-
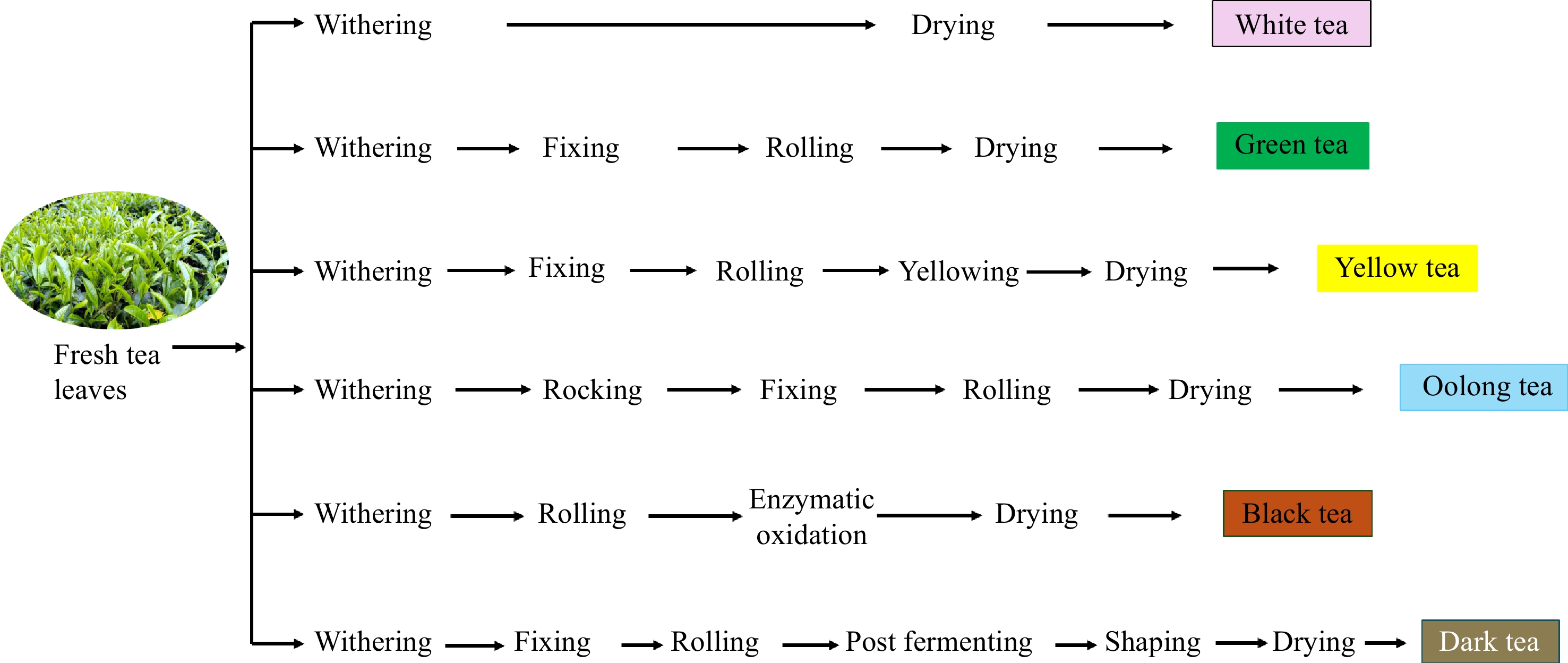
Figure 1.
Six types of tea processing.
-
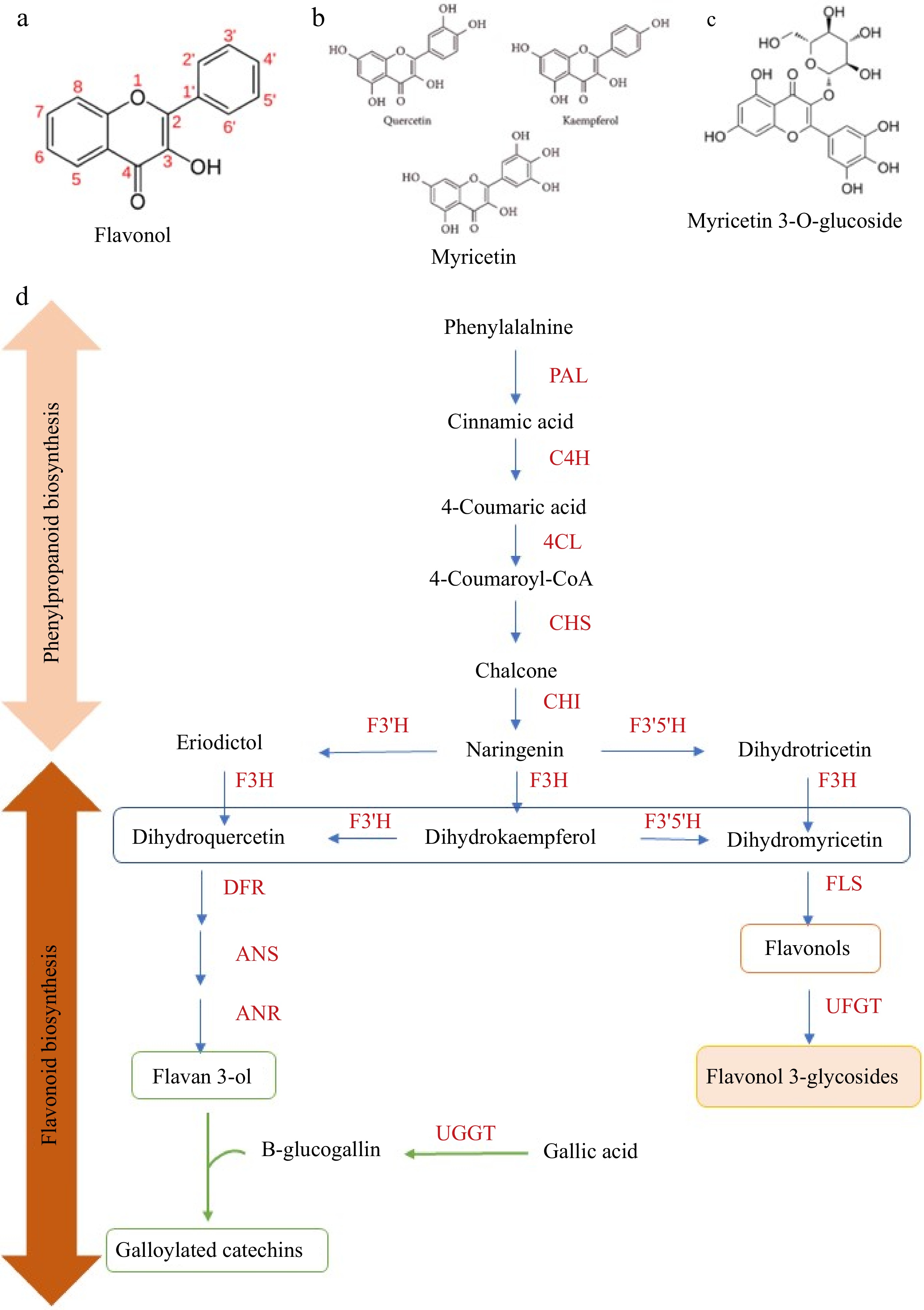
Figure 2.
The basic skeleton of (a) flavonols, (b) aglycone flavonols, (c) flavonol glycosides, and (d) proposed biochemical changes of flavonoids in tea leaves during withering. Phenylpropanoid and flavonoid biosynthesis play a vital role in the biosynthesis of flavonol aglycones and flavonol glycosides. PAL, phenylalanine ammonia-lyase; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumarate CoA ligase; CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; F3'5'H, flavonoid 3',5'- hydroxylase; F3'H, flavonoid 3'-hydroxylase; FLS, flavonol synthase; DFR, dihydroflavonol 4-reductase; ANS, anthocyanidin synthase; LAR, leucoanthocyanidin reductase; ANR, anthocyanidin reductase; UFGT, flavonol 3-O-glucosyltransferase; UGGT, UDP-glucose: galloyl-1-O-β-D-glucosyltransferase; ECGT, epicatechin: 1-O-galloyl-β-D-glucose O-galloyltransferase.
-
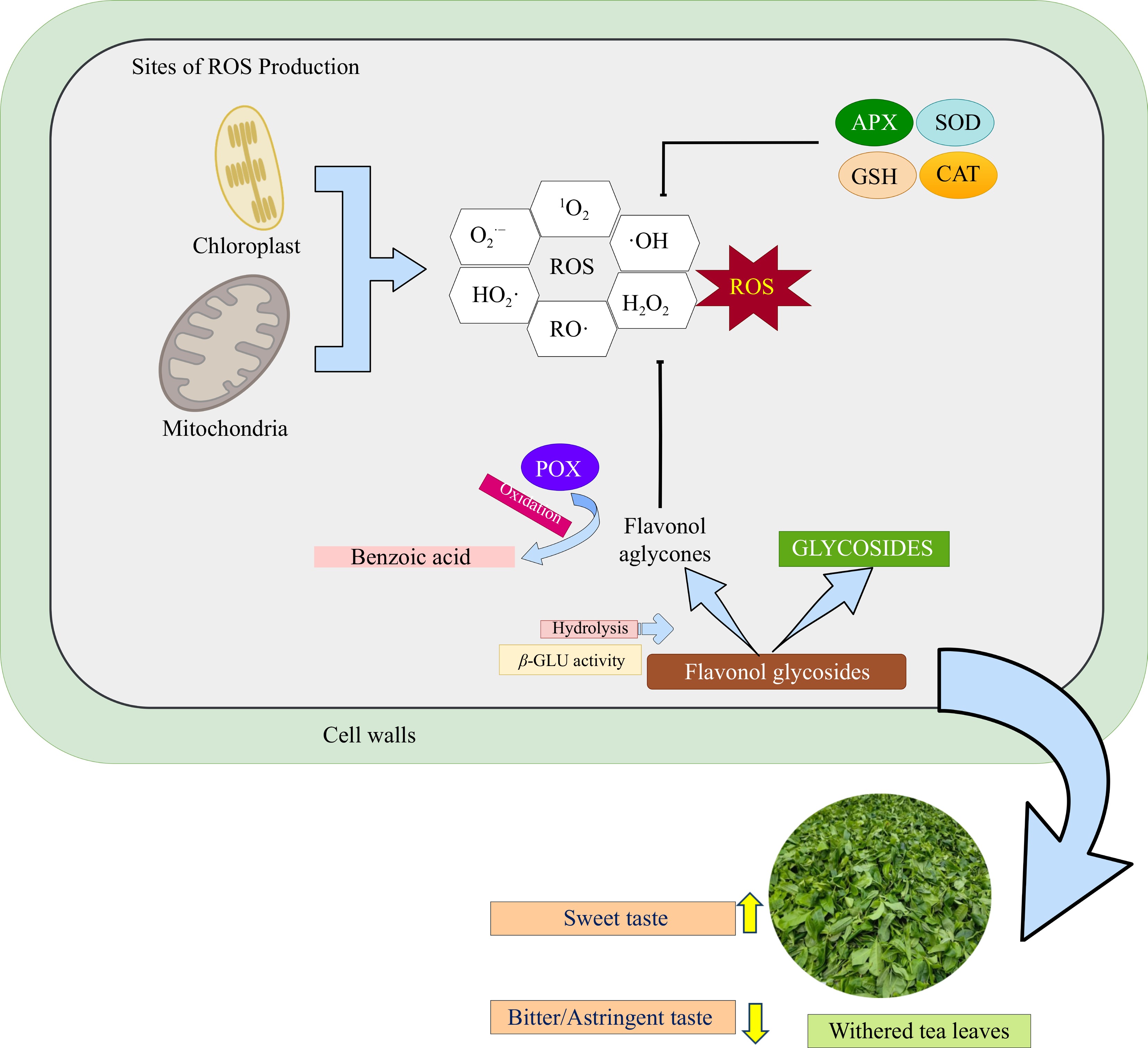
Figure 3.
Simplified schematic of flavonol glycosides degradation and antioxidant defense mechanisms in tea leaves during the withering process. This stage involves the action of enzymatic and non-enzymatic antioxidant systems to mitigate excess reactive oxygen species (ROS)[81,84]. Abiotic stress during withering triggers ROS generation in mitochondria and chloroplasts, which is subsequently mitigated by enzymatic antioxidants, including superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), and glutathione (GSH). Concurrently, flavonol glycosides, acting as non-enzymatic antioxidants, undergo enzymatic hydrolysis catalyzed by β-glucosidase, releasing flavonol aglycones and sugar moieties. Subsequently, the liberated flavonols function as ROS scavengers. Further oxidation of flavonols, mediated by peroxidase (POX), contributes to the formation of benzoic acid and its derivatives[7,93].
-
Type of tea Withering conditions Impact on the quality of tea Ref. Black tea Different degrees of withering with a final moisture content of approximately 65%, 60%, 55%, 50%, and 45% in the withered leaves (withered temp 33 °C, for 12 ± 1 h) - Reduce the total amount of catechins, flavonoids/flavonoid glycosides, theaflavins, and glycosidic bound volatiles (GBVs).
- The withering degree is associated with increasing free amino acids and volatile compounds.
- The 60% moisture content of the withered leaves exhibited the highest sensory quality score and the best bioactivity of black tea.[97] Keemun black tea Dynamic withering technology: Fresh leaves
are spread on dynamic conveyors at a thickness of approximately 20 cm. Tea leaves remain in continuous motion at a speed of 5 m/min
(temp. maintained at 23 ± 1 °C; for 15 h).- Total catechins decrease after the withering process.
- Total theaflavins increase significantly.
- Dynamic withering technology has more floral and fruitier odor-active compounds.[34] Black tea Red-light irradiation (630–640 nm, 725–1,015 lx) for 9 h. Indoor temperature of 24–29 °C and a RH of 46%–50%. - Increase the activity of the PPO and POD enzymes and theaflavin formation during fermentation.
- The number of amino acids is increased by inducing up-regulated expression of phenylalanine and tryptophan synthesis genes and the activity of peptidases.
- Treatment with red light can reduce the astringent and bitterness of black tea and improve the strong, fruity aroma and fresh and mellow taste.[35] Black tea Red-light irradiation (630 nm, approximately 3,000 lx) and dark conditions (temperature maintained at 28 °C, RH: 70%). - Red light predominantly disrupts secondary metabolite biosynthesis, amino acid synthesis and metabolic pathways.
- Lower content of theobromine, catechin, and some flavonoid glycosides
- Generate the accumulation of amino acids and flavins.
- Produce black tea with a mild, thick, and fresh taste.[36] Keemun Black Tea Natural withering (8–19 °C, 24 h, humidity 48%–75%); Sun withering (15–17 °C, 2.5 h,
with 5.68–8.36 Klx, humidity 48%–56%); Warm air withering (18–22 °C; 7 h in the withering trough).- Warm air withering reduced the concentration of catechins and flavonoid glycosides, increasing the sweet taste of tea infusion.
- The sun withering produced Keemun black tea with intense bitterness and astringency compared to the warm air withering (sweeter taste).[32] Black tea Ethylene (treated with continuous air ethylene at 10 μL/L). UV-C irradiation (254 nm) at varying doses (1 and 15 kJ/m2). - UV-C (15 kJ/m2) treatment resulted in rapid color changes from green to red/brown and increased catechin oxidation of catechins into theaflavin.
- Ethylene and UV-C treatments can shorten the withering time while improving the accumulation of flavor components, leading to higher-quality tea with a faster processing time.[37] Green tea Indoor natural environment (25 °C, 60% RH, natural light of 200 lx); Artificial climate box
(15 °C, 70% RH, at dark); Artificial climate box with two yellow light emitting diodes (LED) lamps (15 °C, 70% RH, and LED light of 250 lx/9 W); Controlled atmosphere (15 °C, 70% RH, 3% CO2, at dark).- Low-temperature withering reduces the level of oxidation of polyphenols by blocking the activity of polyphenol oxidase and maintaining the expression of flavanol synthase genes. The relative total amount of flavonoids reduced after withering.
- Low temperature and yellow light enhanced the synthesis of γ-aminobutyric acid, the metabolism of phenylalanine-methyl salicylate, and tryptophan-indole.
- Combination treatments can maintain and enhance the taste quality of green tea products and increase the aroma of green tea products due to the highest relative amount of terpene volatiles and amino acid-derived volatiles.[31,49] White tea Sunlight withering (SW, 17.5 Klx, 48 h); Withering tank (WWT, by blasting fresh air at
30 °C, 48 h); Indoor withering (IW, room temp: 21–27 °C, 52%–37% humidity).- SW significantly improved astringent flavonoids and flavone glycosides in white tea.
- SW exhibited a more floral scent due to the increase in geraniol and linalool.
- WWT had a grassy scent due to higher levels of hexanal.[18,98] White tea Applied yellow light color with an intensity
of 5,000 lx during withering (duration 36 h,
0.5 m distance between the tea and light,
1 cm thickness of tea in the sieve, temperature 25 °C, a wind speed of 1 m/s, and a humidity of 60%).- Yellow light withering produces a superior aroma and a large amount of methyl salicylate, geraniol, and nerolidol.
- Increasing the expression of flavonoid 3,5-hydroxylase (F3'5'H) affected the high accumulation levels of quercetin, kaempferol, and luteolin in white tea.
- Affects the decrease in catechin synthesis but increases the expression of genes for theaflavin synthesis, such as PPO (CSS0019276.1 and CSS0002951.1) and POD (CSS0021668.1, CSS0007582.1, and CSS0011664.1).[21] Oolong tea Sun withering was carried out in the sun at different ambient temperatures, 43 and 30 °C, for 15, 30, 60, and 120 min. - Primary aroma components increased during 15 min of solar withering compared to indoor withering.
- Sun withering can significantly increase the expression of essential genes associated with the aroma-related metabolic pathway. Up-regulation of heat shock and other resistance protein-related genes quickly suppresses their expression.
- Short-term solar withering can improve the development of scent throughout the oolong tea manufacturing process.[40] Wuyi rock tea (oolong tea) Sunlight withering (SW, 5–7 leaf thickness, light intensity 57,000 lx, withering time 45 min, turning tea leaves every 15 min); Charcoal fire withering (FW, temperature in the withering chamber around 35 °C, withering time 200 min with manual turning every 100 min); Withering (WW, hot air temp. 35 °C, 5–7 cm of leaves thickness, withering time 225 min, every 75 min for turning tea leaves). - SW significant increase in gene expressions such as ubiquinone and other terpenoid-quinone biosynthesis (ko00130), pyruvate metabolism (ko00620), starch and sucrose metabolism (ko00500), and tryptophan metabolism (ko00380) pathways.
- SW effectively increases the number of nucleotides and derivatives, terpenoids, organic acids, and lipids, resulting in the mellowness of tea, the fresh and brisk taste, and aroma.
- WW treatment influences a high amount of gene expression of the glutathione metabolism (ko00480) and phenylpropanoid biosynthesis (ko00940) pathways.[99] Table 1.
The withering treatment affects various tea processing processes and impacts biochemical changes and tea quality.
-
Identified flavonol glycosides Abbreviation Type of tea GT BT OT WT Myricetin-3-O-glucosyl-rhamnosyl-galactoside M-gal-rha-glu 0.12−0.26 0.16−0.17 0.33−0.36 n.d. Myricetin-3-O-glucosyl-rhamnosyl-glucoside M-glu-rha-glu 0.12−0.36 0.09−0.31 0.44−1.17 0.10 Myricetin-3-O-rhamnosyl-glucoside M-rha-glu 0.09−1.09 0.10−0.33 0.20−0.99 0.17 Myricetin-3-O-galactoside M-gal 0.16−1.24 0.10−0.49 0.21−1.66 0.27 Myricetin-3-O-glucoside M-glu 0.12−1.93 0.14−0.51 0.45−0.74 0.84 Quercetin-3-O-glucosyl-rhamnosyl-galactoside Q-gal-rha-glu 0.12−2.78 0.09−2.57 2.09−2.99 0.58 Quercetin-3-O-glucosyl-rhamnosyl-glucoside Q-glu-rha-glu 0.09−4.41 0.15−3.81 n.d. n.d. Quercetin-3-O-rhamnosyl-rhamnosyl-galactoside Q-gal-rha-rha 0.26−1.27 0.13−0.84 n.d. 13.86 Quercetin-3-O-rhamnosyl-rhamnosyl-glucoside Q-glu-rha-rha 0.10−0.97 0.13−0.3 n.d. n.d. Quercetin-3-O-rhamnosyl-galactoside Q-gal-rha 0.06−0.34 0.15−0.45 n.d. n.d. Quercetin-3-O-rhamnosyl-glucoside + Kaempferol-3-O-glucosyl-rhamnosyl-galactoside Q-glu-rha + K-gal-rha-glu 0.22−4.88 0.40−2.99 1.73−1.75 0.77 Quercetin-3-O-galactoside Q-gal 0.26−2.21 0.26−0.91 0.35−0.48 0.03 Quercetin-3-O-glucoside Q-glu 0.11−2.51 0.17−1.51 0.33−0.36 0.25 Kaempferol-O-rhamnosyl-rhamnosyl-galactoside K-gal-rha-rha 0.06−4.74 0.05−1.32 n.d. 5.73 Kaempferol-3-O-glucosyl-rhamnosyl-glucoside K-glu-rha-glu 0.05−8.65 0.25−5.87 n.d. n.d. Kaempferol-3-O-rhamnosyl-galactoside K-gal-rha 0.05−2.65 0.08−0.45 n.d. n.d. Kaempferol-O-rhamnosyl-rhamnosyl-glucoside K-glu-rha-rha 0.06−2.68 0.10−1.88 1.51−3.33 n.d. Kaempferol-3-O-galactoside K-gal 0.11−2.45 0.10−2.45 0.00−0.58 n.d. Kaempferol-3-O-rhamnosyl-glucoside K-glu-rha 0.06−2.61 0.06−2.61 0.32−0.48 0.07 Kaempferol-3-O-glucoside K-glu 0.10−1.46 0.17−1.33 0−0.38 n.d. Total flavonol glycosides TFG 3.78−17.81 5.57−14.77 11.18−12.06 23.30 TFG: Total flavonol glycosides; GT: Green tea; BT; Black tea; OT: Oolong tea; WT: White tea; n.d.: Not detected. The results are based on 474 tea samples reported by Fang et al.[19]. Table 2.
Flavonoid glycosides are found in various tea products (mg/g dry weight).
-
Antioxidants Reaction with ROSa Subcellular locationa Function in ROS scavengingb Enzymatic antioxidants Superoxide dismutase
(SOD; EC 1.15.1.1)2O2·− + 2H+ → O2 + H2O2 Chloroplast, peroxisomes, cytosol, mitochondria, apoplast Converts superoxide to H2O2 Catalase
(CAT; EC 1.11.1.6)2 H2O2 → 2H2O + O2 Peroxisomes, mitochondria dismutation high levels of H2O2 into H2O and O2 Ascorbate peroxidase
(APX; EC 1.11.1.11)H2O2 + AsA → 2H2O + MDHA Chloroplast, peroxisomes, cytosol, mitochondria, apoplast Converts H2O2 to H2O using AsA as a reducing agent Monodehydroascorbate reductase
(MDHAR; EC 1.6.5.4)MDHA + NAD(P)H → AsA + NAD(P)+ Mitochondria, cytoplasm, chloroplast Regenerates ascorbate from MDHA Dehydroascorbate reductase
(DHAR; EC 1.8.5.1)2GSH + DHA → GSSG + AsA Mitochondria, cytoplasm, chloroplast Reduces DHA using an electron donor from GSH to maintain the ascorbate pool Glutathione peroxidase
(GPX; EC 1.11.1.9)H2O2 + GSH → H2O + GSSG Cytosol, mitochondria Reduces H2O2 and lipid peroxides Glutathione S-transferase
(GST; EC 2.5.1.18)R-X + GSH → GS-R + H-X Chloroplast, cytosol, mitochondria Detoxifies lipid hydroperoxides Peroxidases
(POX; EC 1.11.1.7)2PhOH + H2O2 → 2PhO· + 2H2O
2PhO· → cross-linked substances
PhO· + AsA → PhOH + MDHA
PhO· + MDHA → PhOH + DHACell wall, apoplast, vacuole Uses phenolics to reduce H2O2 Polyphenol oxidase
(PPO; EC 1.14.18.1)PhOH + O2 → Catechols
Catechols + O2 → Q + H2OThe thylakoid membrane of the chloroplast, cytosol, and vacuole Oxidizes phenolics, reduces ROS load Non-enzymatic antioxidants Ascorbic Acid AsA + O2·− → MDHA + H2O Chloroplast, peroxisomes, cytosol, mitochondria, apoplast Detoxifies H2O2 via the action of APX Glutathione (GSH) 2GSH + H2O2 → GSSG + 2H2O Cytosol, chloroplast, mitochondria, peroxisome, vacuole, apoplast Acts as a detoxifying co-substrate for enzymes like peroxidases, GR, and GST α-Tocopherol Tocopherol-OH+ROO· →
Tocopherol-O·+ROOHChloroplasts and mainly in mitochondrial membranes Guards against and detoxifies the products of membrane LPO Carotenoids Carotenoid + 1O2 →
Carotenoid + 3O2Chloroplast Detoxifying various ROS and capturing the lipid peroxyl radical (LOO·) Proline Proline + ·OH → Proline radical+H2O Mitochondria, cytosol, chloroplast Efficient scavenger of OH· and1O2 and prevents damage due to LPO Flavonoids Flavonoid-OH + ROS →
Flavonoid-O· + H2OVacuole and chloroplast Direct scavengers of H2O2 and1O2 and OH· APX: Ascorbate peroxidase; AsA: Ascorbic acid; DHA: Dehydroascorbate; DHAR: Dehydroascorbate reductase; GSH: Reduced glutathione; GSSG: Oxidized glutathione; GR: Glutathione reductase; MDHA: Monodehydroascorbate; PhOH: Phenolic compounds; PhO·: Phenoxyl radical; Q: Quinone; LPO: Lipid peroxidation; ROOH: Hydroperoxides; ROO·: Peroxyl radical; ROS: Reactive oxygen species. Notation of a and b according to the reference[79,81−83,100]. Table 3.
Mechanism of enzymatic and non-enzymatic antioxidants with their roles and localization in scavenging major reactive oxygen species (ROS).
-
Mechanism Principle Step-by-Step Reaction Hydrogen Atom Transfer (HAT) Direct hydrogen atom donation from the hydroxyl group to neutralize ROS Ar–OH + R· → Ar–O· + RH Single Electron Transfer (SET) Electron donation to R· Ar–OH + R· → Ar–OH·+ + R− Single Electron Transfer Proton-Transfer (SET-PT) Electron transfer followed by proton loss Ar–OH → Ar–OH·+ → Ar–O· + H+ Sequential Proton Loss Electron Transfer (SPL-ET) Deprotonation followed by electron transfer Ar–OH → Ar–O− → Ar–O· + e− Ar–OH: Antioxidant; R·: Radical. Reaction antioxidants according to Estévez et al.[85], Stepanic et al.[86]. Table 4.
Mechanism of antioxidant scavenging of reactive oxygen species (ROS).
Figures
(3)
Tables
(4)