Kombucha 10% (v/v) kombucha, fermented at room temperature for 15 d. Green tea pH = 2.94, total acid: 11.72 g/L,
alcohol free, glucuronic acid: 1.37 g/L, gluconic acid: 41.42 g/L, DSL: 3.44 g/L, ascorbic acid: 0.61 g/L, acetic acid: 10.42 g/L, succinic acid: none.[35] Black tea pH = 2.70, total acid: 16.75 g/L,
alcohol free, glucuronic acid: 1.58 g/L, gluconic acid: 70.11 g/L, DSL: 5.23 g/L, ascorbic acid: 0.70 g/L, acetic acid: 11.15 g/L, succinic acid: 3.05 g/L.Oolong tea pH = 2.89, Total acid: 12.24 g/L, no alcohol,
glucuronic acid: 0.07 g/L, gluconic acid: 48.75 g/L, DSL: 4.02 g/L, ascorbic acid: 0.60 g/L, acetic acid: 10.48 g/L, succinic acid: none.Tea wine 25°Brix, 0.5% yeast,
fermentation at 25 °C.Black tea Alcohol 14.0% vol, theanine content: 0.241 mg/mL. [38] Tea wine The sugar level was 17 °Bx, 0.3 % yeast, fermentation at 24 °C for 13 d. Green tea The alcohol content was 8.5 %vol, the concentration of tea polyphenols was 2,902.35 mg/L. [36] Tea vinegar 4% alcohol by total volume, 5% acetic acid strain, 30 °C for 9 d. Black tea Theaflavins were significantly decreased, thearubigins were decreased, and theabrownines were increased. [22] Tea vinegar 0.9% yeast, initial sugar 15%. The initial alcohol content was 6% vol,
9% acetic acid bacteria, fermentation at 28 °C for 12 d.Summer and
fall green
tea fragmentsThe acidity content was 59.10 g/L, tea polyphenol content was 2.95 g/L, alcohol content was 0.08% vol, amino acid nitrogen content was 0.14 g/dL, and caffeine content was 0.80%. [72] GAE: Gallic acid equivalent, DW: Dry Weight, TPC: total polyphenols content, TFC: total flavonoids content. Overall, while kombucha, tea wine, and tea vinegar can be produced from six predominant tea types, an assessment of factors such as taste, fermentation duration, and polyphenol content highlights specific advantages of black tea. Nevertheless, green tea is distinguished by its higher catechin levels and superior antioxidant properties, making its transition from tea soup to fermented tea beverages during microbial fermentation (Fig. 2).
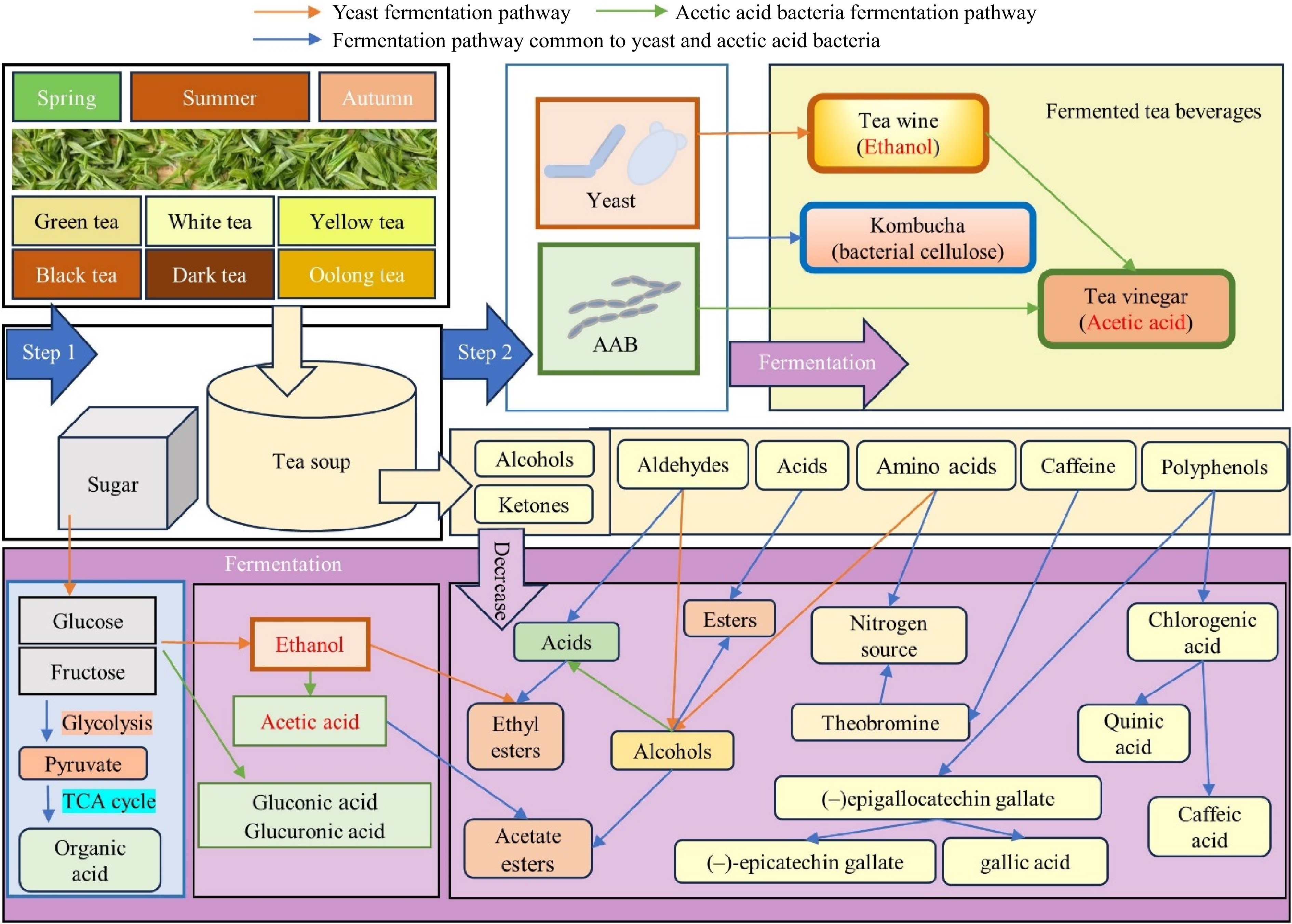
Figure 2.
Fermentation processes in tea beverages: from raw materials to finished products. (1) In the production of kombucha, tea wine, and tea vinegar, commonly used tea leaf raw materials include various types of tea such as green, black, oolong, dark, and white teas, processed differently, as well as seasonally surplus summer tea leaves. (2) These teas provide the substrate for fermentation. For kombucha, sugar tea soup, yeast, and a mix of acetic acid bacteria are added to initiate fermentation. In contrast, the addition of yeast alone ferments into tea wine while combining the ethanol from yeast fermentation with acetic acid bacteria further produces tea vinegar[16]. (3) During fermentation, glucose and fructose are converted into ethanol by yeast and transformed into gluconic and glucuronic acid by acetic acid bacteria. Additionally, ethanol is oxidized to acetic acid[14,27]. Throughout the microbial metabolism process, various compounds such as alcohols, ketones, aldehydes, acids, amino acids, caffeine, and polyphenols in the tea undergo transformations, being preserved, increased, or decreased. Caffeine is converted into theobromine and theophylline, serving as nitrogen sources for the microbes. Amino acids provide nitrogen and are transformed into alcohols, while aldehydes convert into alcohols and acids. Reactions between alcohols and acids with ethanol and acetic acid result in the formation of new ethyl esters and acetate compounds[15,30,31,33,35,55,72]. Tea polyphenols, predominantly flavonoids, and phenolic acids[67], undergo significant changes: catechin ester bonds break, (–)epigallocatechin gallate hydrolyzes into epigallocatechin and gallic acid, and chlorogenic acid converts into caffeic acid and quinic acid[19]. This intricate fermentation process not only alters the chemical composition of the raw materials but also enhances the flavor and nutritional value of the final products, underscoring the uniqueness and complexity of tea beverage fermentation.
-
Fermented tea beverages, including kombucha, tea wine, and tea vinegar, owe their health benefits to bioactive compounds that are produced during the fermentation process. These health benefits encompass antioxidant properties, antibacterial activity, anti-inflammatory effects, and blood sugar regulation. Additionally, these beverages contribute to gut health by promoting the growth of beneficial bacteria (Table 2). Fermented tea beverages may also aid in detoxification and support liver health, attributed to the increased levels of polyphenols, organic acids, and other metabolites produced by microbial fermentation.
Table 2. Healthy benefits of fermented tea beverage kombucha in vivo.
Model Intervention and dosage Significant results and biological activity Ref. Glycemic index and insulin index after a standard carbohydrate meal Sample: 11 healthy adults (four males and seven females),
Period: 120 min after test meals
Group 1: 330 ml of soda water;
Group 2: diet lemonade soft drink;
Group 3: organic kombucha.
Additional capillary blood samples were collected at regular intervals
(15, 30, 45, 60, 90, and 120 min) after commencement of the reference solution or test meal.Soda water (GI: 86 and II: 85)
soft drink (GI: 84 and II: 81)
kombucha (GI: 68, and II: 70)
↓GI (Glycemic index)
↓II (insulin index).[92] HFHF Sample: 40 wistar rats,
Period: 10 weeks,
n = 10 for all groups, ad libitum consume,
Group 1: standard diet (AIN-93M);
Group 2: high-fat and high-fructose diet (HFHF);
Group 3: HFHF + GTK diluted in water (30% v/v);
Group 4: HFHF + BTK diluted in water (30% v/v).GTK, BTK: ↑propionic acid
↑Firmicutes: Bacteroidetes,
↑Erysipelotrichaceae,
↓Bacteroidaceae ,
↓S24-7, Desulfovibrionaceae.
GTK: ↓Proteobacteria.
BTK: ↑Euryarchaeota, Lachnospiraceae.[93] HFHF Sample: 32 wistar rats,
Period: 10 weeks, ad libitum consume,
Group 1: AIN-93M: standard diet (n = 8);
Group 2: HFHF (n = 24);
then regroup as follows: HFHF; HFHF + GTK diluted in water (30% v/v);
HFHF + BTK diluted in water (30% v/v).GTK, BTK: improving the insulin sensitivity, reduced the percentage of lipid vesicles in the liver, reverting the liver steatosis from grade 2 to 1, active CPT1 express,
↑glucose tolerance, TAC in plasma and liver, SOD and CAT in liver,
↓ALT, NO, NLR,
GTK: ↑ADIPO-R2, BTK: ↑SREBP1c.[94] LPS-induced sepsis Sample: Specific pathogen-free C57BL/6 adult mice,
Period: 60 d,
n = 10 per group, with equal numbers of male and female.
Group 1: free-drinking water, the sham;
Group 2: free-drinking kombucha (FD), (replaced every 2 d);
Group 3: intragastric administration (IA) of kombucha (100 μL/100 g daily);
Group 4: free-drinking water, LPS-induced sepsis (LPS);
Group 5: FD + LPS;
Group 6: IA + LPS.
At day 60, Group 1−3 injected phosphate-buffered saline, Group 4−6 injected LPS (20 mg/kg).
Kombucha was added to drinking water at a volume ratio of 1:20.↑CD4+ T cells, B cell, macrophages,
↓CD8+ T cells,
↓IL-1β, TNF-α, CCL-2, IL-10, and CXCL10 in the lung tissues, alleviated the symptoms of lung histopathological damage
↓pIκBα, p-NF-κB expression.
inhibits LPS-induced NF-κB activation.
↓Bacteroidetes,
↑Cyanobacteria and Alistipes,
Prevotellaceae enrich in IA and FD group.[95] High fat high sugar diet injected with Streptozotocin to Type 2 Diabetes Sample: 60 Kunming mice,
Period: day 40 to 68 (4 weeks), randomly divided into five groups ( n = 12). The mice feed with HFHSD was intraperitoneally injected with STZ (50 mg/kg·bw) 4 times (day 28, day 31, day 34 and day 37) to induce T2DM.
Group 1: the normal control (NC), normal diet and gavage administration with 11.1 mL/kg·bw saline;
Group 2: DC, HFHSD and gavage administration with 11.1 mL/kg·bw saline;
Group 3: MET, HFHSD and gavage administration with 0.13 g/kg·bw metformin;
Group 4: KT, HFHSD and gavage administration with 11.1 mL/kg·bw kombucha;
Group 5: tea soup (TS), HFHSD and gavage administration with 11.1 mL/kg·bw tea soup.↓HOMA-IR, ↑HOMA- β, ↓AST, ALT.
↑liver glycogen content,
↓pancreatic index,
↑GPR41/GPR43 mRNA express,
↑GLP-1, PYY,
↑ZO-1, Claudin-1, Occludin,Muc2,
↓IL-1β, IL-6, TFN-α mRNA in intestinal inflammation,
↓LPS, TNF-α and IL-6 in serum.
↑Firmicutes, Lactobacillus, Butyricicoccus, Bifidobacterium,
↓Proteobacteria.
↑acetic acid and butyric acid in SCFAs.[96] NAFLD Sample: 12 male C57BLKS db/db mice,
Group 1: Control, the control diet (n = 4); MCD, the methionine/choline-deficient (MCD) diet (n = 8) for 4 weeks;
Group 2: MCD + water (n = 4);
Group 3: MCD + KT, Kombucha powder 2 g/kg by oral administration,
every 24 h for 3 weeks (n = 4).↓Fat accumulation in the livers.
↓Firmicutes,
↓Erysipelotrichia,
↓Allobaculum,
Turicibacter and Clostridium, ↑Bacteroidetes, ↑Lactobacillus, ↑Mucispirillum.[97] High-fat diet NAFLD Sample: 20 male C57BL/6 mice,
Period: 12 weeks (At the end of the 10th week, for nine consecutive days),
n = 5 for all groups.
Group 1: RC, the control group + tap water;
Group 2: RC + K, control group + kombucha (0.2 mL containing 107–108 microorganisms/mL);
Group 3: HFD, HFD + tap water;
Group 4: HFD + kombucha (HFD + K).Reduced the presence of intra hepatocyte lipid droplets, collagen deposition in the liver's perivascular spaces, and hepatic FXR gene expression. [98] DSS induced the leaky gut syndrome Sample:16 male NMR mice,
Period: day 7 to 21 (14 d);
Old mice: normal group (n = 8); DSS colitis induction group (n = 8): DSS no treatment group and DSS + fKT.
Young mice: normal group (n = 8); DSS colitis induction group (n = 8): DSS no treatment group and DSS + fKT.Ameliorates tissue changes associated with PMNs infiltration, crypt loss, epithelial defects, mucosal destruction, apoptosis, edema, and increased mucosal thinness due to DSS.
↑ZO-1 and ZO-2 express.[99] Antioxidant properties
-
Fermentation generally increases phenolic content and antioxidant activity[58]. Kombucha, for example, demonstrates strong antioxidant properties[35]. Green tea kombucha, after seven days of fermentation, exhibits less DPPH radical inhibition than unfermented green tea, while black tea kombucha has higher antioxidant activity compared to its unfermented counterpart[34,48,100]. This may be due to the higher phenolic content in black tea kombucha[93]. Green tea kombucha is found to have the highest antioxidant capability among kombuchas made from different tea types[29,47]. Similarly, with green tea wine having significantly higher ABTS values compared to other tea wines[31]. Tea vinegar's antioxidant and antibacterial functions surpass those of tea soup and conventional food vinegar[101], likely due to its rich phenolic and organic acid content[22].
Anti-inflammatory effects
-
Kombucha intervention significantly reduces serum levels of lipopolysaccharide (LPS), interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α cytokines, and markedly decreases the expression levels of inflammatory factors IL-1β, IL-6, and TNF-α mRNA in the colon[95,96]. It significantly restores T cell levels and macrophage counts, elevates CD4+ T cell and B cell levels, significantly lowers CD8+ T cell levels, markedly suppresses the upregulation of CCL-2, IL-10, and CXCL10, decreases phosphorylated-IκBα (pIκBα)expression levels and inhibits nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)signal transduction[95].
Blood sugar regulation
-
In human clinical trials, consuming unpasteurized kombucha significantly reduces Glycemic Index (GI ) and Insulin Index (II) after a standard high-GI diet for 2 h[92]. In animal models induced by a high-fat diet, kombucha can reduce weight, significantly decrease fasting blood glucose (FBG) and food intake, reduce HOMA-IR, increase HOMA-β, raise glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) levels in the intestine, significantly repair pancreatic damage, and protect the pancreas[95,96]. It improves insulin sensitivity, lowers insulin resistance, and enhances glucose tolerance[94,98].
Liver protection
-
Kombucha intervention can reduce total fat tissue in high-fat, high-sugar diet mice, decrease serum triglyceride levels, significantly reduce AST levels, enhance glycogen synthesis, and effectively improve liver function[96]. It restores liver fat degeneration from grade 2 to grade 1, increases plasma and liver total antioxidant capacity (TAC), reduces the neutrophil/lymphocyte ratio (NLR), and inflammatory marker levels. Black tea kombucha reduces SREBP1c expression, while green tea kombucha increases ADIPO-R2 expression[94]. Kombucha decreases citrate synthase and phosphofructokinase-1 enzyme activity, downregulates G protein-coupled bile acid receptor (Gpbar1, also known as TGR5) and farnesol X receptor (FXR) gene expression, reduces liver collagen fiber deposition, and liver fat accumulation[97,98].
Regulation of gut microbiota
-
Both green tea and black tea kombucha can regulate the intestinal microbiota, improving intestinal health in Wistar rats fed a high-fat, high-fructose (HFHF) diet[93]. Kombucha can restore colon damage in Type 2 diabetes mellitus (T2DM) mice, significantly increase the relative expression levels of tight junction proteins (ZO-1, Claudin-1, Occludin), and mucin proteins (Muc2), improving intestinal barrier damage[96]. Kombucha can improve tissue changes associated with "leaky gut syndrome" induced by dextran sodium sulfate (DSS), such as polymorphonuclear cells (PMNs) infiltration, crypt loss, epithelial defects, upregulating ZO-1 and ZO-2 expression[99].
In HFHF and T2DM animal models, kombucha can increase the ratio of Firmicutes to Bacteroidetes, decrease Proteobacteria, significantly increase intestinal microbial richness, such as Lactobacillus, Butyricicoccus, Lachnospiraceae, Bifidobacterium, and others[93,96,97]. It can significantly increase the content of short-chain fatty acids (SCFAs) acetate, butyrate, and propionate, and promote the growth of bacteria producing propionate, thereby exerting anti-inflammatory effects[93,95,96].
Antibacterial effects
-
The antibacterial activity of kombucha is similar to that of acetic acid, exhibiting inhibitory effects on intestinal pathogenic bacteria (Escherichia coli, Shigella dysenteriae, Salmonella Typhi, and Vibrio cholerae)[35,102]. Black tea kombucha shows strong inhibitory effects on Candida krusei, C. glabrata, C. albicans, C. tropicalis, and Hemophilus influenzae[34]. The main antibacterial compounds present in the polyphenolic fraction of kombucha were catechin and isorhamnetin[102]. These findings suggest that acetic acid and polyphenols in kombucha offer significant potential health benefits in inhibiting intestinal pathogenic bacteria.
-
As consumer health awareness increases, kombucha's popularity has surged in the global beverage market. Simultaneously, low-alcohol beverages have gained traction among younger and female consumers, enhancing their market value. Additionally, vinegar — rich in amino acids and various organic acids — has become a popular pre-meal choice. In response to the growing demand for health-focused and natural products, tea wine and tea vinegar are carving out niches as innovative trends.
The advanced utilization of summer and autumn teas through microbial fermentation is another notable development. This process not only reduces the bitterness and astringency by lowering catechin levels but also encourages the transformation of organic acids, alcohols, amino acids, and other substances, thus creating uniquely flavored tea beverages. Moreover, fermentation decreases the caffeine content in these drinks, categorizing them distinctly as fermented products. The flavor profile of these beverages is greatly enhanced by volatile compounds, which are key contributors.
However, the fermentation conditions and the type of tea used can significantly affect the formation and balance of these compounds. Research into how bioactive components in tea are transformed by yeasts and acetic acid bacteria is highly promising. Moreover, using metabolic engineering to amplify the biotransformation of phenolic compounds, especially to increase phenolic acid content, is an area ripe for further exploration.
-
The authors confirm contribution to the paper as follows: data curation and writing the original draft: Guo Q; investigation & visualization: Yuan J, Ding S, Nie Q, Xu Q, Pang Y, Liao X, Liu Z; resources, supervision: Liu Z; funding acquisition, supervision, writing–review: Cai S. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
The authors gratefully acknowledge the financial support from Guangxi Innovation Driven Development Special Fund Project (No. AA20302018), the National Key R&D Program of China (2018YFC1604405), National Natural Science Foundation Project of China (31471590, 31100501), and Self-Science Foundation of Hunan Province, China (2019jj50237).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Guo Q, Yuan J, Ding S, Nie Q, Xu Q, et al. 2024. Microbial fermentation in fermented tea beverages: transforming flavor and enhancing bioactivity. Beverage Plant Research 4: e029 doi: 10.48130/bpr-0024-0026
Microbial fermentation in fermented tea beverages: transforming flavor and enhancing bioactivity
- Received: 21 February 2024
- Revised: 10 June 2024
- Accepted: 24 June 2024
- Published online: 09 August 2024
Abstract: This study mainly explores three types of fermented tea beverages: kombucha, known for its distinctive sweet and sour flavor; tea wine, valued for its rich taste and low alcohol content; and tea vinegar, notable for its unique vinegar aroma. These beverages are produced through fermentation using teas as a base, facilitated by yeast and acetic acid bacteria. The research investigates how these microbes utilize tea as a nitrogen source, enhancing the content of tea polyphenols, reducing caffeine, and generating a rich array of organic acids and volatile compounds. This process imparts fermented tea beverages with unique flavors and augmented health benefits. Moreover, the bacterial cellulose film created by the symbiotic relationship between yeast and acetic acid bacteria opens up innovative avenues for the deep processing and high-value utilization of tea leaves.