-
With the increasing demand for fine chemicals, industrial accidents, such as fires, explosions, significant casualties, and environmental damage continue to occur. These disasters attract people's attention to the safety problems in the chemical manufacturing process and promote the sustainable development of emergency technologies in process safety management[1]. The in-depth study of accident causes and their evolution process are the basis for formulating process safety policies and regulations, developing safety technologies and solutions in emergencies within chemical processing plants[2].
Thermal runaway of exothermic reactions generally refers to uncontrolled self-heating that is caused by cooling system failure[3], feeding error, reactant accumulation[4], appearance of hot spots[5,6], mixing failure, human error, or system parametric sensitivity[7,8], which may lead to a self-perpetuating cycle between the increase in temperature and acceleration of reaction rate.
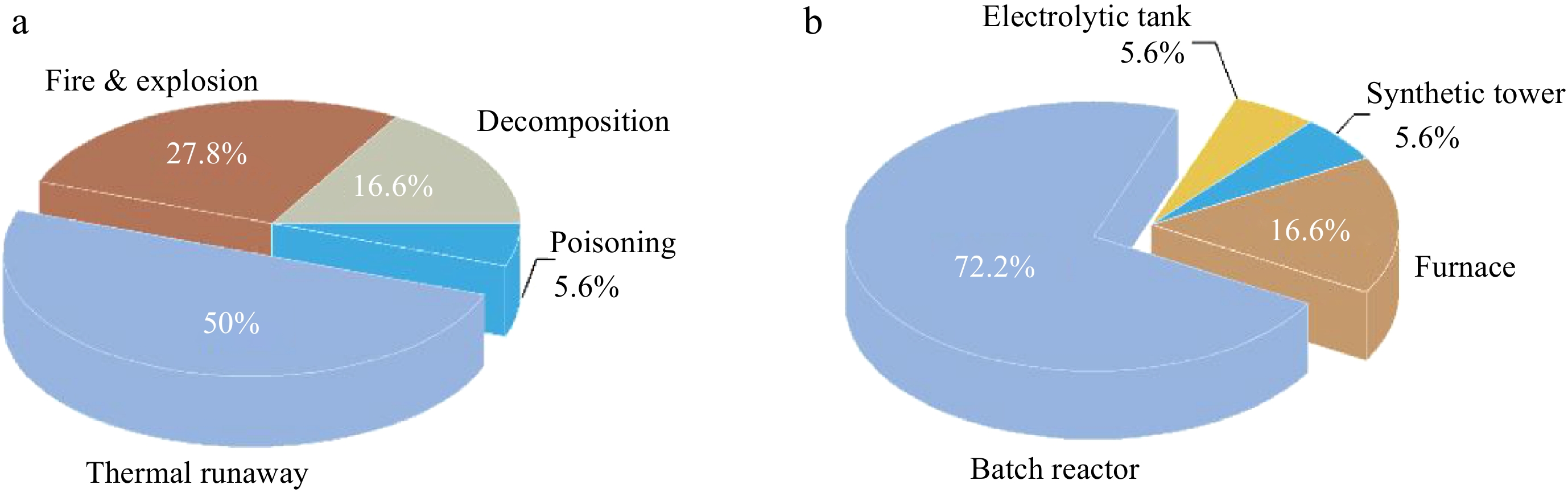
According to the analysis of 169 accidental events between 1974 and 2014 in the French chemical industry[9], over 25% of accidents in French chemical plants resulted from reaction thermal runaway, and then developed into disasters such as leakage, fire, and explosion. With the vigorous development of fine chemical and pharmaceutical industry in Mainland China over the past two decades, the number of industrial accidents caused by reaction thermal runaway is gradually increasing.
To identify the risks of different types of chemical reactions, the Chinese government formulated two documents in 2009 and 2013, respectively, which include 18 processes with high accident risk, their hazard characteristics and loss prevention methods[10,11]. As shown in Fig. 1, the accident types of high-risk processes are composed of thermal runaway, reactant decomposition, fire, explosion, and poisoning. More than two-thirds of the 18 high-risk processes are exothermic reactions and half of them have the risk of thermal runaway. Furthermore, decomposition of reaction substances is one of the main reasons for reaction thermal runaway. Compared with other reaction devices, thermal runaway occurring in batch reactors has the most serious consequences, owing to high accumulation of reactants and low volumetric heat transfer efficiency. In recent years, with the development of technology and industrial upgrading, several typical accidents caused by reaction thermal runaway occurred in China, which damaged people's confidence in the chemical industry. Zhang et al.[12] statistically analyzed the accidents, in China, caused by reaction thermal runaway between 1984 and 2019 . The results showed that both the frequency of thermal runaway accidents and the mortality of accidents were increased. Reaction thermal runaway in chemical processes has been a major threat to process safety that cannot be ignored.
It is therefore necessary to develop safety technologies which can control reaction thermal runaway in emergencies and mitigate the subsequent hazards, injury of personnel, or other disastrous consequences. Since the theory of reaction thermal runaway was initiated by Semenov in 1928[13], technologies of emergency disposal for reaction thermal runaway have developed for almost 100 years. In the past two decades, the majority of efforts have been made to overcome the aforementioned potential hazards caused by reaction thermal runaway. For instance, emergency relief systems for discharging overpressure in reactors, reactivity and high temperature reactants have been widely used in process safety design and reactor manufacturing. Rupture discs, also called bursting discs, are widely used in pressure vessels, pressure equipment, and pressure piping in process industries for controlling the reaction pressure. Very recently, reaction inhibition attracted much more attention and provide a new methodology to halt or control reaction thermal runaway. Reaction inhibition presents several advantages such as high efficiency, controllable release, and enhanced heat transfer of reactors. However, an integrated summary to provide a deep understanding of emergency response technologies of reaction thermal runaway is still missing in published literature. In this work, a comprehensive review is delivered to outline the recent progress in emergency response technologies for reaction thermal runaway, major principles and potential applications of those loss prevention methods.
-
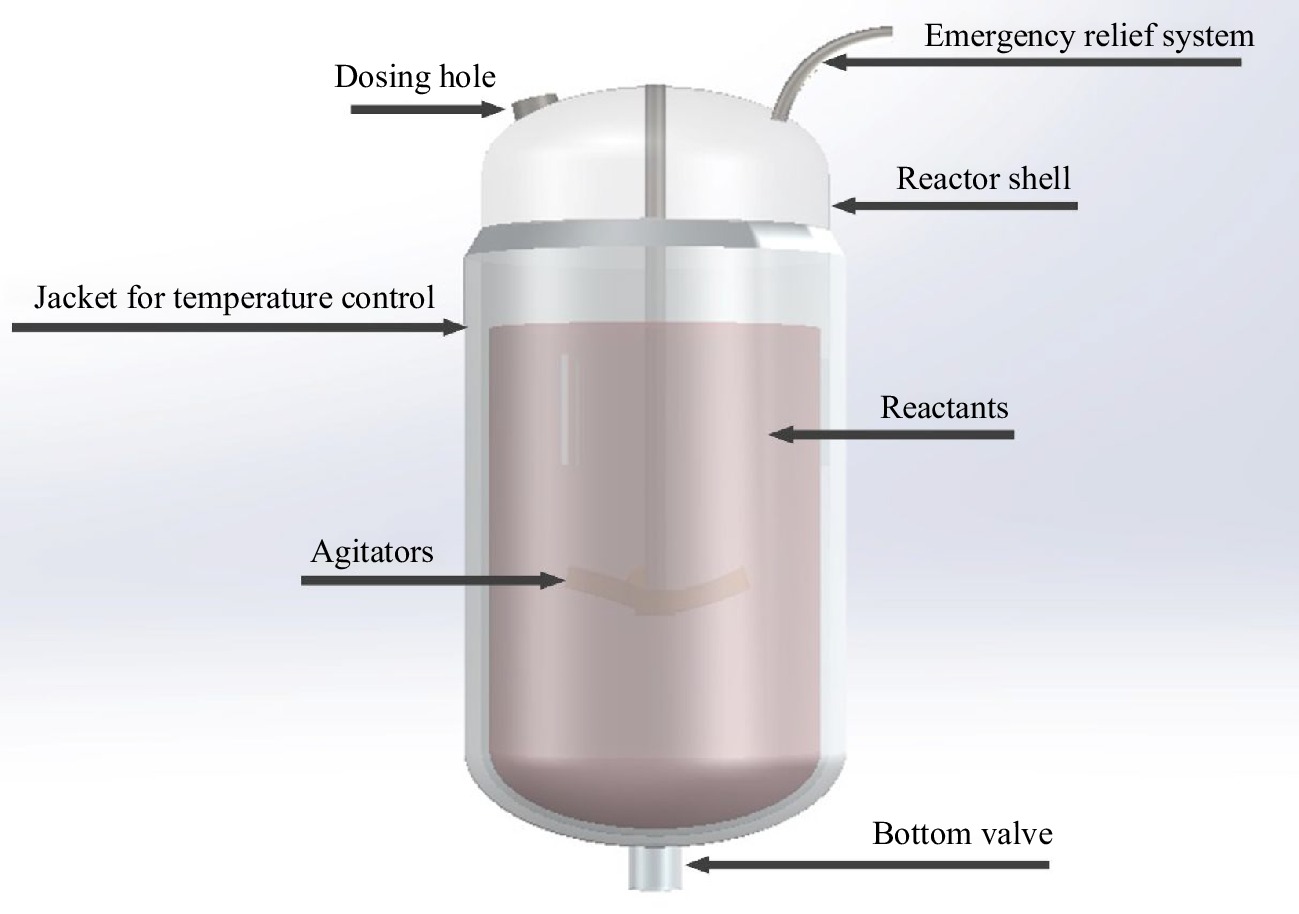
The essence of reaction thermal runaway is the vicious cycle between uncontrolled reaction rate and uncontrolled reaction temperature. The structure of a batch reactor is shown in Fig. 2, which is the most widely used in the pharmaceutical industry, pesticide industry, and other fine chemical industries. It can be seen that the basic construction of a batch reactor includes the reactor shell, jacket for temperature control, dosing hole, emergency relief system, agitators, bottom valve, and reactants. The reactor shell is the container of reactants and used to bear the pressure generated by chemical reactions. The jacket for temperature control of the reactor is used to provide energy to maintain the continuous progress of the reaction. The dosing hole on the top cover of the reactor is used to add reaction required raw materials and observe the state of the reactants. The pressure relief pipeline is used to balance the pressure between the reactor and the atmosphere in normal production process. However, the pressure relief pipeline is used to reduce overpressure in the reactor in the case of an emergency. The reaction stirring or agitator is used for mixing and mass transfer of different reactants, and promoting heat exchange between reactants and cooling jackets. The bottom valve is used to discharge the reaction product. In case of emergency, it is used to discharge the active substance to the containment device for diluting, quenching, or inertion. Chemical reactions are carried out between the molecules of reactants. The capacity of reactants should comply with the specified dosage. With the continuous progress of the reaction, the chemical composition and physical property of reactants are constantly changing.
The common causes of reaction thermal runaway can be classified into three categories. The first one is the hot spot in the reactor caused by insufficient mixing of reactants or agitator failure. The second is weak cooling capacity of the cooling jacket failure, resulting in reaction heat accumulation in the reactor. The third is excessive feeding caused by human error or metering device failure. Regardless of whether the reaction thermal runaway occurs in batch reactors, tubular reactors, continuous-flow stirred tank reactors, or fixed bed catalytic reactors, the emergency response and loss prevention methods for reaction thermal runaway follow three basic principles: relieving overpressure in the reactor, controlling the reaction temperature, and retarding the reaction rate.
Relief of overpressure
-
For liquid phase reactions, overpressure in the reactor is usually caused by the vapor pressure of the superheated liquid reactants. For gas phase reactions, the pressure in the reactor is generally positively correlated with the reaction rate. When thermal runaway happens, the reaction rate and reaction temperature are out of control, which may accelerate the vaporization of the reactants, resulting in a sharp increase in the vapor space pressure of the reactor. When the inner pressure exceeds the maximum limit stress of the shell material, the catastrophic explosion of reaction vessel will happen. The shock wave formed by overpressure released during the explosion is the main cause of casualties and property loss around the plant area.
Therefore, releasing the overpressure is one of the most basic operations of the emergency disposal for reaction thermal runaway. Releasing the overpressure so that the pressure difference between the inside and outside of the reactor shell is within the limit of yielding, and ensures the equipment integrity. The vapor of the reactants is largely combustible or toxic. Therefore, the released vapor from the relief system also needs follow-up treatment procedures, such as collection by storage tanks, combustion by flare systems, and absorption or degradation by special devices.
Control of reaction temperature
-
The uncontrolled increase of reaction temperature is the direct cause of the acceleration of the reaction rate and overpressure in the reactor. The reaction temperature is adjusted according to the heat exchange between the cooling jacket and the reaction material. Therefore, insufficient cooling capacity or cooling failure will lead to heat accumulation in the reactor. The temperature uniformity of the reaction mixture is realized by stable stirring. Hence, insufficient stirring or stirring failure will lead to uneven mixing of reactants and hot spots in reactors. This phenomenon is more likely to occur in polymerization processes, especially in bulk polymerization and solution polymerization. Due to the high monomer content in these polymerization processes, the viscosity of the reactants changes greatly during the polymerization process. Generally, the viscosity of reactants rises with the increase of the polymerization degree. The increased viscosity requires greater stirrer torque with larger agitator power. At the same time, the increase of viscosity results in poor heat exchange effect between the reactants and the reactor. It is an effective method for the inhibition of reaction thermal runaway.
Quenching the runaway reaction
-
The above-mentioned two principles, relief of overpressure and control of the reaction temperature, can be classified as passive emergency disposal strategies, which are committed to mitigating the catastrophic consequences of reaction thermal runaway. However, starting from the root of reaction thermal runaway, quenching the active substance and inhibiting the progress of the chemical reaction could extinguish the 'flame' of runaway reaction.
According to Arrhenius equation, as expressed in Eq. (1), the reaction rate constant is affected by the pre-exponential factor, apparent activation energy, and thermodynamic temperature. With the exception of the control of reaction temperature mentioned above, the reaction rate constant can be reduced by diluting the concentration of reactants, which may decrease the probability of collision between different reactive molecules. In addition, quenching the reactive intermediates could reduce the reaction rate by increasing the activation energy of the reaction.
$ k=A{e}^{-\frac{{E}_{a}}{RT}} $ (1) where k is the reaction rate constant, A is the pre-exponential factor, Ea is the apparent activation energy, R is the molar gas constant, T is the thermodynamic temperature.
-
According to the phase state of the venting reactants, the pressure relief system can be divided into single-phase flow system and two-phase flow system. According to the composition of the venting reactants, the pressure relief system can be divided into gas system, vapor system, and mixed system. According to the flow regime of the venting reactants, the pressure relief system can be divided into laminar flow system and flow system.
Leung et al.[14−16] developed a calculation model of the mass flow rate for safety venting in vapor systems, which has been widely used in chemical process design.
The team from American Design Institute of Emergency Relief System (DIERS) put forward the development of the venting model for gas relief systems[17−24]. Based on the DIERS model, two methods of gas relief systems were proposed. According to the two methods, the gaseous venting flow was treated as homogeneous two-phase flow for convenience of calculation, where the result accuracy can be guaranteed. The mixed system is more complex than the other two systems. Leung et al. and Fauske et al.[25−28] proposed computational models for mixed systems on the basis of the vapor system model, respectively. On the basis of previous theoretical studies, Moncalvo & Friedel[29] studied single-phase flow and two-phase flow relief of polyvinylpyrrolidone (PVP) aqueous solution in a fully opened safety valve. Gustin[30] studied the venting process of the thermal runaway caused by cooling failure in the reaction system of phenol and formaldehyde.
Véchot et al.[31] investigated the runaway and venting process of a hybrid chemical system (30% cumene hydroperoxide) in a 110 mL closed cell. The sensitivity of the maximum temperature and the maximum temperature rise rate to the size of the exhaust port were discussed. The closed and open cell tests according to the DIERS approach were carried out using a Vent Sizing Package 2 (VSP2) adiabatic calorimeter (Fauske & Associates, LLC), as shown in Fig. 3, which has been widely used and become one of the most powerful devices for the research of safety venting.
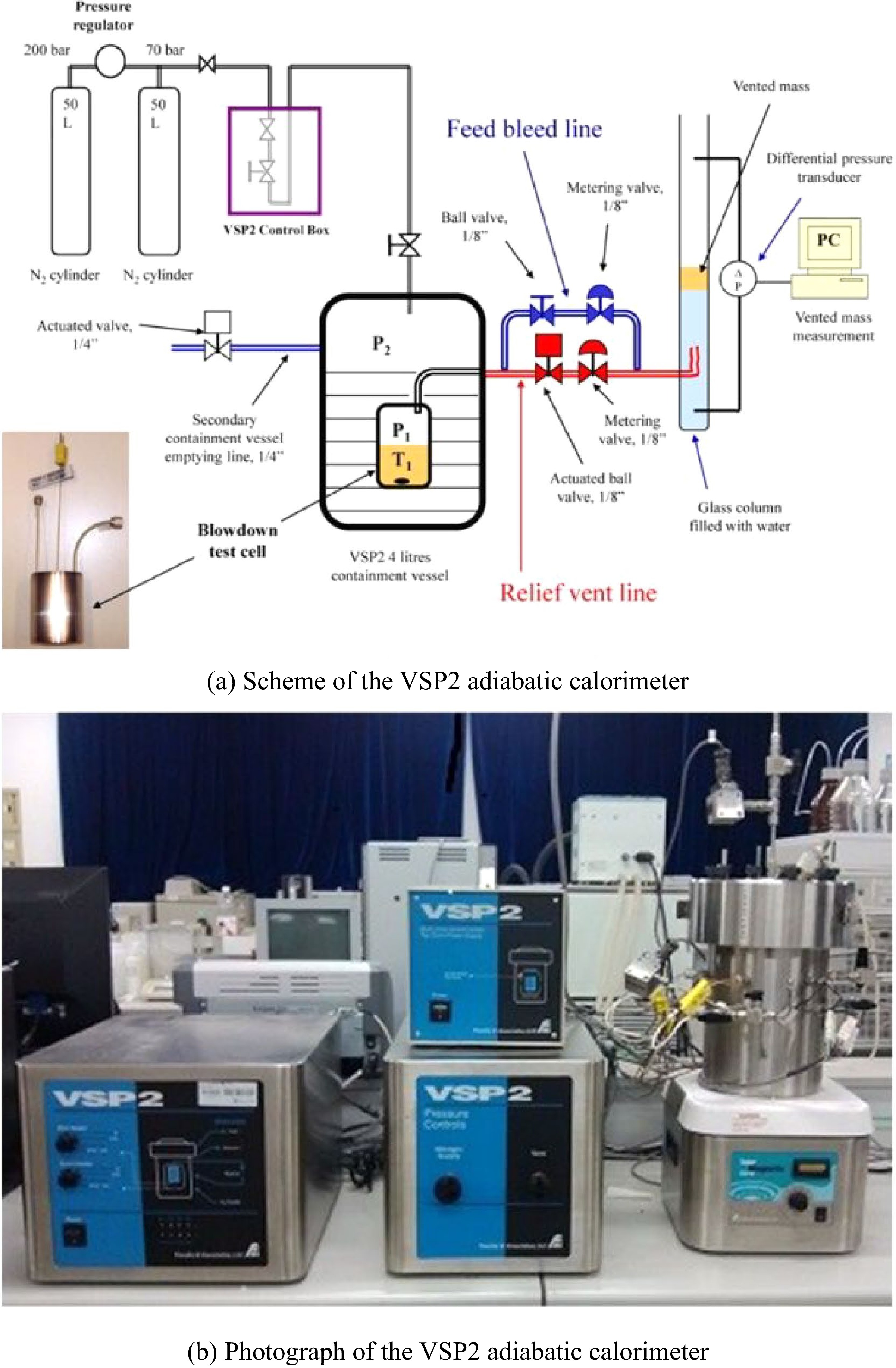
Figure 3.
Schematic set up of the VSP2 adiabatic calorimeter[31].
Jiang et al.[32] studied the pressure relief of the vinyl acetate polymerization process and calculated the venting area using VSP2. Wei & Jiang[33] studied the safety relief of di-tert-butyl peroxide (DTBP) using adiabatic accelerating rate calorimeter. The results showed that the decomposition process of DTBP was a mixed system. Deng et al.[34] studied the thermal decomposition of dicumyl peroxide (DCP) and found that decomposition products is a gas-liquid mixture. Cao et al.[35,36] systematically analyzed the effects of the cracking pressure and duct length on explosion pressure at different positions according to the test of the hydrogen with different concentrations in an explosion venting device with a duct. Kim et al.[37] proposed a manufacturing process of an integrated safety vent including backward extrusion and coining process, which can be used in lithium-ion battery system. As a complex electrochemical reaction process, safety vents have been an effective means to control unpredictable explosions caused by the thermal runaway of lithium-ion batteries[38].
Emergency cooling
-
In order to control the rapid rising of reaction temperature when thermal runaway occurs, adding coolant into the reactor and maintain good mixing are the main emergency response methods. As the thermal runaway in industrial reactors is extremely dangerous, lab-scale reaction calorimetry experiments[39−41] and computational fluid dynamics (CFD) simulations are preferred and have been widely studied.
Rakotondramaro et al.[42] performed an experiment to analyze the situation of stirring and cooling failure in epoxidation process, the results showed that the secondary thermal runaway can be delayed with high stirring speed. Milewska & Molga[43] performed CFD numerical simulations and experiments to study the influence of stirring rate on the comprehensive convection heat transfer coefficient of the reactor. Jiang et al.[44] studied the styrene polymerization process using Fluent software. The effect of reaction viscosity on the temperature distribution in reactors was studied. The optimal temperature monitoring point and the injection position of coolant were determined. Hristov & Mann[45] studied the influence of coolant injection on the reaction temperature rise, which associated the mixing efficiency with the emergency cooling. This research established a simple coolant diffusion model. Torré et al.[46,47] used a single-phase flow CFD model to research the dependence of the jet trajectory on the injection parameters and defined a global mixing criterion to quantify the mixing quality and to assess the influence of the jet trajectory on the cooling efficiency.
Dakshinamourthy et al.[48−50] did a series of studies on the shortstopping and jet mixers in preventing reaction thermal runaway in stirred batch reactors. As shown in Fig. 4, the influence of the nozzle diameter, injection angle, injection position, and amount of coolant on the cooling effect and mixing effect for the polymerization of propylene oxide.
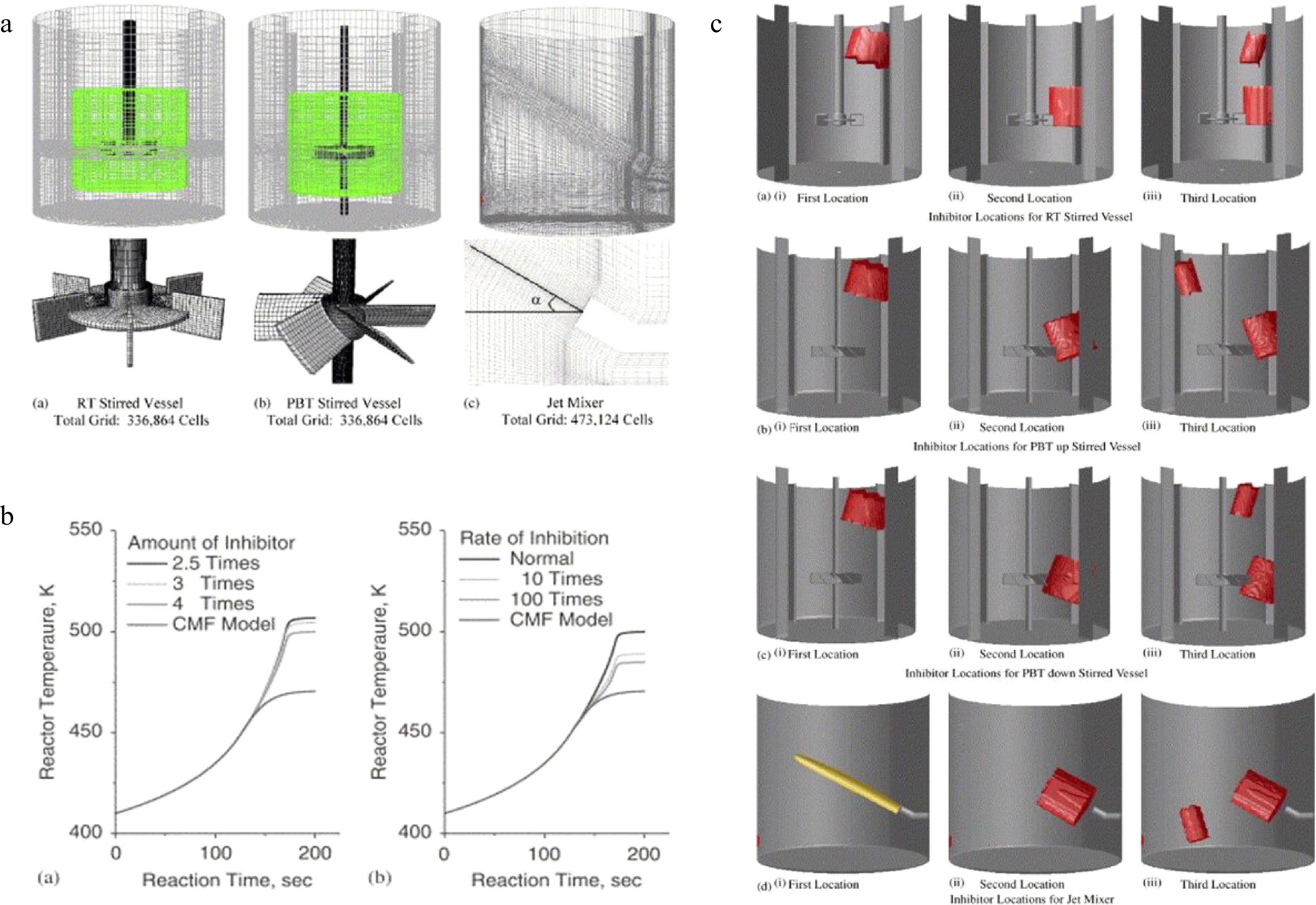
Figure 4.
CFD simulation of shortstopping and jet mixers in preventing runaway reactions, (a) solution domain and grid, (b) predicted evolution of average reactor temperature, and (c) locations of shortstopping and jet mixers.
Ni et al.[51] studied the thermal polymerization of styrene in a lab-scale batch reactors using CFD. The critical point of the runaway reaction was determined and used to inhibit the thermal runaway of styrene polymerization by injecting cooling diluents at the liquid surface. As shown in Fig. 5, the influence of injection rate, injection position, and amount of cooling diluents injected on the thermal runaway inhibition of the reaction was investigated and elucidated.
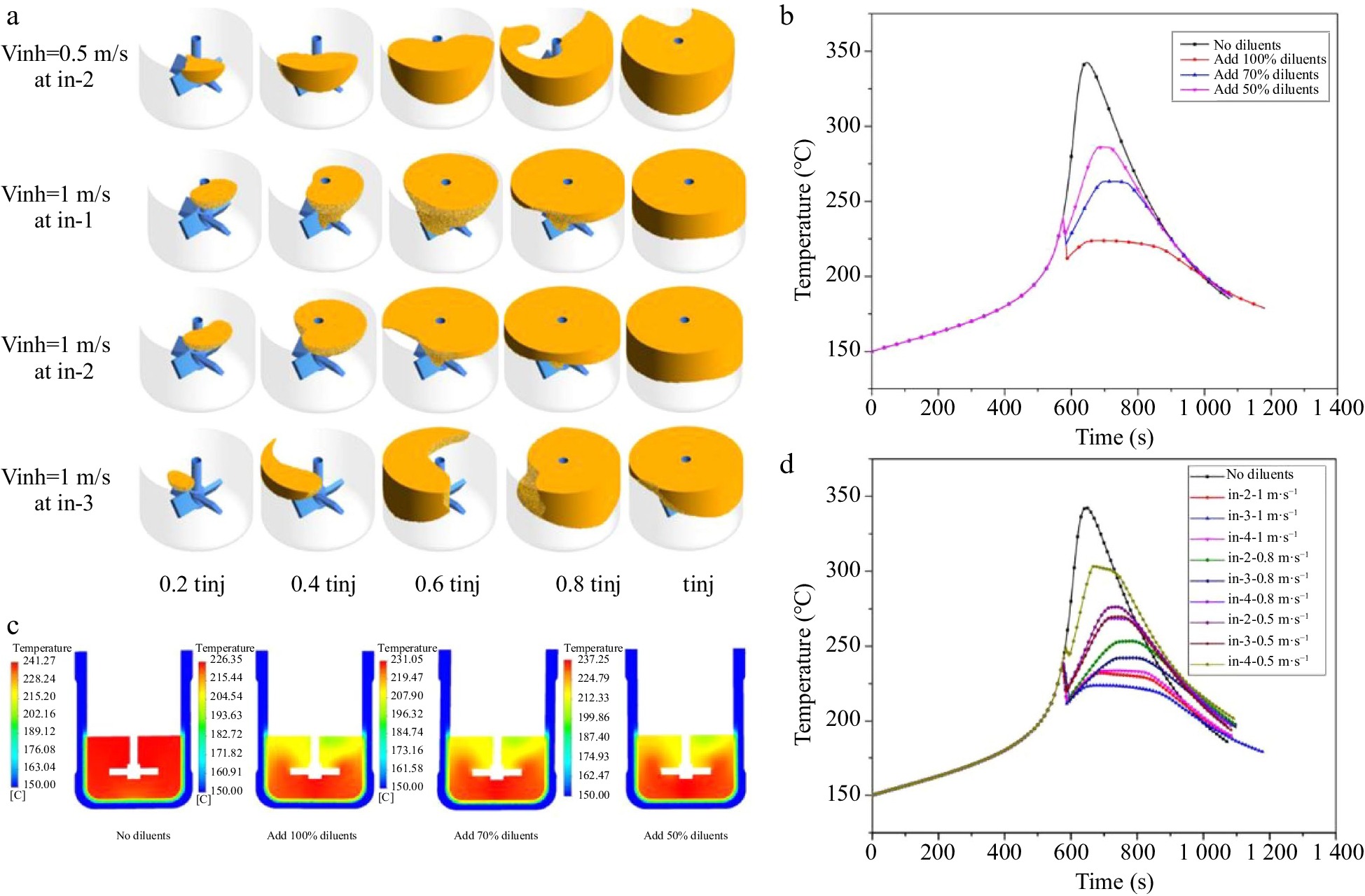
Figure 5.
Simulation on runaway inhibition of styrene polymerization, (a) inhibited regions at various times, volume average temperature for (b) different amounts of cold diluents and (d) different cold diluent addition locations, and (c) temperature profile of Y-axis.
Reaction inhibition
-
Even if the overpressure or reaction temperature can be controlled by emergency pressure relief systems or cooling systems, the runaway reaction continues to progress and there may be significant possibility of secondary thermal runaway. Therefore, reaction inhibition is being considered as an alternative or additional measure of safety venting and emergency cooling.
Since emergency cooling has been described above, the reaction inhibition mentioned in this section focuses on chemical inhibition.
Reaction inhibition involves injecting a chemical inhibitor to the reactor at the early stage of the thermal runaway. The inhibitor used can either halt the reaction completely or reduce the reaction rate to delay further runaway for a time period, allowing time for other emergency response[52]. The applicability of chemical inhibitor is dependent on the reaction mechanism. Normally, radical catcher is suitable for free radical reactions (such as polymerizations and Grignard reactions), pH regulator is suitable for pH-dependent reactions, and systems where the catalyst can be deactivated or removed by the inhibitor.
Some attempts of reaction inhibition have been made on polymerization processes[53].
Kammel et al.[54] took the radical solvent-polymerization of styrene in o-xylene as an example. The kinetic, caloric, and rheological parameters of the polymerization process were determined using a reaction calorimeter and a rheometer. The inhibition experiments were carried out under different conditions of different inhibitor concentration, injection timing, and injection speed.
Snee & Cusco[55] employed tertiary butyl catechol as the inhibitor for the polymerization of styrene initiated by benzoyl peroxide. The results showed that increasing the stirring speed could accelerate the dispersion of the inhibitor, alleviate the formation of local hot spots, and make uniform the temperature distribution of the reactor.
Ampelli et al.[56] used the reaction calorimetry coupled with a particular system for early warning detection of thermal runaway to investigate the effect of two chemical inhibitors (hydroquinone and 1,4-benzoquinone) on the reaction rate of the methylmethacrylate polymerization process.
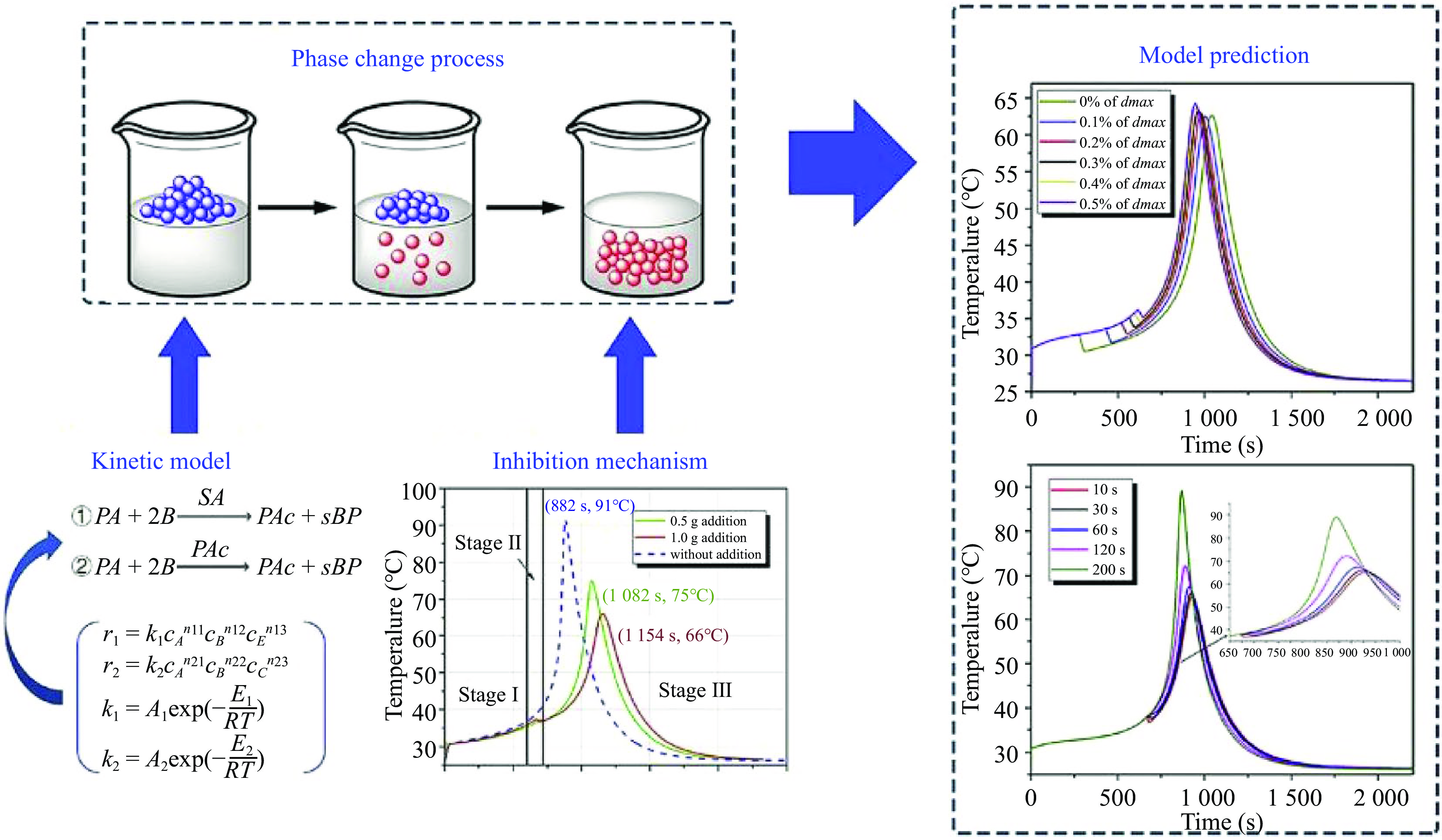
Inspired by the application of encapsulated phase change materials to control the thermal runaway of methanol oxidation and methyl methacrylate[57], our group have fabricated the microencapsulated phase change materials (microPCMs) with inorganic shell or melamine formaldehyde (MF) resin shell (Fig. 6). Furthermore, microPCMs were used to control the reaction rate of a homogeneous esterification of propionic anhydride with n-butanol catalyzed by sulfuric acid in batch or semi-batch reactors[58−60]. The experimental results showed that the mechanism of microPCMs on the inhibition of reaction thermal runaway was the synergy of physical cooling and chemical inhibition.
-
Reaction thermal runaway has been a common hazard leading to process safety-related accidents. In this work, the principles of the emergency response technologies for reaction thermal runaway were summarized. According to the published literature, several loss prevention methods of reaction thermal runaway were developed, but there are still many aspects worthy of further investigation. Suggestions for future work can be summarized into the following aspects:
(1) The calculation model of safety venting mostly obtained by theoretical derivation and verified by lab-scale experiments. With high integration, large scale and complex process of the chemical industry, experiential equipment and test methods for large scale safety venting testing is necessary.
(2) With the development of multiphase fluid model and CFD simulation technology at different scales, the mixing process of coolant and reactant in complex reaction processes could be further studied, such as the heat and mass transfer in microreactors.
(3) The combination of thermal storage and material functionalization may provide an intensive and practical approach for thermal management and runaway inhibition in complex chemical processes.
The authors gratefully acknowledge the financial support from the Suzhou science and technology innovation project of social development (SS202153) and Innovation training program for college students of Changshu Institute of Technology.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Tech University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Chen Q, Wang J, Gao M, Liu L, Tao J. 2022. Review on loss prevention of chemical reaction thermal runaway: Principles and application. Emergency Management Science and Technology 2:10 doi: 10.48130/EMST-2022-0010
Review on loss prevention of chemical reaction thermal runaway: Principles and application
- Received: 28 August 2022
- Accepted: 27 September 2022
- Published online: 14 October 2022
Abstract: Reaction thermal runaway has been extensively characterized as a major hazard for fine chemical industry. It is necessary to develop safety technologies for the control of reaction thermal runaway in emergencies and mitigating the subsequent hazards. To date, literature review on the loss prevention methods of chemical reaction thermal runaway is insufficient. In this paper, a comprehensive review is delivered to outline the progress of emergency response technologies for reaction thermal runaway in recent years, major principles and potential applications of those loss prevention methods. It is expected that this review article has certain reference value for the further understanding of thermal runaway, the design of mitigation systems, and the formulation of emergency response strategy for runaway reactions.
-
Key words:
- Loss prevention /
- Thermal runaway /
- Chemical reaction /
- Emergency response /
- Reaction inhibition