-
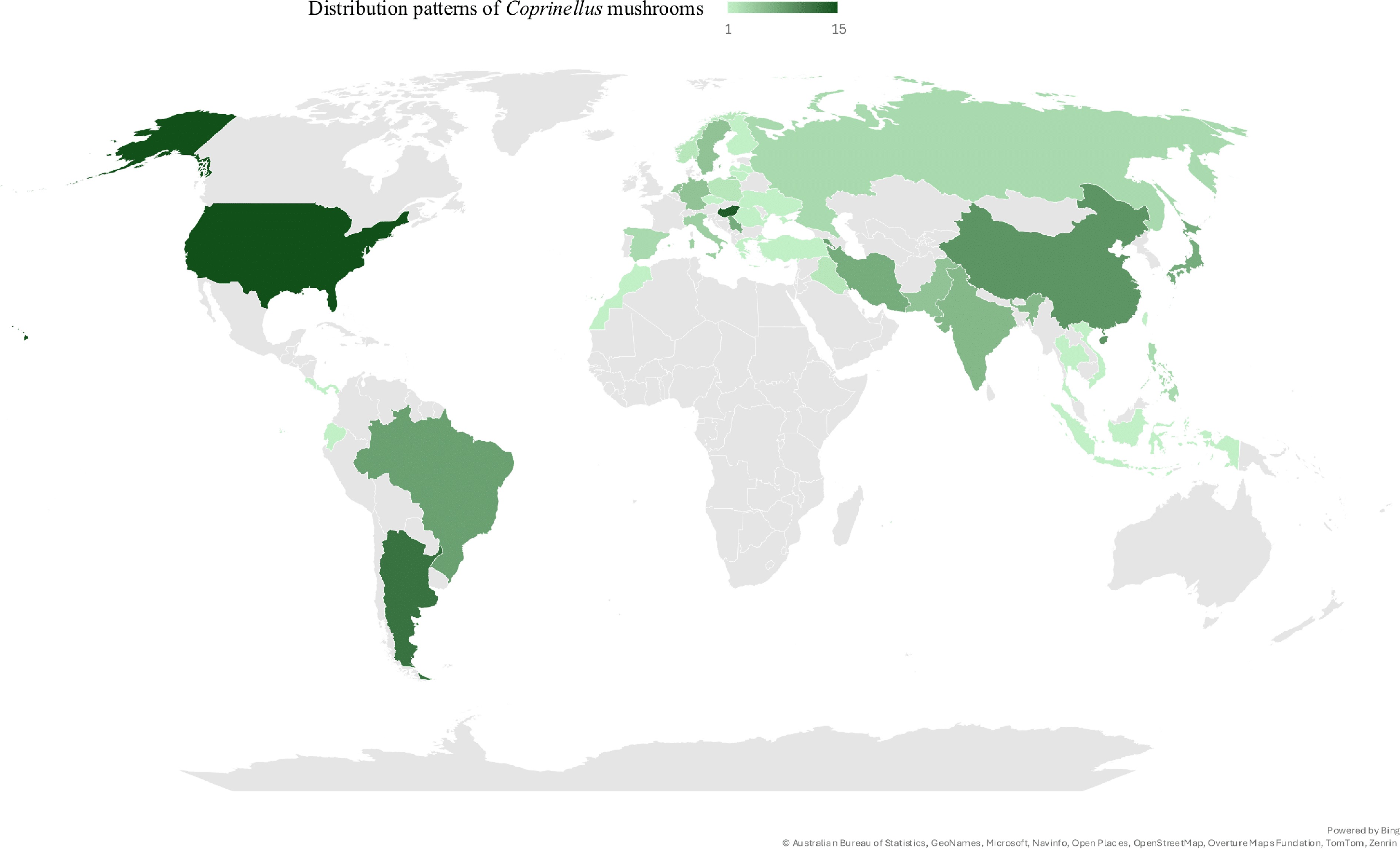
Figure 1.
Global occurrence patterns of Coprinellus mushrooms, visualized using filled map tools in Microsoft Excel 2019.
-
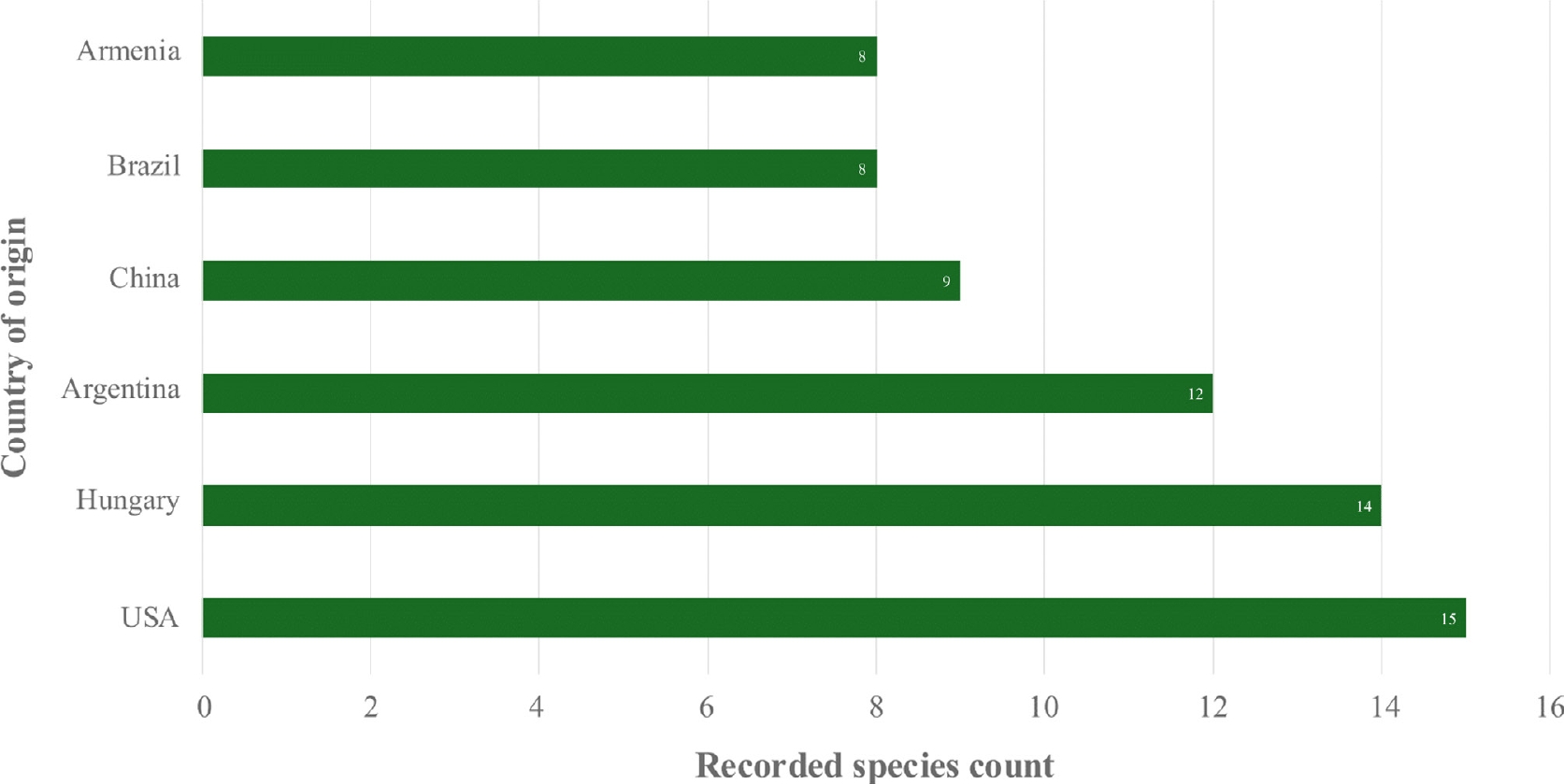
Figure 2.
Countries with the highest reported species diversity of Coprinellus.
-
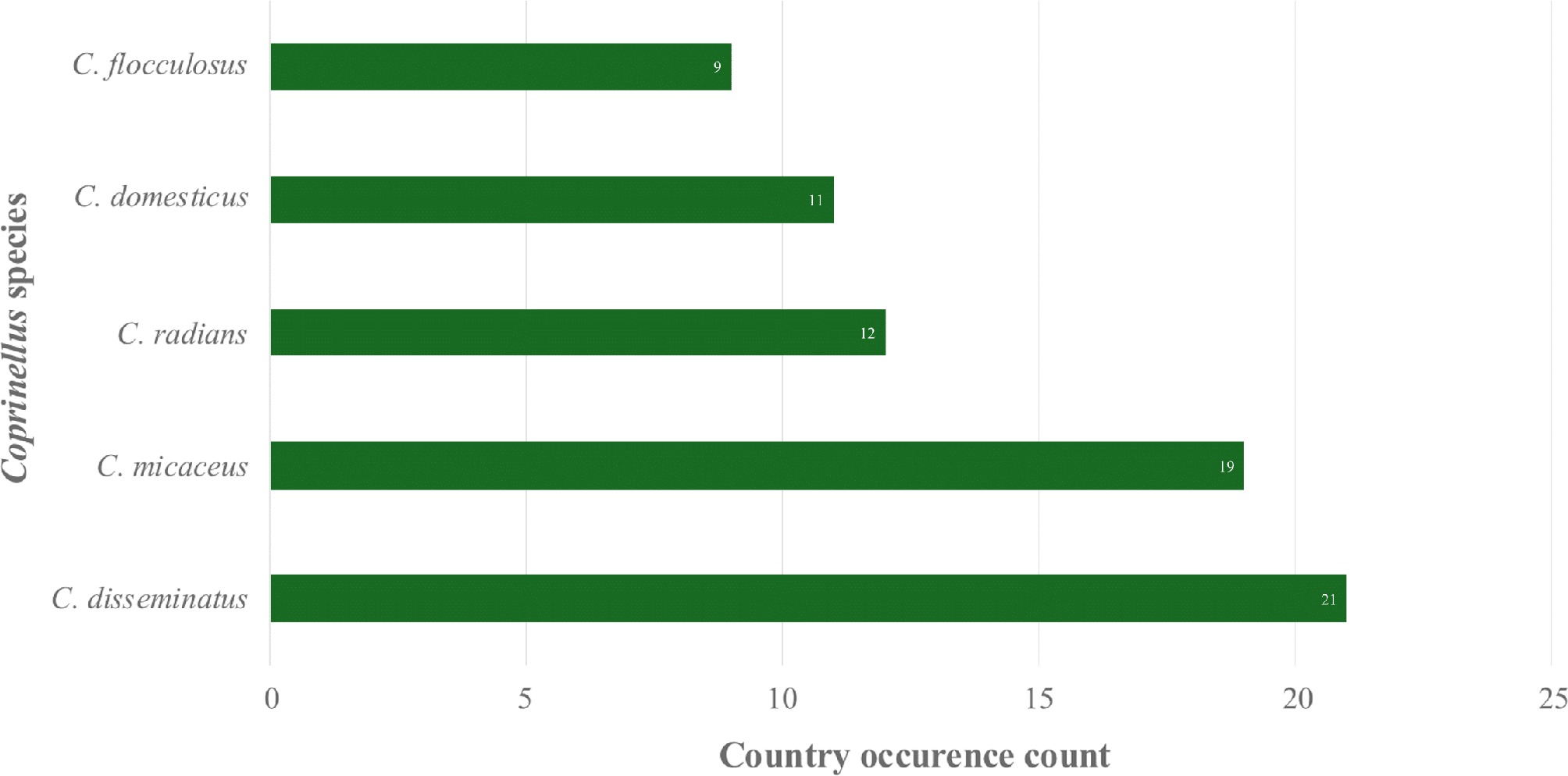
Figure 3.
Coprinellus species with the broadest global occurrence.
-
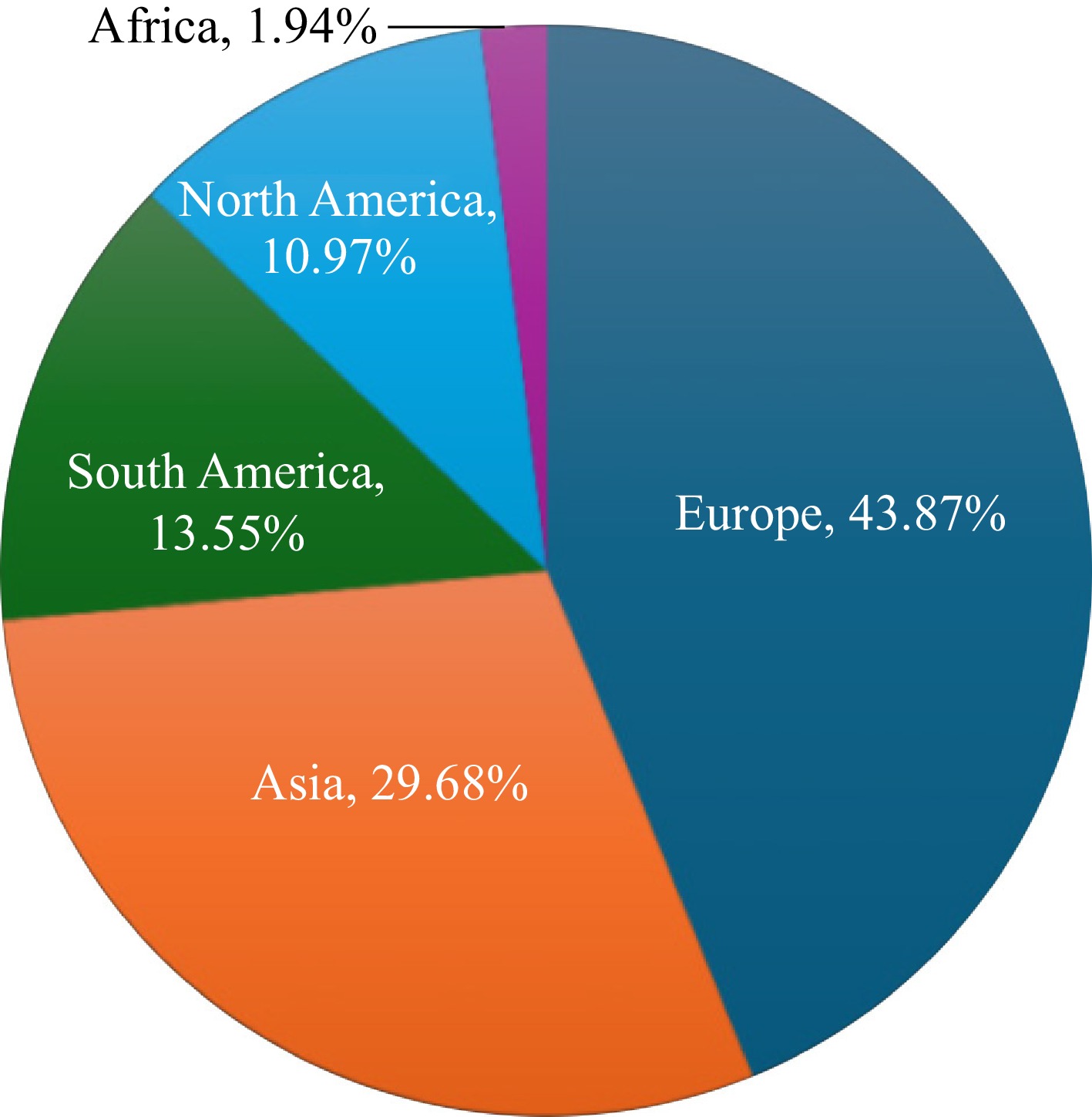
Figure 4.
Percentage of Coprinellus species recorded across continents.
-
Coprinellus species Country of origin Ref. 1. Coprinellus alkalinus (Anastasiou) Voto (2021) n.r. [54] 2. Coprinellus alvesii Voto (2019) Brazil [55] 3. Coprinellus andreorum Sammut & Karich (2021) Malta [56] 4. Coprinellus apleurocystidiosus Voto (2021) n.r. [54] 5. Coprinellus aquatilis (Peck) Voto (2019) Finland [57] Norway [57] USA (New York) [58] 6. Coprinellus arenicola Wartchow & A.R.P. Gomes (2014) Brazil [27] 7. Coprinellus aureodisseminatus T. Bau & L.Y. Zhu (2024) China [59] Ecuador [40] 8. Coprinellus aureogranulatus (Uljé & Aptroot) Redhead, Vilgalys & Moncalvo (2001) Netherlands [32] China [12] Vietnam [60] Philippines [61] 9. Coprinellus austrodisseminatus T. Bau & L.Y. Zhu (2024) China [59] 10. Coprinellus bipellis (Romagn.) P. Roux, Guy García & Borgar. (2006) Morocco [62] 11. Coprinellus campanulatus S. Hussain & H. Ahmad (2018) Pakistan [13] 12. Coprinellus carbonicola (Singer) Voto (2020) Argentina [27] 13. Coprinellus castaneus (Berk. & Broome) Voto (2020) Mauritius [63] 14. Coprinellus chaignonii (Pat.) Voto (2019) n.r. [64] 15. Coprinellus crassitunicatus Voto (2021) n.r. [54] 16. Coprinellus criniticaulis Voto (2021) n.r. [36] 17. Coprinellus curtoides Voto (2021) USA (Hawaii) [14] 18. Coprinellus curtus (Kalchbr.) Vilgalys, Hopple & Jacq. Johnson (2001) Hungary [32] USA (North Carolina) [65] Japan [66] Armenia [29] Italy [8] Argentina [67] Sweden [13] 19. Coprinellus deliquescens (Bull.) P. Karst. (1879) India [3] Argentina [67] 20. Coprinellus deminutus (Enderle) Valade (2014) Hungary [32] 21. Coprinellus dendrocystotus (Voto) Voto (2023) n.r. [68] 22. Coprinellus dilectus (Fr.) Redhead, Vilgalys & Moncalvo (2001) Poland [9] Netherlands [35] Germany [35] 23. Coprinellus disseminatisimilis S. Hussain (2018) Pakistan [13] 24. Coprinellus disseminatus (Pers.) J.E. Lange (1938) China [69] Russia [40] Hungary [32] Lithuania [70] Latvia [71] Sweden [40] USA (North Carolina) [72] Armenia [5] Japan [73] Serbia [44] Iran [74] Indonesia [75] Iraq [20] India [76] Philippines [77] Brazil [78] Russia (Karelia) [41] USA (Hawaii) [79] Korea [79] Costa Rica [80] Argentina [67] 25. Coprinellus domesticus (Bolton) Vilgalys, Hopple & Jacq. Johnson (2001) Hungary [32] Panama [81] USA (North Carolina) [65] Iran [82] Armenia [29] West Africa (Côte d'Ivoire) [83] Spain [47] Japan [84] Serbia [45] Argentina [67] Netherlands [13] 26. Coprinellus duricystidiosus Voto (2021) n.r. [54] 27. Coprinellus ellisii (P.D. Orton) Redhead, Vilgalys & Moncalvo (2001) USA
(North Carolina)[85] Japan [73] Armenia [29] 28. Coprinellus ephemerus (Bull.) Redhead, Vilgalys & Moncalvo (2001) India [3] Italy [8] Argentina [67] 29. Coprinellus fimbriatus (Berk. & Broome) Redhead, Vilgalys & Moncalvo (2001) India [3] 30. Coprinellus flocculosus (DC.) Vilgalys, Hopple & Jacq. Johnson (2001) Hungary [32] USA (North Carolina) [39] Iran [74] Iraq [20] Poland [11] Armenia [29] Italy [8] Spain [47] Norway [13] 31. Coprinellus furfurellus (Berk. & Broome) Redhead, Vilgalys & Moncalvo (2001) n.r. [21] 32. Coprinellus heptemerus (M. Lange & A.H. Sm.) Vilgalys, Hopple & Jacq. Johnson (2001) USA [86] Hungary [33] Italy [8] 33. Coprinellus limicola (Uljé) Doveri & Sarrocco (2011) n.r. [28] 34. Coprinellus magnoliae N.I. de Silva, Lumyong & K.D. Hyde (2021) Thailand [87] China [59] 35. Coprinellus maysoidisporus Voto (2021) n.r. [54] 36. Coprinellus micaceus (Bull.) Vilgalys, Hopple & Jacq. Johnson (2001) Hungary [32] China [59] Armenia [85] Japan [73] Iran [20] USA (Virginia) [88] Russia (Volograd) [43] Turkey [89] India [3] Greece [90] Japan [79] Korea [91] UK (Wales) [92] Germany [37] Romania [93] Serbia [45] Brazil [36] Argentina [67] Philippines [7] 37. Coprinellus neodilectus Voto (2019) Brazil [55] 38. Coprinellus occultivolvatus Voto (2021) n.r. [54] 39. Coprinellus ovatus M. Kamran & Jabeen (2020) Pakistan [22] 40. Coprinellus pallidissimus (Romagn.) P. Roux, Guy García & S. Roux (2006) Spain [48] 41. Coprinellus papillatus Voto (2021) n.r. [54] 42. Coprinellus parapellucidus Voto (2021) n.r. [54] 43. Coprinellus parcus T. Bau, L.Y. Zhu & M. Huang (2024) China [59] 44. Coprinellus parvulus (P.-J. Keizer & Uljé) Házi, L. Nagy, Papp & Vágvölgyi (2011) Netherlands [24] 45. Coprinellus phaeoxanthus A.R.P. Gomes & Wartchow (2016) Brazil [10] 46. Coprinellus plicatiloides (Buller) Voto (2020) n.r. [94] 47. Coprinellus pseudomicaceus (Dennis) Voto (2019) Brazil [36] 48. Coprinellus punjabensis Usman & Khalid (2021) Pakistan [95] 49. Coprinellus pusillulus (Svrček) Házi, L. Nagy, Papp & Vágvölgyi (2011) Hungary [33] 50. Coprinellus pyrrhanthes (Romagn.) Redhead, Vilgalys & Moncalvo (2001) n.r. [21] 51. Coprinellus radians (Desm.) Vilgalys, Hopple & Jacq. Johnson (2001) Armenia [6] Hungary [32] China [52] USA (North Carolina) [65] Germany [38] USA (Virginia) [88] Taiwan [96] Germany [39] Serbia [45] Brazil [36] Argentina [67] Sweden [13] 52. Coprinellus rufopruinatus (Romagn.) N. Schwab (2019) n.r. [65] 53. Coprinellus saccharinus (Romagn.) P. Roux, Guy García & Dumas (2006) Ukraine [17] Argentina [67] 54. Coprinellus sclerobasidium (Singer) Voto (2020) Argentina [67] 55. Coprinellus silvaticus (Peck) Gminder (2010) Hungary [32] Sweden [16] Iran [82] Serbia [45] Czech Republic [97] 56. Coprinellus subangularis (Thiers) Voto (2020) USA (Texas) [19] 57. Coprinellus subcurtus Voto (2019) USA (Hawaii) [55] 58. Coprinellus subradians Voto (2021) n.r. [54] 59. Coprinellus subrenispermus (Singer) Voto (2020) Argentina [67] 60. Coprinellus tenuis S. Hussain (2018) Pakistan [13] 61. Coprinellus tibiiformis Voto (2021) n.r. [54] 62. Coprinellus truncorum (Scop.) Redhead, Vilgalys & Moncalvo (2001) Serbia [46] Hungary [34] Iran [4] India [3] Argentina [67] Sweden [13] 63. Coprinellus valdivianus (Singer) Voto, Dibán & Maraia (2023) n.r. [68] 64. Coprinellus velutipes T. Bau & L.Y. Zhu (2024) China [59] 65. Coprinellus verrucispermus (Joss. & Enderle) Redhead, Vilgalys & Moncalvo (2001) Hungary [32] 66. Coprinellus xanthothrix (Romagn.) Vilgalys, Hopple & Jacq. Johnson (2001) USA (North Carolina) [39] Japan [73] Iran [82] Armenia [29] Germany [39] Serbia [45] Hungary [31] Netherlands [13] 67. Coprinellus xylophilus Voto (2021) Hungary [13] n.r., not reported. Table 1.
Global distribution of Corpinellus species.
-
Coprinellus species Sample type Bioactive compounds Ref. Coprinellus species Sample type Bioactive compounds Ref. C. curtus HPLC (water extract) Mannitol [30] C. truncorum Hot water extracts of fruiting body, submerged mycelia, and fermentation broth Apigenin [46] Stearic acid Baicalein Myristic acid Chrysoeriol C. disseminatus Crude ethanol (CdEtOH) and water extract (CdAq) Phenol [106] Vitexin Flavonoids Apigenin-7-O-glucoside Methanol extract Chrysoeriol [44] Luteolin-7-O-glucoside Luteolin7-O-glucoside Apiin Apigenin7-O-glucoside Baicalin Amentoflavone Quercetin p-Hydroxybenzoic acid Isorhamnetin p-Coumaric acid Quercitrin Protocatechuic acid Kaempferol-3-O-glucoside Chlorogenic acid Hyperoside HPLC (water extract) Mannitol [30] Quercetin-3-O-glucoside Fructose Naringenin Saccharose Catechin Oleic acid Epicatechin Stearic acid Amentoflavone C. domesticus HPLC (water extract) Mannitol [30] Daidzein Oleic acid Genistein C. ellisii HPLC (water extract) Mannitol [30] p-Hydroxybenzoic acid Stearic acid Protocatechuic acid C. micaceus HPLC (water extract) Mannitol [30] Vanillic acid Fructose Gallic acid Stearic acid Gentisic acid Myristic acid p-Coumaric acid HPLC (hot water extract) Protocatechuic acid [91] o-Coumaric acid Chlorogenic acid Caffeic acid (−)−Epicatechin Esculetin Naringin Scopoletin C. radians HPLC (water extract) Mannitol [30] Umbelliferon Fructose Quinic acid Oleic acid 5-O-Caffeoylquinic acid Stearic acid Ethanol extracts of the fruiting body and mycelia Apigenin [108] C. xanthorix HPLC (water extract) Mannitol [30] Baicalein Saccharose Chrysoeriol Oleic acid Amentoflavone Stearic acid Aqueous extract Crysoeriol [51] Myristic acid Apigenin-7-O-glucoside Coprinellus sp. Crude extract Coprinsesquiterpin A (1) [107] Luteolin-7-O-glucoside Coprinsesquiterpin B (2) Hyperoside Coprinsesquiterpin C (3) p-Hydroxybenzoic acid Coprinsesquiterpin D (4) Protocatechuic acid Coprinsesquiterpin E (5) Gallic acid p-Coumaric acid o-Coumaric acid Quinic acid 5-O-Caffeoylquinic acid Table 2.
Bioactive compounds present in Coprinellus mushrooms.
-
Coprinellus species Bioactivity Extract/compounds Findings Ref. C. disseminatus Promote seed germination n.r. Promote seed germination of Cremasta appendiculata up to 71.61% ± 0.92%. [53] Biobleaching C. disseminatus SH-1 NTCC-1163 (enzyme-A) and SH-2 NTCC-1164 (enzyme-B) Under solid-state fermentation, two newly developed low-cellulose xylanases (enzymes A and B) reduced the kappa number of wheat straw soda-AQ pulps by 24.38% and 27.94%, respectively, following XE treatment. [76] Antiproliferative Biomass ethyl acetate (BEA) extract The BEA extract exhibited antiproliferative activity (GI50 < 50 μg/mL) against all tested solid tumor cell lines (A549, HBL-100, HeLa, T-47D, WiDr), except for SW1573, which showed a slightly higher GI50 value of 52 μg/mL. [41] Antioxidant Biomass ethyl acetate (BEA) extract, and
Culture-broth Ethyl
acetate (CEA) extractIn the galvinoxyl radical assay, C. disseminatus (CEA) exhibited an antioxidant capacity of 10.281 ± 0.237 μM Trolox equivalents (TEAC). Meanwhile, in the ABTS assay, the BEA extract of C. disseminatus demonstrated the strongest activity, with a TEAC value of 126.67 ± 7.69 μM. [41] Antioxidant Crude ethanol (CdEtOH) The extract demonstrated strong superoxide anion scavenging activity (IC50 = 1.40 ± 0.66 μg/mL), followed by hydroxyl radical (7.37 ± 1.46 μg/mL) and FRAP (9.74 ± 0.79 μg/mL) scavenging. In contrast, it showed moderate nitric oxide (273.30 ± 21.53 μg/mL) and weaker DPPH (397.28 ± 64.17 μg/mL) scavenging effects. [106] Antioxidant Water extracts (CdAq). The extract demonstrated antioxidant activity, with the strongest activity against hydroxyl radicals (OH, IC50 = 4.02 ± 0.29 μg/mL) and FRAP reduction (4.02 ± 0.60 μg/mL). Moderate activity was observed for superoxide anion (SOA, 24.84 ± 2.38 μg/mL) and nitric oxide (NO, 21.28 ± 6.08 μg/mL), while DPPH scavenging was least potent (250.37 ± 15.74 μg/mL). [106] Cytotoxicity Crude ethanol (CdEtOH) The extract exhibited time and assay-dependent cytotoxicity against MCF-7 cells. In MTT assays, potency improved with prolonged exposure (24 h: IC50 > 249.47 ± 11.52 μg/mL; 72 h: 217.90 ± 24.79 μg/mL). Meanwhile, the effect of exposure time was observed in SRB assays, where the IC50 decreased from 511.37 ± 6.46 μg/mL (24 h) to 205.90 ± 35.98 μg/mL (72 h). Notably, the 72-hour results converged across both assays, indicating sustained exposure enhances cytotoxic efficacy regardless of the detection method. [106] Cytotoxicity Water extracts (CdAq) The cytotoxic effects on MCF-7 breast cancer cells were evaluated using MTT and SRB assays at different time points. In the MTT assay, the compound showed minimal cytotoxicity at 24 h (IC50 > 900 μg/mL) but exhibited moderate activity after 72 h of exposure (IC50 = 718.07 ± 37.36 μg/mL). The SRB assay demonstrated stronger concentration-dependent cytotoxicity, with IC50 values decreasing from 625.26 ± 26.80 μg/mL at 24 h to 211.01 ± 25.07 μg/mL at 72 h. These results indicate that the compound's anti-proliferative effects are both time-dependent and assay-dependent, with the SRB method showing greater sensitivity in detecting cytotoxic activity compared to the MTT assay. [106] Lignocellulolytic activity Xylanase and cellulase When cultured in a glucose-containing medium, the mycelium exhibited XLE and CLE activities of 815.074 ± 7.102 U/mL and 9.704 ± 0.030 U/mL, respectively. [69] C. disseminatus and
C. micaceusBiotransformation of polychlorinated dibenzo-p-dioxin n.r. C. disseminatus achieved nearly complete degradation of dibenzo-p-dioxin (DD) within two weeks. Additionally, both C. disseminatus and C. micaceus converted 2,7-dichlorodibenzo-p-dioxin (2,7-DCDD) into a monohydroxylated derivative, suggesting the activity of the cytochrome P450 system in this process. [73] C. micaceus Lignocellulolytic activity n.r. Exhibited high production of cellulolytic enzymes, including endo-β-1,4-glucanase (0.69 ± 0.04 U/mL after 10 d with optimal pH 5.0) and endo-β-1,4-xylanase (1.17 ± 0.21 U/mL after 10 d with optimal pH 6.0), along with the lignolytic enzyme laccase (0.81 ± 0.20 U/mL after 28 d with optimal pH 3.0). This robust enzymatic activity indicates a strong potential for lignocellulose degradation. [90] Antioxidant Methanol and hot water extract Both methanol and hot water extracts exhibited lower DPPH scavenging activity than BHT but showed superior metal chelating effects at all concentrations. Their reducing power was also weaker than BHT at 0.125–0.2 mg/mL. [91] Antidiabetic Methanol and hot water (fruiting body) extract At a concentration of 2.0 mg/mL, the methanol and hot water extracts of C. micaceus reduced α-glucosidase activity by 62.26% and 67.59%, respectively. In comparison, acarbose, the positive control, showed an 81.81% inhibition at the same concentration. [91] Anticholinesterase Methanol and hot water extract In the AChE inhibitory assay, the methanol and hot water extracts of C. micaceus demonstrated 94.64% and 74.19% inhibition, respectively, at a concentration of 1.0 mg/mL. In comparison, galanthamine, the control drug, showed 97.80% inhibition at the same concentration. [91] Anti-tyrosinase Methanol and hot water extract At a concentration of 2.0 mg/mL, the methanol and hot water extracts exhibited strong tyrosinase inhibition, with rates of 91.33% and 91.99%, respectively. In comparison, kojic acid (the positive control) showed a higher inhibition rate of 99.61% at the same concentration. [91] Antioxidant (Nitric oxide inhibition) Methanol and hot water extract The methanol and hot water extracts dose-dependently suppressed nitric oxide (NO) production in lipopolysaccharide (LPS)- stimulated RAW264.7 cells. [91] C. truncorum Cytotoxicity Polysaccharide and exopolysaccharide Both PSH and ePSH demonstrated notable cytotoxic effects on human-derived HepG2 cancer cells (three-way ANOVA, p < 0.05). The C. truncorum PSH and ePSH were particularly effective, achieving a maximal reduction in cell viability of approximately 50% at 450 μg/mL after 24 h of treatment. [108] Anticholinesterase Polysaccharide extracts The polysaccharide extracts (PSH) from C. truncorum exhibited significant acetylcholinesterase (AChE) inhibitory activity, with an IC50 value of 0.61 mg/mL in liquid assays. [51] Antifungal MeOH (Fruiting body) The MIC and MFC values of CtMeOH extracts against Fusarium proliferatum BL1, Fusarium verticillioides BL4, Fusarium proliferatum BL5, and Fusarium graminearum were both found to be 198.00 mg/mL. In contrast, lower values of 99.00 mg/mL for both MIC and MFC were recorded for Alternaria padwickii (ALT). [112] Antifungal EtOH (Fruiting bodies) The CtEtOH exhibited a minimum inhibitory concentration (MIC) of 99.00 mg/mL against Alternaria padwickii (ALT) [112] Antioxidant Hot water extract The antioxidant activity of different fungal extracts was evaluated using DPPH radical scavenging and FRAP assays. The fruiting body extract exhibited moderate antioxidant activity, with a DPPH IC50 value of 65.90 ± 2.13 µg/mL and a FRAP value of 26.72 ± 0.47 mg AAE/g. In contrast, the submerged mycelium demonstrated significantly stronger antioxidant effects, showing a much lower DPPH IC50 (7.52 ± 2.46 µg/mL) and a higher FRAP value (30.63 ± 0.88 mg AAE/g), indicating greater free radical scavenging and reducing power. Meanwhile, the fermentation broth exhibited intermediate DPPH scavenging activity (IC50 42.39 ± 1.75 µg/mL) but the lowest FRAP value (6.03 ± 0.18 mg AAE/g), indicating a comparatively weaker reducing capacity. [46] Coprinellus sp. Anti-inflammatory Coprinsesquiterpin Coprinsesquiterpins 1–5 were tested for their ability to reduce inflammation in vitro by suppressing NO production in LPS-stimulated RAW264.7 macrophages. Among them, Coprinsesquiterpins 1, 3, and 5 showed significant anti-inflammatory effects, with IC50 values of 34.7, 27.1, and 12.8 μM, respectively. In contrast, Coprinsesquiterpins 2 and 4 were less effective, displaying IC50 values above 40 μM. [107] n.r., not reported. Table 3.
Biological activity of Coprinellus mushrooms.
Figures
(4)
Tables
(3)