-
As one of the non-alcoholic beverages with health benefits[1], tea (Camellia sinensis (L.) O. Kuntze.) has become more popular worldwide and an extensive beverage produced in China. Generally, according to the degree of fermentation, Chinese tea can be divided into six categories: green tea, white tea, yellow tea, black tea, oolong tea, and dark tea[2]. Along with the development of tea processing techniques, a kind of tea with flower fragrance, known as scented tea, has been produced. Among that, jasmine-scented tea is one of the most well-known scented teas[3,4]. In addition, numerous types of scented tea have been created and named according to the source of flower fragrance, such as Citrus aurantium L. var. amara Engl. (CAVA) scented tea, Osmanthus fragranced tea[5], Rose scented tea[6], Calyx scented tea[7], orchid scented tea[8], and Tartary buckwheat scented tea[9]. Comparatively, the production of scented tea is complicated. Taking jasmine scented green tea as an example, the traditional process requires stacking green tea with jasmine for several hours, followed by separation, heat-drying, dissipating, and cooling processes as shown in Fig. 1. The multi-plicating processes are conducted to obtain jasmine scented green tea for higher aroma intensity[3,10]. To date, flower essential oil nanocomposites have been used for the production of scented tea leaves. Specifically, jasmine-essential-oil-casein nanocomposites can be prepared by ultrasonication, and then the prepared jasmine essential oil nanocomposites are placed with the tea. Jasmine-scented tea is prepared by mixing it with tea leaves in a bag, which exhibits a better aroma sensory effect[11].
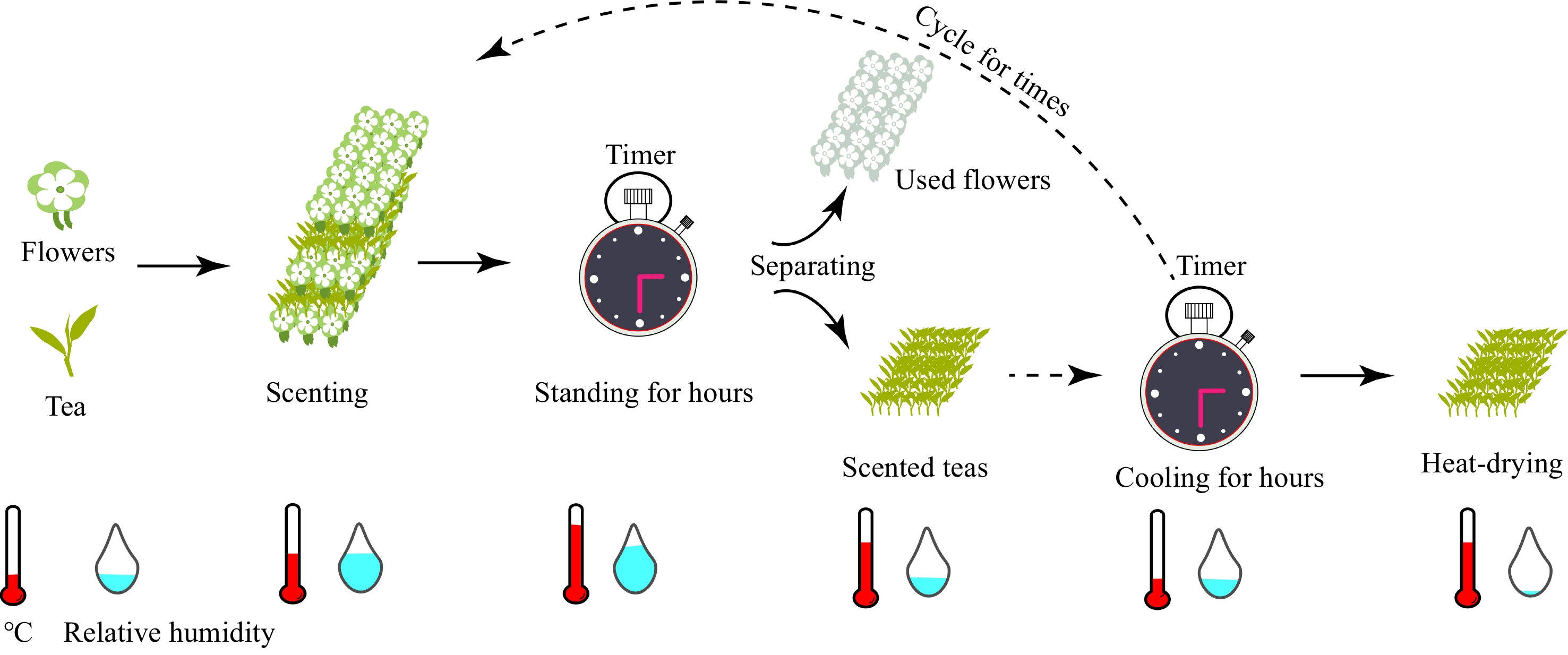
Figure 1.
Reduced form model of the scenting processes. The flowers are firstly mixed with tea and scented for hours at high temperature and relative humidity, then, the scented teas are separated and the flowers are used. The scented teas will then be mixed with flowers again. Finally, the scented teas will be heat-dried for hours to fix the aroma with tea leaves.
Regardless of traditional or modern crafts, the presentation of the fragrance in scented tea mainly depends on important processes such as the adsorption and desorption of aroma compounds by tea leaves. This process involves a complex adsorption mechanism[12], which is the primary subject of this review. In addition, tea also can be applied in other aspects as an adsorbent. For instance, tea waste can adsorb methylene blue in an aqueous solution through main interaction mechanisms such as electrostatic attraction, hydrogen bonding, ion exchange, and π-π bonding[13]. Hu et al.[14] found that oolong tea waste can also be used for the adsorption of methylene blue. The adsorption of radioactive cesium and the defluorination of tea infusion[15] can be achieved by using the cross-linking effect of tea leaves and metal ions. Yu & Chen[16] has reviewed the research progress on the biosorption of heavy metals in solution by tea waste, which also reflected the advantages of tea as a heavy metal adsorbent. The application of tea wastes to adsorb heavy metal ions has promoted the utilization of tea resources to a certain extent and effectively solved the problem of waste of tea secondary resources. However, the adsorption mechanism involved in the application of tea to adsorb exogenous compounds is still inconclusive.
Regarding the mechanism of tea aroma adsorption and aroma retention, Zhou et al.[17] confirms the complexity in the process of tea aroma adsorption and indicates that physical adsorption mainly depends on surface adsorption and the existance of capillary coagulation. Simultaneously, similar effects can be found in its water extracts[18]. Chemisorption also plays a key role in the process of scenting. Generally, after treatment by high-temperature desorption and adsorption, the scented tea base with high water content and water extract can adsorb more essential oils, more components, and better storability, which provides irrefutable evidence for chemical adsorption theory. It was found that the lower water content of the tea base leads to the stronger water adsorption capacity. While the water adsorption capacity of tea leaves is not directly proportional to the aroma adsorption capacity. The tea base has an obvious aroma adsorption ability within a wide range of water content, and the difference lies in the aroma intensity and aroma composition released by the flower. Furthermore, physical characteristics such as the porosity of tea also play an important role in aroma adsorption[19]. Tang[20] analyzed the physical adsorption theory of tea, the fixative contained in tea adsorbs aroma, the chemical adsorption theory, the vitality of flowers, and the theory of polymer embedding, respectively. The physical adsorption theory holds that gaseous molecules and solid surface molecules can be adsorbed by van der Waals gravity[21], but this is only a secondary phenomenon and not the main effect. Numerous experiments have shown that the relationship between the water content of tea and the ability to adsorb aroma is contradictory to the physical adsorption theory, which proves that physical adsorption does not play a major role in the process of tea adsorbing aroma compounds[22]. The chemical adsorption theory believes that new chemical bonds can be formed between gas molecules and solid surface molecules to generate adsorption effects. The theory of maintaining the vitality of flowers believes that the stronger vitality of flowers, the better scenting effect, which should be attributed to the longtime of aroma release. The theory of polymer embedding and binding is an in-depth analysis of the adsorption capacity of tea leaves at the molecular level, which believes that the polar groups contained in the aroma components can be combined with the polymer chains in tea leaves. The polar groups are bound by forming hydrogen bond effects. In addition, the relatively complex distribution of polymer chains in tea leaves can effectively prevent the volatilization of small molecule volatile components. There was also research[23] on the mechanism of aroma adsorption for scented tea with two theories. Among that, one of the most important theories, the physical adsorption mechanism believes that solid surface adsorption, gradient osmosis mechanism and capillary condensation adsorption are the main mechanisms of aroma adsorption[24]. When the surface of tea leaves are in contact with air or liquid compounds, some components in the gas or liquid will aggregate on the surface. Due to the differences in temperature, humidity, and aroma concentration between tea residues and flowers, these gradient differences result in the gradient osmosis by the aroma compounds transfer effect (Fig. 2). The other chemical adsorption mechanism proposes three main theories: water extract as adsorbent, water as adsorbent, and other compounds as adsorbent[10]. This theory mainly indicates atoms or ions in aqueous solution that have low valence saturation causes the remaining aroma components to composite through hydrogen bonding.
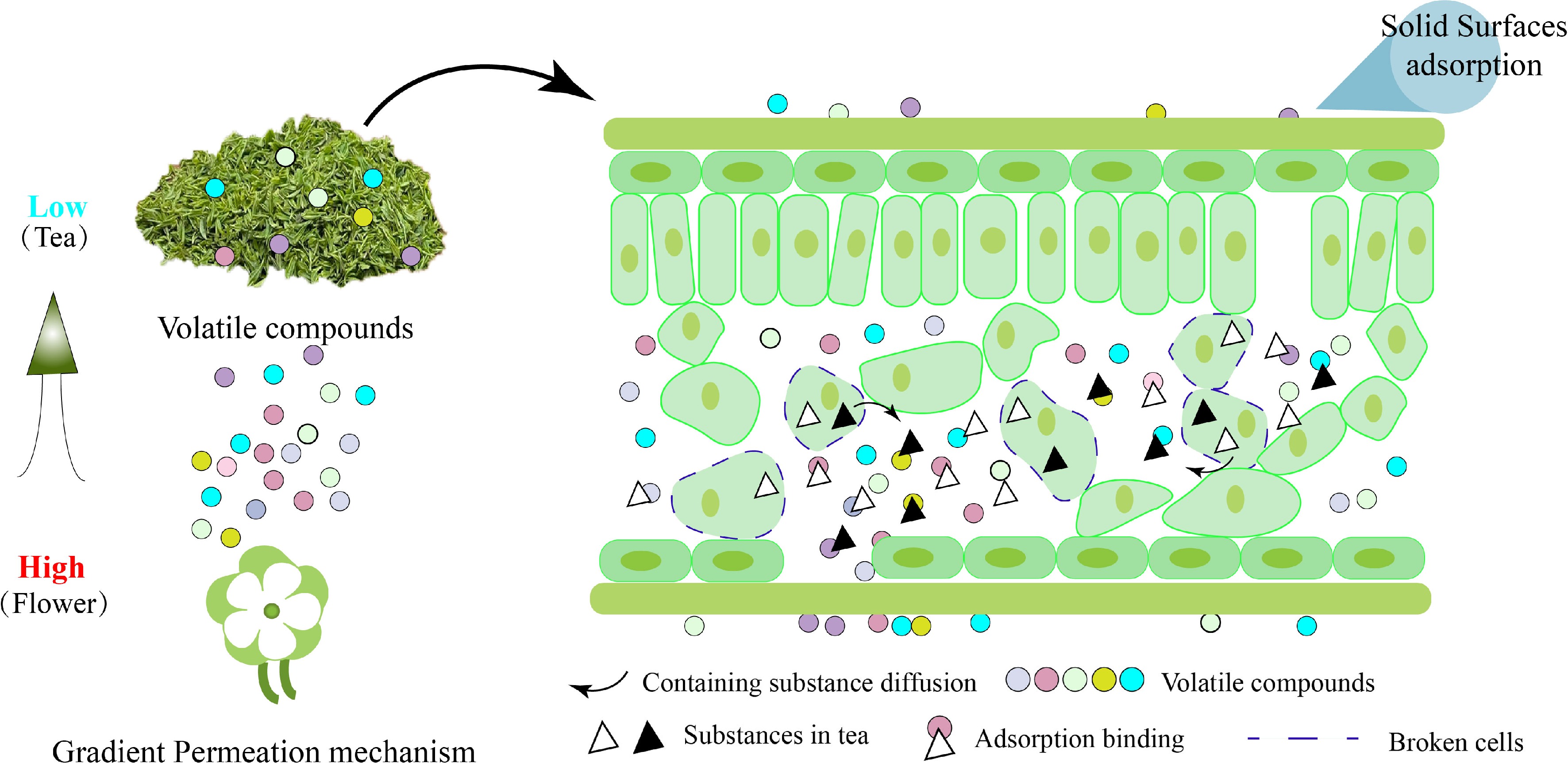
Figure 2.
Schematic diagram of waste tea residue adsorbing aroma substances. The aroma compounds will volatilize from higher concentrations to lower. The physical adsorption mainly happened on the solid surfaces of tea leaves. The chemical adsorption mainly depended on the bonding effect of volatile compounds with containing substances diffused from broken cells.
As early as the 1970s, the tea adsorption mechanisms of aroma compounds were being discussed. Scholars world wide used various scientific methods to verify and analyze the adsorption mechanism of aroma compounds in tea leaves. With the continuous improvement in methods of scientific research, these adsorption theories can be roughly divided into physical adsorption and chemical adsorption. To date however, no theories can perfectly explain the process of tea adsorption of aroma compounds. This review summarizes the adsorption mechanism of exogenous compounds in tea, which provides a theoretical basis for the processing of tea scenting. Practically, the mechanism of tea as an adsorbent can be used to develop adsorption materials targeted to different adsorbates. More importantly, this review would promote the application of tea resources and adsorption mechanisms.
-
The discussions of adsorption theory could help to expand the application range and rational utilization of tea residue, and meanwhile the directional development of adsorbent.
Physical adsorption
-
Classical physical chemistry believes that gas molecules are adsorbed by solid molecular surface compounds through van der Waals with a weakly interacting force. However, it’s not adequate to make tea surface molecules adsorb and fix aroma compounds through this force alone. In the physical adsorption theory, solid compounds have no selectivity for the adsorption of aroma compounds. From the perspective of solid surface adsorption[25], the solid compounds such as activated carbon, microporous resin, activated carbon fiber, and nanocellulose, can rely on their complex pore structure and huge specific surface area. The higher specific surface area can adsorb more aroma compounds[26]. Capillary coagulation adsorption theory[27] is also an adsorption theory based on the pore structure of solid matter. This theory insists the formation of capillary phenomenon from the internal pore structure of solid matter and gas or liquid matter due to surface tension so that aroma from tea leaves is fixed in the pore structure. The traditional floral tea scenting process uses a mass of flowers and tea leaves for mixing and scenting. Due to the mixture and accumulation of tea leaves and flowers, the ambient temperature of the scenting process increases, meanwhile, accompanied by the phenomenon of increased humidity (Fig. 1). Therefore, some scholars put forward the theory of gradient permeation[28] , which refers to the fact that the fragrance between flowers and tea leaves is gradually infiltrated from high-concentration flowers to low-concentration tea leaves due to other factors such as environment and concentration. Simultaneously, tea leaves absorb and fix the aroma.
Chemical adsorption
-
The chemical adsorption theory is proposed based on the application of the continuous scenting technology of wet billets. Floral tea leaves scented with this technology have outstanding fragrance retention, which cannot be explained only by the physical adsorption theory. The chemical adsorption theory holds that aroma components transform into composites in the form of chemical bonds, thus fixing the aroma compounds in the pore structure of tea leaves. Researchers verified for the first time that the aroma absorption of tea mainly depends on the hydrogen bond complexation between tea contents and aroma compounds through experiments on dewatered extracts and ether extracts. Additionally, wet green tea contains small amounts of moisture that promotes the formation of hydrogen bonds with the hydroxyl and carbonyl groups of aroma compounds through polar bonds, to adsorb and fix more aroma compounds. Apart from the water molecules, some scholars proposed the theory that polyphenols, palmitic acid, terpenes, and other physiological and biochemical processes are used to fix aroma compounds in tea, which still needs further discussion. Since then, with the advancement of scientific research technology, based on the theory that protein molecules embed and bind aroma substances, Tang[20] proposed the chemical adsorption of hydrogen bond formation between the polar groups of protein molecular chains and aroma substance molecules in tea. This theory explains the phenomenon preferably that scented tea leaves produced by the continuous scenting technique with wet billets have a better effect.
-
The exploration and development of adsorption theory is closely related to the development of research technology. Effective experimental verification methods will help to provide a more objective and accurate theoretical basis that can improve the reliability of adsorption theory. Obtaining visualized data will make the theory more convincing. This article will review some of the research methods used in the experiments on the application of tea as an adsorbent. In the future, this research method will be applied to the theoretical research on the adsorption of aroma compounds by tea.
Research on physical adsorption theory
-
Tea leaves contain a complex pore structure, which plays an important role in the adsorption of volatile compounds. The characterization of the pore structure provides the theoretical basis for this adsorption theory with a strong basis in tea leaves. The research on the adsorption of volatile organic compounds by activated carbon fibers (ACFs)[29] shows that the larger total specific surface area and micropore volume of the surface of ACFs, the higher adsorption capacity for volatile organic compounds. The smaller pore structure generates better adsorption performance on volatile organic compounds[19].
Plant tissue paraffin section technology is an effective tool to characterize the cross-sectional structure of objects. Ding et al.[30] performed anatomical structure characterization on the bud leaves and mature leaves of Rucheng Baimao tea leaves, and found an obvious presence of irregular pore structures internally. Lin[31] explored the anatomical structure of nine types of tea, including Anji Baicha and Fuding Dabaicha through the slicing technique, and found obvious pore structures in all these slices. Meanwhile, the paraffin section technology only observes the section structure of tea leaves at the two-dimensional level, which would be performed to further characterize the internal pore structure of tea leaves. Therefore, a more intuitive method is needed to characterize the internal structure of tea leaves. Scanning electron microscope (SEM) is an excellent solution to such problems. In the research on the removal of ciprofloxacin by tea biochar[32], SEM technology was used to characterize the used tea biochar, and the internal irregular pore structure was clearly observed. In the study of waste tea residue biochar on cadmium[33], SEM technology was used to observe the morphology of waste tea residue biochar at different temperatures, which confirmed that the morphology of waste tea residue biochar was more three-dimensionally displayed feature. Hence, SEM technology has advantages in morphological characterization. Similarly, in the research on tea adsorption aroma compounds, especially treated tea leaves, SEM technology could be used to observe the internal pore structure of tea and provide data support for physical adsorption theory.
Research on chemical adsorption theory
-
The chemical adsorption theory is more complex than the simple physical adsorption theory. From the biochemical change process to the group bonding reaction, a large number of experiments have confirmed the pivotal role of the chemical adsorption theory in the process of tea adsorption of aroma compounds. Nevertheless, effective relevant experimental data support is still lacking for the research of the chemical adsorption theory, and the existing experimental data prevents researchers from understanding the occurrence of adsorption behavior. Aroma components adhere to the surface of tea leaves, while some aroma compounds enter the internal pore structure and remain in tea leaves through physical adsorption. However, the physical adsorption processes cannot explain the phenomenon that aroma compounds remain in tea leaves for a long time. Therefore, the chemical adsorption theory plays an important role in the adsorption process of tea aroma compounds to a certain extent. The formation mechanism of composites between aroma compounds and tea ingredients through bonding is worthy of further study. Intelligibly, the making processes of the major tea types are different. The tea manufacture from fresh tea leaves to made tea basically involves a series of processes such as greening, withering, rolling, and drying, which leads to the destruction of internal cell structure along with the release of intracellular compounds in tea leaves. A large amount of active ingredients will exist in the internal pore of tea leaves as free form during processing, which is conducive to the formation of composites between aroma compounds and these contained compounds through chemical bonding. Hence, it was inferred that the processing technique of various teas also led to differences in their internal components, along with diverse variations in the contents and types of polar groups. Simultaneously, the combination degree of these groups with aroma compounds also shows certain changes. All the factors contribute to the differences in the effects during the making of scented tea leaves. This hypothesis would provide an experimental basis for the chemical adsorption theory, which is worthy of further exploration.
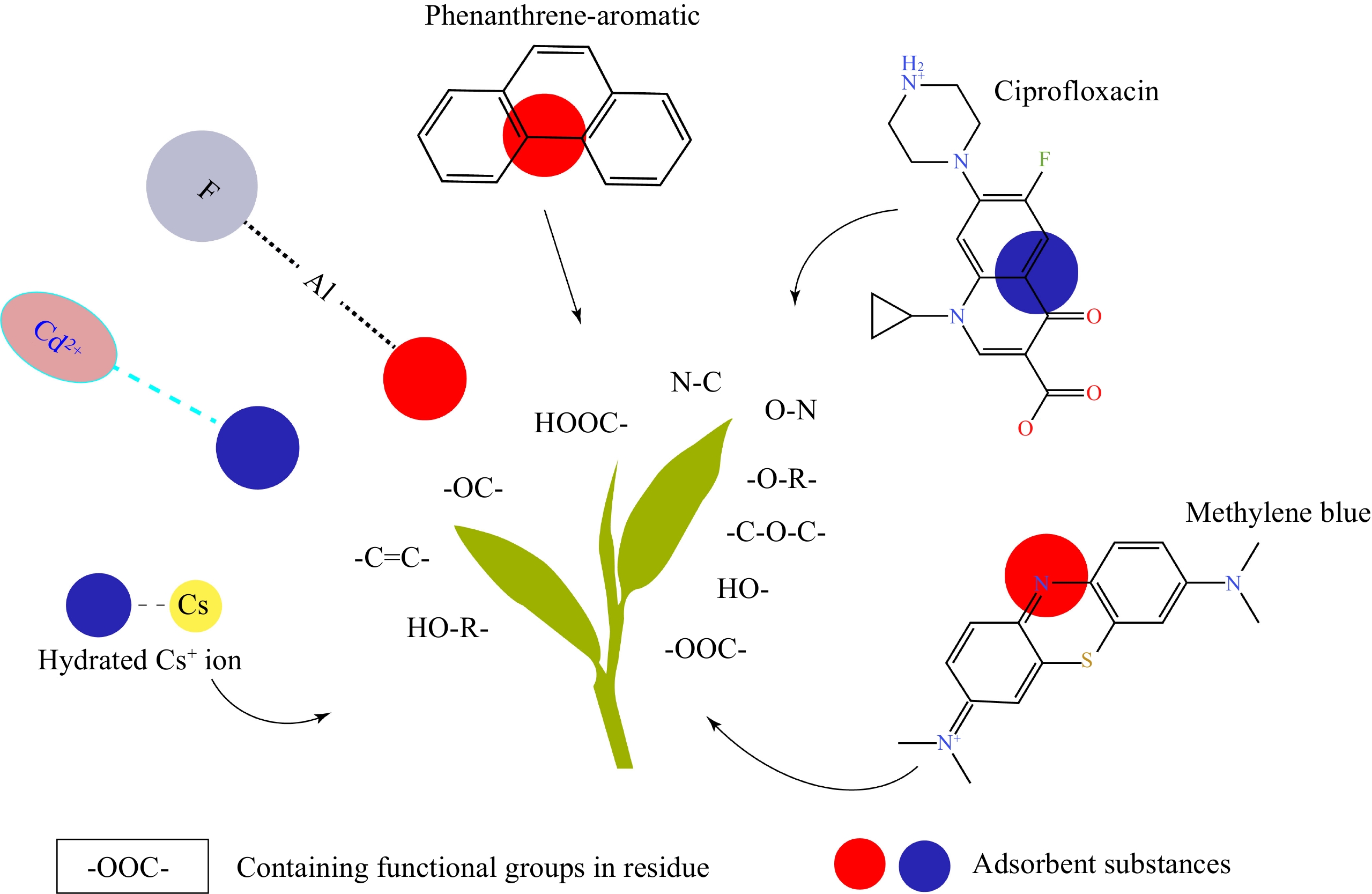
Xi & Chen[34] used the Fourier transform infrared spectrometer (FTIR) to investigate the polar groups of tea leaves after acid hydrolysis treatment, and detected -OH, CH2, ester C=O, aromatic C=C, and C=O , phenol -OH , C-OC and other functional groups. The SEM scanning image of oolong tea dregs shows that the porosity of oolong tea is extremely low, which is consistent with the functional group results detected by FTIR, however, the difference is mainly -SO3 and C-N bonds[14]. In addition, as a result of the existence of the internal pore structure, the hydrophobic sites on the inner surface of tea are exposed, and the π-π stacking effect may provide an important site for the aroma compounds to attach to the pore structure in tea leaves[19] after the aroma compounds enter the pore structure. The π-π stacking effect fixes the aroma of tea leaves. However, this theory not only depends on the exposure of the hydrophobic sites of tea, but also requires further experiments on the π electronic structure of aroma compounds. There is still a lack of experimental data support. In the earlier experiment, Keiluweit & Kleber[35] found that the bonding strengths of cation-π interaction and π-π electron donor-acceptor interaction are greater than that of hydrogen bonding. FTIR technology can be used to detect the difference between the functional groups on the inner surface of the pore structure and compare their changes before and after scenting, to infer the compounds involved in the formation of complexes.
Headspace solid-phase microextraction combined with GC-MS technology (Solid phase microextraction-gas chromatograph-mass spectrometer, SPME-GC-MS) is an efficient method to detect the composition and content of aroma compounds[36]. GC-MS is irreplaceable in the field of aroma substance detection. The composites rely on tea leaves in that they absorb aroma compounds through the bonding effect, however, the aroma compounds involved in the formation of composites remain to be explored. To detect the main aroma compound forming composites, the tea leaves were detected by SPME-GC-MS under the same conditions before and after scenting[37]. Therefore, it is necessary to perform SPME adsorption on the source of the aroma compounds under the same conditions. Meanwhile, a comparative analysis based on the SPME-GC-MS detection results of the scented tea can be conducted to identify the difference in aroma compounds composition. After that, the changes in the composition infer the aroma compounds absorbed by tea leaves.
-
The process of aroma adsorption still could not be visualized which greatly limits our understanding of the adsorption mechanism. Based on the fluorescence principle, the aroma compounds treated with fluorescence treatment revealed that the fluorescent materials competitively combine with the functional groups on the surface of the internal pore structure of the tea, which hinders the absorption of aroma compounds. The bonding process with functional groups on the surface of the internal pore structure cannot be further explored with the principle of functional group bonding. This experimental verification method is complicated to operate and difficult to implement. After long-term scientific research practice, researchers have summed up a variety of mathematical models to describe a certain process and realize its scientific analysis. To describe the process of tea adsorption aroma, the means of adsorption isotherm model and kinetic model analysis are fitted and analyzed, which can also help researchers to further understand the adsorption process. Numerous adsorption isotherm equations have been widely used to explain the occurrence of adsorption equilibrium, while all these equations have advantages and limitations[38].
The adsorption isotherm model can be used to investigate the adsorption mechanism, predict the maximum adsorption capacity of the adsorbent, estimate the affinity between the adsorbent and the adsorbate, and optimize the design of the adsorption system[13], as well as explore the influence of different time, temperatures, humidities, and other conditions on the adsorption of aroma by tea leaves. In a specific environment, the fragrance source of the flower releases aroma compounds, and at the same time, the tea leaves absorb and volatilize the aroma compounds. After reaching equilibrium, the change of aroma compounds in tea leaves was detected by SPME-GC-MS. Then, the adsorption isotherm model was fitted to obtain the change law of aroma compounds and describe the process of tea aroma adsorption. The differences in the making processes of major tea, directly lead to differences in the composition and content of aroma compounds among various types of tea leaves. Obvious differences are found in the process of absorption in tea. These differences can be judged by fitting the adsorption isotherm model. In the process of scenting, the release of aroma compounds changes with the variation of temperature, humidity, equilibrium vapor pressure, etc. The releasing process belongs to non-uniform release[39], and the Toth equation is suitable for systems with such inhomogeneous properties. The Toth equation is as the following Eqn (1):
$ q=\dfrac{{q}_{s}bp}{{[1+{\left(bp\right)}^{t}]}^{1/t}} $ (1) where, q is the adsorption capacity, qs is the saturated adsorption capacity, b is the adsorption affinity constant, and t is the heterogeneity parameter explaining the relationship between the adsorbent and the adsorbate. Additionally, there are still many models used to fit the adsorption equilibrium process, which will not be discussed in detail here, and the list is as Table 1.
Table 1. Adsorption equilibrium process equations.
Adsorption models Equation The main parameters Field of application Ref. Freundlich model $ s={K}_{F}{C}^{N} $ s is the adsorption concentration (μg/g), C is the liquid phase equilibrium solution concentration (μg/mL), KF is the adsorption capacity coefficient [(μg/g)/(μg/mL)],
N (dimensionless) is the description Freundlich exponent of isothermal nonlinearity.Monolayer adsorption and
non-uniform surface adsorption[40] Langmuir model $ \dfrac{{c}_{e}}{Q\mathrm{e}}=\dfrac{{c}_{e}}{{Q}_{max}}+\dfrac{1}{{Q}_{max}{K}_{L}} $ Where Ce is the equilibrium concentration, Qe is the adsorption amount of the dye at equilibrium, Qmax is the maximum adsorption amount of the dye, and
KL is the Langmuir constant.Uniform surface adsorption [14] Freundlich model $ \mathit{ln}{Q}_{e}=\mathrm{ln}{K}_{F}+\dfrac{1}{n}\mathrm{ln}{C}_{e} $ Among them, KF is the Freundlich constant, and
n is the heterogeneity factor.Monolayer adsorption and
non-uniform surface adsorption[41] Dubinin–Radushkevich model $ {l}_{n}{Q}_{e}=\mathrm{ln}{Q}_{m}-K{\varepsilon }^{2} $ In the formula, K is the coefficient related to the free energy of adsorption (mol/kJ2), Qm is the maximum
adsorption capacity (mg/g), ε is the Polanyi adsorption potential.Adsorption behavior of exogenous substances on surfaces [42] Temkin model $ {Q}_{\mathrm{e}}=\dfrac{RT}{{B}_{T}}\mathrm{ln}\left({A}_{T}{C}_{e}\right) $ In the formula, BT is the Temmin constant (J/mol),
AT is the Temkin isotherm equilibrium binding
constant (L/mg).Non-uniform surface adsorption [43] -
The adsorption capacity of the tea residue could be applied with multiple uses, moreover, the modified tea residue has better stable adsorption performance.
As shown in Table 2 and Fig. 3, tea leaves can be prepared as an adsorbent through saponification, high-temperature carbonization, acidolysis, alkali treatment, powderization, etc., for the adsorption of heavy metals and environmental pollutants. Hence, the modified tea leaves play a huge role in purifying wastewater and air. Despite a variety of treatment methods applied to the modification of tea leaves, most of the current methods are based on destroying the original structure of tea leaves. In a recent research on the antibacterial properties of metal-phenolic network nanoparticles, the author used multi-component to mix with metal ions in alkaline water at room temperature and the assembly of nanoparticles was completed within a few seconds[44]. TEM images show regular particle morphology and high stability after long-term storage. The research indicates that polyphenols in tea can stably bind to exogenous substances under the condition of applying weak external force. The X-ray photoelectron spectroscopy results showed that metal ions and polyphenols combined through benzene-O, C-S contact mode. In the future, this stable binding effect between exogenous substances with functional groups like N-C, -OOC-, -C-O-C-, -OC-, HO- and so on, also provides a wider way for the application of adsorption Additionally, adsorbents can be developed for different adsorbates based on this theory.
Table 2. Applications of tea as adsorbent.
Applications Modified tea adsorbent Adsorbate structure Ref. Adsorption of hydrophobic organic compounds by Tea Tea powder Phenanthrene 
[40] Cd and cadmium by waste tea biochar Continuous high temperature pyrolysis of tea leaves to remove ash and make biochar CdCl2 
[33,45] Tea waste removal of heavy
metals in wastewaterPickling, alkali washing, vulcanization, etc. treat overnight wasteto deprotonate the surface of tea waste and activate the adsorption sites Pb, Cu, Zn, Cr, Ni [16] Tea-aluminum biosorbent Preparation of tea-aluminum biosorbent by chemical coprecipitation 
[15] Removal of methylene blue from aqueous solution by tea residue Deionized water is used to remove ash from the tea waste, and the pigment, the ophylline and caffeine in the tea waste are removed by boiling water, and finally dried. 
Methylene Blue[13,14] Adsorption of organic pollutants and polycyclic aromatic hydrocarbons (PAH)
by modified tea leavesTea industry waste is treated by Sohit extraction, saponification and acid hydrolysis PAH 
[34] Selective adsorption of radioactive cesium by crosslinked tea leaves Fresh tea leaves and discarded tea leaves are stirred in concentrated sulfuric acid at high temperature, and then washed with deionized water until it's neutral CsCl, CsOH [46] Removal of ciprofloxacin from aqueous solution by waste tea biochar Tea biochar was obtained from high temperature pyrolysis of tea by argon gas Ciprofloxacin 
[32] -
The solid adsorbent has excellent performance and wide applicability, and needs to be developed further. Moreover, it’s meaningful that the research and applications of the solid adsorbent adsorb exogenous compounds mechanisms. As a renewable resource, tea residue has unique advantages as a solid adsorbent. Through the mechanism research in tea adsorption aroma compounds, researchers have investigated the combination of tea and aroma compounds to determine the adsorption mechanisms. Based on the aroma adsorption mechanisms of tea, there is still a lack of evident data support. In the near future, SEM, FTIR, X-ray photoelectron spectroscopy (XPS), laser confocal micro-Raman (LCM-Raman) spectroscopy and other technologies could be used to achieve the structural analysis and characterization of the combination between aroma molecules and tea content, which will help to obtain the visualization of molecular binding and arrangement rules. The scented teas prepared based on adsorption mechanisms show the aroma characteristics that cannot be found in traditional scented teas. In addition to the application of floral tea scenting, the mechanisms of exogenous compounds in tea have a wider range of applications. The technical requirements for purification treatment in air, water, and soil is rising with waste recycling. Therefore, based on this mechanism, the directional development of modified tea cross-linked adsorbents that can be used to adsorb specific compounds will have application prospects.
-
The authors confirm contribution to the paper as follows: writing - original draft preparation: Xu L; writig - review and editing: Xu L, Ma C, Chen X, Du Q, Song C, Li X, Xu YQ; investigation: Xu L, Ma C; supervision: Du Q, Li X, Xu YQ; conceptualization: Li X, Xu YQ; project administration: Li X, Xu YQ; validation: Li X, Xu YQ. All authors reviewed the results and approved the final version of the manuscript.
-
Data availability is not applicable to this article as no new data were created or analyzed in this study.
This research is supported by the Science and Technology Program of Wen County (2023--X.QKJ--02), the Science and Technology Cooperation Project of Zhejiang Province (2024SNJF028), the Science and Technology Program of Enshi (D20220025) and the Science and Technology Program of Gansu Province (22CX8NA034).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Xu L, Ma C, Chen X, Du Q, Song C, et al. 2024. Theories and applications of tea residue adsorbing aroma compounds: a review. Beverage Plant Research 4: e030 doi: 10.48130/bpr-0024-0030
Theories and applications of tea residue adsorbing aroma compounds: a review
- Received: 08 May 2024
- Revised: 01 July 2024
- Accepted: 22 July 2024
- Published online: 14 August 2024
Abstract: Tea leaves have a good natural adsorption capacity due to their numerous pore structures and high specific surface area. Along with the advancement of tea process technology, more and more sites of adsorption and binding have been found in tea leaves, thus further improving the adsorption performance. Based on the original biological structure and internal components of tea leaves, the adsorption mechanism in tea leaves for exogenous compounds is mainly divided into physical adsorption theory and chemical adsorption theory. Compared with original tea leaves, modified tea leaves significantly improve adsorption performance with a specific adsorption function, which could improve the utilization rate of tea leaves and their waste residues. Techniques such as scanning electron microscopy provide basic data support for exploring the adsorption mechanism of tea leaves at the level of material structure and a theoretical basis for the development of specific adsorbents. Additionally, the application of thermodynamic adsorption models and adsorption kinetic equations could help to understand the process of tea adsorbing exogenous compounds and to visualize the adsorption process. The adsorption of exogenous additives by tea residue could make use of the affinity between functional groups. In future, these mechanisms will lay the foundation for its application in the field of substance adsorption and improve the utilization efficiency of renewable resources similar to tea leaves.