-
People's diets and lifestyles have changed significantly due to the rise in living standards, and as a result, constipation is becoming more common, drawing more and more attention[1]. Less than three bodily functions per week, complicated bowel motions, lumpy stools, abdominal pain, bloating, nausea, and indigestion are signs of constipation[2]. Slow Transit Constipation (STC) is an endemic condition with a complex pathophysiology that involves multiple factors. Extreme constipation symptoms have been linked to myocardial infarction, sudden death, endocrine abnormalities, skin acne, and abdominal distension[3, 4]. Long-term use of solvent and stimulant laxatives can bring serious side effects, damaging the intestinal mucosa, nerves, and flora balance, leading to subsequent constipation[5, 6]. Functional foods are increasingly being used to manage constipation and enhance gastrointestinal function.
Kiwi berry (Actinidia argute), also called soft kiwi and kiwi pear is a perennial deciduous vine belonging to the Actinidiaceae family and Actinidia genus[7]. It is widely distributed in China, especially in the northeast area, and has the wealthiest resources[8]. Compared with the kiwi fruit, the appearance of small fruit skin is smooth and hairless, with no need to peel, and can be eaten whole[9, 10]. Ripe and fresh kiwi berry is tender and juicy with a unique flavor. It is not only nutritious but also rich in polysaccharides, polyphenols, anthraquinones, alkaloids, and other active ingredients, with anti-tumor, anti-virus, anti-lipid peroxidation, and other pharmacological effects[11]. Therefore, functional foods like kiwi berries, which have no side effects on human intestinal health, were selected to explore their mechanism of constipation prevention and alleviation.
Loperamide served as the model drug in this study. It is an effective antidiarrheal drug in common use. Excessive use however significantly inhibits intestinal peristalsis and causes constipation[12]. After modeling, it has been demonstrated that excessive loperamide can cause a large loss of fluid in the intestines of mice, and the stools became dry and hard to pass[13]. Defecation status (number of stool particles, water content) and colonic propulsion rate are vital indicators to measure the degree of constipation. The gastrointestinal (GI) tract is a complex enteric neural network with numerous enteric neurotransmitters, which strongly influence colon motility[14−16]. Enteric neurotransmitters are associated with intestinal motility and the transport of intestinal contents[17]. Motilin (MTL) and substance P (SP) are excitatory neurotransmitters promoting intestinal motility. Vasoactive intestinal peptide (VIP) is an inhibitory neurotransmitter[18, 19]. Enterochromaffin cells on the colonic mucosa express 5-hydroxytryptamine (5-HT), and when activated, 5-HT is released and promotes colonic motility[20]. Aquaporin-3 (AQP3) is one of the critical aquaporins, which involves the water absorption of the intestinal tract, and its overexpression will cause the colon to absorb too much water, making it difficult to excrete feces[21]. In order to investigate how kiwi berries influence gastrointestinal function and fecal parameters, loperamide was used to induce constipation in mice so that the researchers could conduct their research. At the same time, pertinent neurotransmitters and aquaporins were found in animals and cells, providing theoretical evidence for creating and using items related to kiwi berry-induced constipation management.
-
Loperamide (CAS number, 34552-83-5) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Sigma Aldrich was contacted in order to acquire activated carbon with the CAS number 7440-44-0 (St. Louis, MO, USA). The Nanjing Jiancheng Bioengineering Institute was visited in order to obtain ELISA kits for the following: SP, MTL, 5-HT, AQP3, and VIP (Nanjing, China). Roche supplied the TUNEL detective kit that was purchased (Basel, Switzerland). TRIpure was purchased from BioTeke (Beijing, China). M-MLV reverse transcriptase from the Beyonce II strain came from Beyotime Bio (Shanghai, China).
Preparation of kiwi berries
-
For the purpose of this investigation, Longcheng No. 2 kiwi berries were gathered from Dandong, which is located at 124°23' East, 40°07' North in China. The fresh weight of 200 g of kiwi berries were divided into two equal parts and utilized for the Kiwi group and the Loperamide + Kiwi group, respectively. The kiwi berry extract was obtained by vacuum microwave drying and ultrafine grinding. Based on previous studies, polysaccharides and polyphenols from kiwi berries were extracted[22,23]. The extraction rate of kiwi berry polysaccharide was 0.97%. The extraction rate of kiwi berry polyphenol was 1.92%.
Constipated BALB/c mice experiment
-
We obtained healthy male BALB/c mice from Liaoning Changsheng Biotechnology Co., LTD. [Certificate No. SCXK (Liao) 2020-0001, China]. The mice were 7 weeks old, weighed between 18 and 22 g, and were of SPF grade. Every mouse was kept in a room that was kept at a constant temperature of 25 °C and humidity of 50%. A full week was given to the mice to acclimate before the researchers carried out the subsequent tests and observations.
The mice were distributed at random among four distinct groups, totaling eight individuals. They were given the following treatments via oral gavage once a day for the duration of the two-week prevention phase: Control group (Con)-0.25 mL sterile saline; Model group (Lop)-0.25 mL 10 mg/kg loperamide; Kiwi berry group (Kiwi)-0.25 mL 300 mg/kg kiwi berry; Lop and Kiwi berry group (Lop + Kiwi)-0.125 mL. Every experimental procedure was carried out in a way that adhered to the guidelines suggested by the Animal Care Committee, which were made available to everyone. The Ethical Committee of Shenyang Agricultural University (Shenyang, China) approved all of the research projects at the university that included the use of animals for testing and observation.
Determination of constipation-related parameters
-
After two weeks, the weight of each mouse was checked at 9:00 in the morning. For the first 18 h, mice were only given water to drink. The powdered activated charcoal was administered to the mice as treatment. After that, one mouse was placed in each cage and each cage was placed in a clean environment. The amount of time that passed between the initial application of carbon powder and the first feces was noted. Within the first three hours following oral gavage, a number of fecal granules as well as the water content were found. Three hours were spent drying the fecal pellet in a 90 °C oven. The following formula was used to determine how much water was present in the final product:
$\begin{split}& \rm Water\;content\;of\;feces({\text{%}})\\=&\rm\frac{Wet\;weight\;of\;feces-Dry\;weight\;of\;feces}{Wet\;weight\;of\;feces}\times 100{\text{%}}\end{split} $ Determination of the GI transit rate with some minor adjustments was based on findings from earlier research[20]. After being gavaged with activated carbon powder, the mice abstained from food for 18 h. After an additional 60 min, the mice were euthanized so that the cervical dislocation procedure could be performed, and the entire small intestine was dissected. Both the distance that the carbon travelled through the small intestine and its total length were measured. The GI transit rate was calculated using the following formula:
$ \rm GI\;transitrate\;({\text{%}})= \frac{The\;distance\;that\;activated\;carbon\;travels}{length\;of\;the\;small\;intestine\;overall} \times 100{\text{%}} $ Sample collection
-
After the portion of the experiment that involved preventing constipation, fecal samples were taken from each mouse separately, placed in sterile polyethylene tubes, and chilled to a temperature of −80 °C. After the mice had been euthanised, their blood was drawn and centrifuged at 3,000 rpm for 15 min at 4 °C to produce serum samples for biochemical testing. Before performing biochemical testing, TUNEL labeling, and RT-qPCR analyses on colon tissue, it was first cleaned with sterile saline.
Biochemical assays
-
Using ELISA kits that are available on the market, we were able to detect the levels of the enteric neurotransmitters SP, MTL, 5-HT, AQP3, and VIP. In all tests, the procedures were carried out in triplicate and conducted in accordance with the manufacturer's instructions.
TUNEL staining
-
Apoptosis in colon cells was evaluated using the TUNEL test kit. After the tissue has been fixed, it was washed under running water for 4 h, the paraffin was removed from the sections, and the tissue rinsed with PBS three times for 5 min each time. After the piece has been air-dried, 50 μL of the TUNEL mixture was placed on it, covered with a cover glass, and put it in a dark area at 37 °C for 1 h. After that, cleaning with PBS was carried out, and finally drying. The samples were placed in the dark at 37 °C for 30 min after dropping them into 50 μL of DAPI for counterstaining. After a second round of washing with PBS, the sections were removed, and a fluorescent quencher was applied drop by drop while the slide was being mounted. Lastly, observations of the staining effect were made using a microscope (model BX53 from OLYMPUS, Japan), and the results photographed (DP73, OLYMPUS, Japan).
Experiment with interstitial cells
-
Four mice were chosen at random from the lop group to have their interstitial cells extracted by Cajal (ICC). The colon tissue was removed from the mice in the cell ultra-clean station after the mice were euthanised for cervical dislocation. The process of washing the tissue with a PBS solution resulted in the addition of 2 mL of an enzymatic hydrolyzed solution after the washing process was completed. Following that, the tissue from the colon was added. After 30 min of digestion at 37 °C with the I-type collagen enzyme, complete medium containing serum was added, a screen with a 200-mesh hole was used for filtering, and cell filtrate was obtained to be utilized in subsequent research. The cells were divided into four groups: normal saline added (Con), constipation (Lop), kiwi berry (Kiwi), mixture of Lop and kiwi berry (Lop + Kiwi), respectively. The content of each index were measured at 3, 6, and 12 h after adding the samples.
Levels of VIP and AQP3 in mice colon tissue and ICC cells
-
Using real-time quantitative RT-PCR, the levels of VIP and AQP3 in mice colon tissue and ICC cells were determined at 3, 6, and 12 h after the start of the experiment. Utilizing the TRIpure reagent, total RNA was extracted from colon tissue. BeyoRT II M-MLV reverse transcriptase was be utilized in order to convert the extracted RNA (500 ng) into cDNA. The ExicyclerTM 96 fluorescence quantifier from BIONEER Bio in Daejeon, South Korea was used for quantitative fluorescence analysis. The 2−ΔΔCᴛ method was utilized in order to compute the relative gene expression. The sequences of the primers used for AQP3 and VIP are presented in Table 1.
Table 1. Primer sequences.
Primer Forward primer Reverse primer Product size (bp) AQP3 5’-ACCCTGCCCGTGACTTTG-3’ 5’-ACACCAGCGATGGAACCC-3’ 130 VIP 5’-TTCACCAGCGATTACAGC-3’ 5’-GTCGTTTGATTGGCACAG-3’ 118 β-actin 5’-CTGTGCCCATCTACGAGGGCTAT-3’ 5’-TTTGATGTCACGCACGATTTCC-3’ 155 Data analysis using statistics
-
The presentation of all the data will remain as mean SD. GraphPad Prism 9.1.1 was used to perform all of the necessary calculations, which included a one-way ANOVA and Tukey's multiple comparisons test. The correlation investigation was carried out using Pearson's approach. A statistically significant result was regarded to have a p-value of less than 0.05.
-
The mice with constipation had significantly lower body weight (Fig. 1a), feces quantity (Fig. 1c), feces water content (Fig. 1d), and GI transit rate when compared to the control group (Fig. 1e). Additionally, the constipated mice had significantly extended first dark stool time (Fig. 1b). However, the mice treated with kiwi berry reversed these changes. The body weight growth of kiwi and lop + kiwi groups were increased by 148.2% and 112.8% compared with constipated mice, respectively. In comparison to the lop group, the amount of feces produced by the kiwi and lop + kiwi groups increased by 138.5% and 100.0%, respectively, while the fecal water content of the two groups increased by 106.5% and 82.6%. Furthermore, GI transit rates in the kiwi and lop + kiwi groups were increased by 45.3% and 33.9% compared with constipated mice, respectively, and first dark stool time of kiwi and lop + kiwi groups shortened by 57.5% and 37.3% compared with the lop induced mice, respectively.
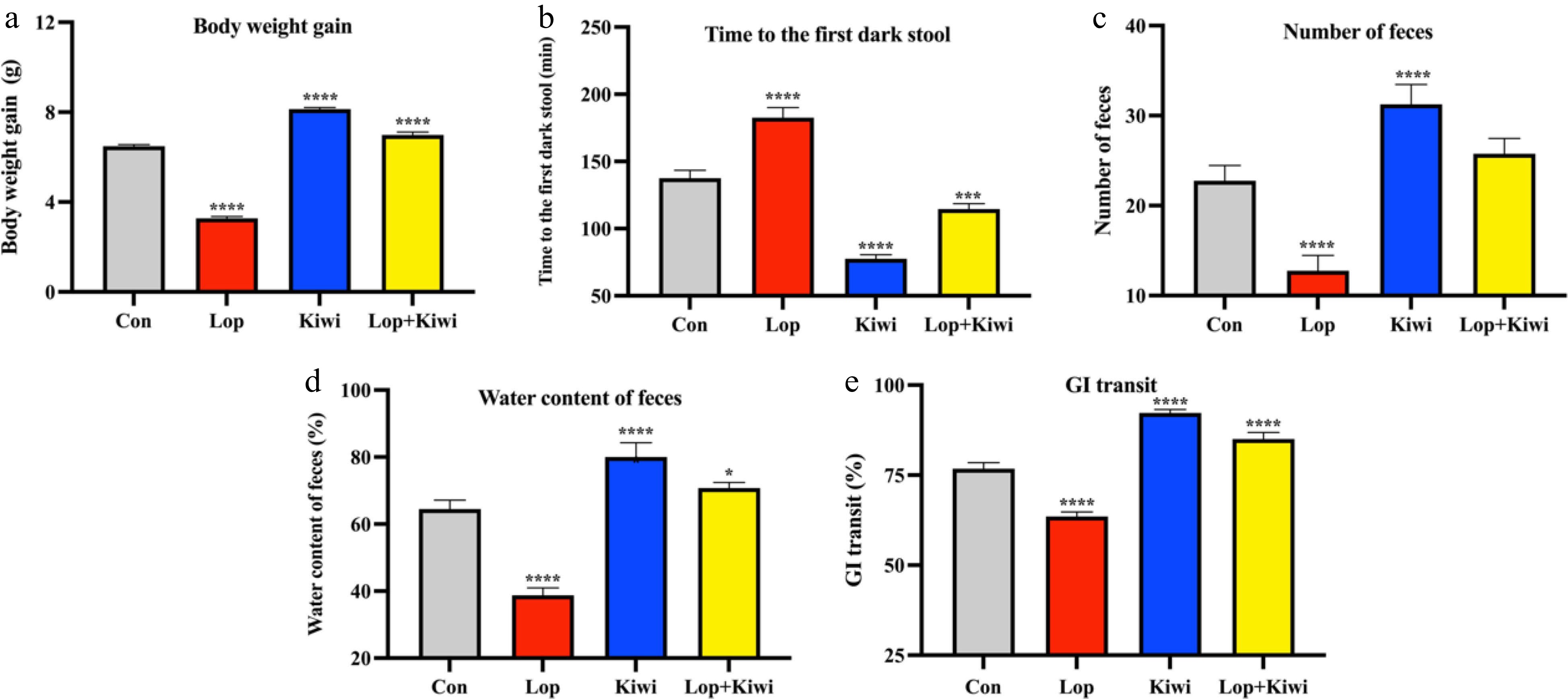
Figure 1.
Changes of constipation-related parameters by kiwi berry. (a) Body weight gain; (b) Time to the first dark stool; (c) Number of feces; (d) Water content of feces; (e) Gastrointestinal transit rate. Data were represented as mean ± SD. **** P < 0.0001, 0.0001 ≤ *** P < 0.001, 0.001 ≤ ** P < 0.01, 0.01 ≤ * P < 0.05, vs Con group.
Kiwi berry effects on neurotransmitter levels
-
In a further investigation on the kiwi berry's potential ability to prevent constipation, the neurotransmitter content of mice's serum and colon was measured. Figure 2 showed that loperamide drastically altered levels of gut hormones when compared to normal animals. Kiwi berries dramatically overcame this decline, raising levels of gastrointestinal hormones in the process. After 14 d, there are noticeable changes in neurotransmitter composition. At the SP content, there was a 69.4% increase in serum samples and a 35.8% increase in colon samples in the kiwi vs lop group (Fig. 2a1 & a2). Serum and colon samples from the kiwi group increased by 119.2% and 47.2%, respectively, at the content of MTL compared to the lop group (Fig. 2b1 & b2). When compared to samples taken from the kiwi group, those taken from the lop group showed a 51.4% and 69.6% increase in the amount of 5-HT found in their serum and colons, respectively (Fig. 2c1 & c2).
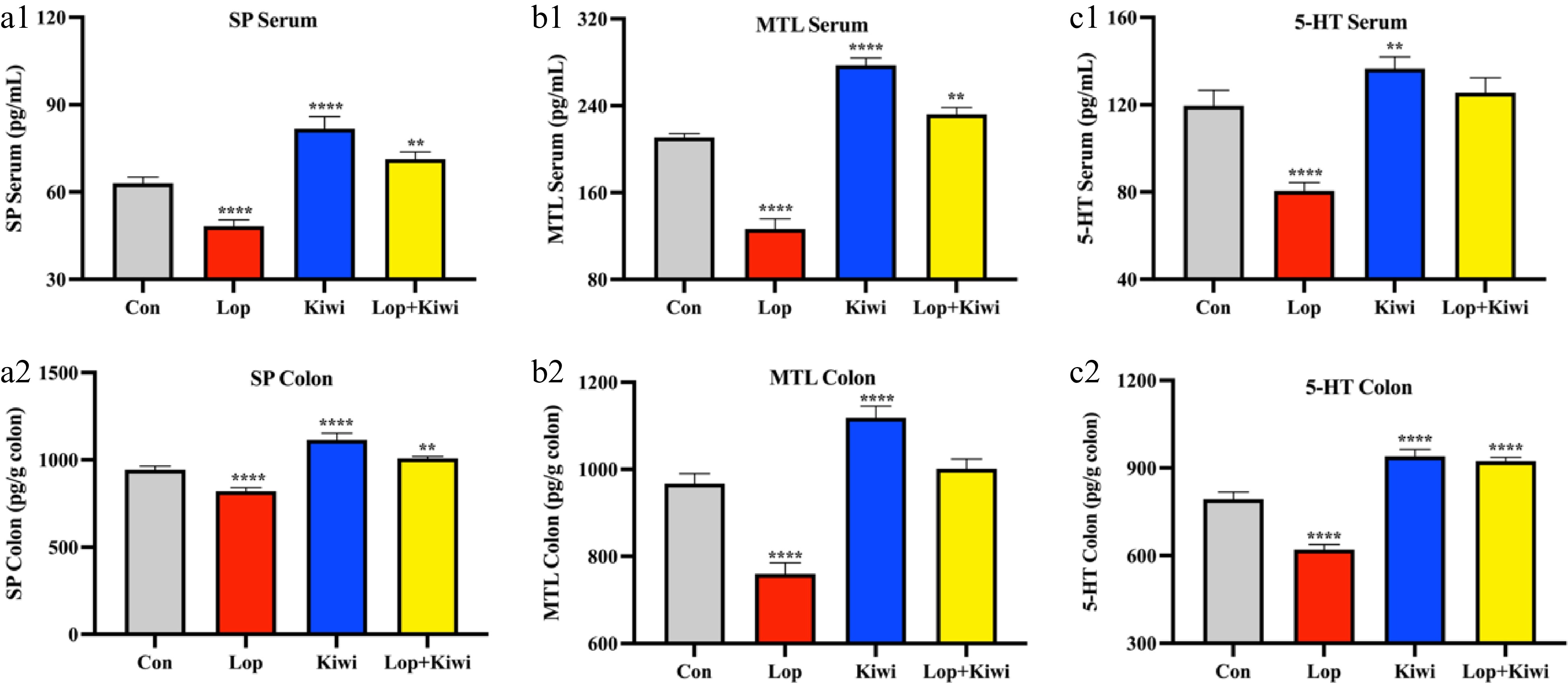
Figure 2.
Effects of kiwi berry on neurotransmitters. Levels of (a) substance P (SP), (b) motilin (MTL) and (c) 5-hydroxytryptamine (5-HT) in serum and colon tissue. Data were represented as mean ± SD. **** P < 0.0001, 0.0001 ≤ *** P < 0.001, 0.001 ≤ ** P < 0.01, 0.01 ≤ * P < 0.05, vs Con group.
Kiwi berry inhibited the loperamide-induced apoptosis of epithelial cells
-
TUNEL labeling was used to identify loperamide-induced apoptosis in the epithelial cells as well as any potential protective effects of kiwi berries (Fig. 3). In the lop group, there was a significant number of apoptotic cells (Fig. 3a). According to Fig. 3b, the rate of apoptosis was significantly lower in the kiwi group (95.3% lower) than it was in the lop group. The apoptosis brought on by loperamide can be successfully reversed by kiwi berries.
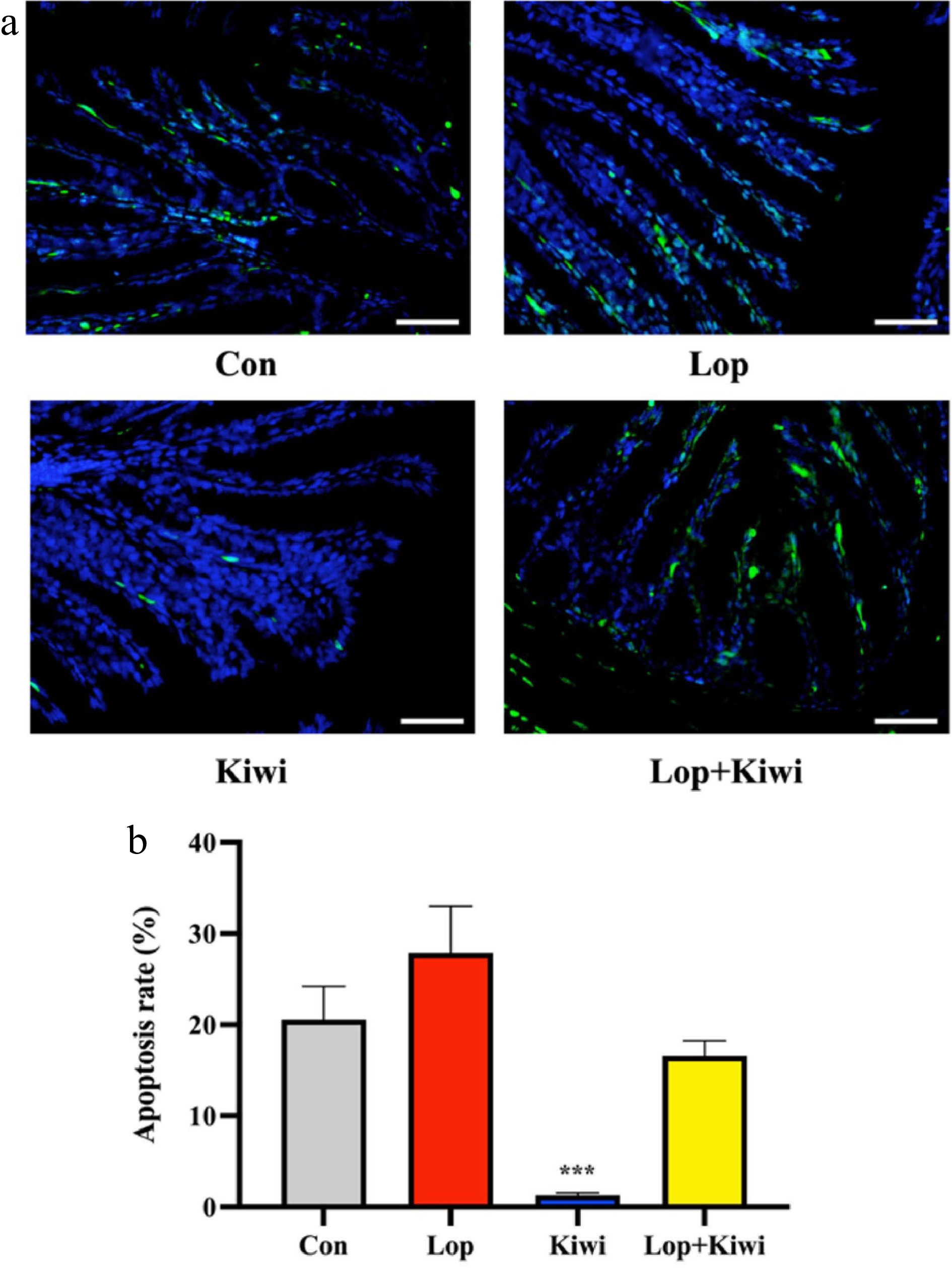
Figure 3.
Cell apoptosis in mice colon tissue. (a) Apoptotic cells showed green fluorescence (the non-apoptotic cells showed blue fluorescence, the pictures are displayed at 400× magnification, scale bar = 50 μm); (b) Apoptosis rate (%). Data were represented as mean ± SD. **** P < 0.0001, 0.0001 ≤ *** P < 0.001, 0.001 ≤ ** P < 0.01, 0.01 ≤ * P < 0.05, vs Con group.
Colon tissue AQP3 and VIP levels
-
In order to evaluate whether or not kiwi berries had any protective benefits against the intestinal tissue damage caused by loperamide, we analyzed the expressions of many key regulators associated to gastrointestinal moisture absorption and the nervous system. Loperamide markedly raised levels of AQP3 and VIP, as shown in Fig. 4a. Kiwi berries also drastically reduced VIP and AQP3 levels. In addition, the higher mRNA levels of AQP3 and VIP in the kiwi group saw a decrease of 65.8% and 41.0%, respectively, when compared to the constipated mice group (Fig. 4b).
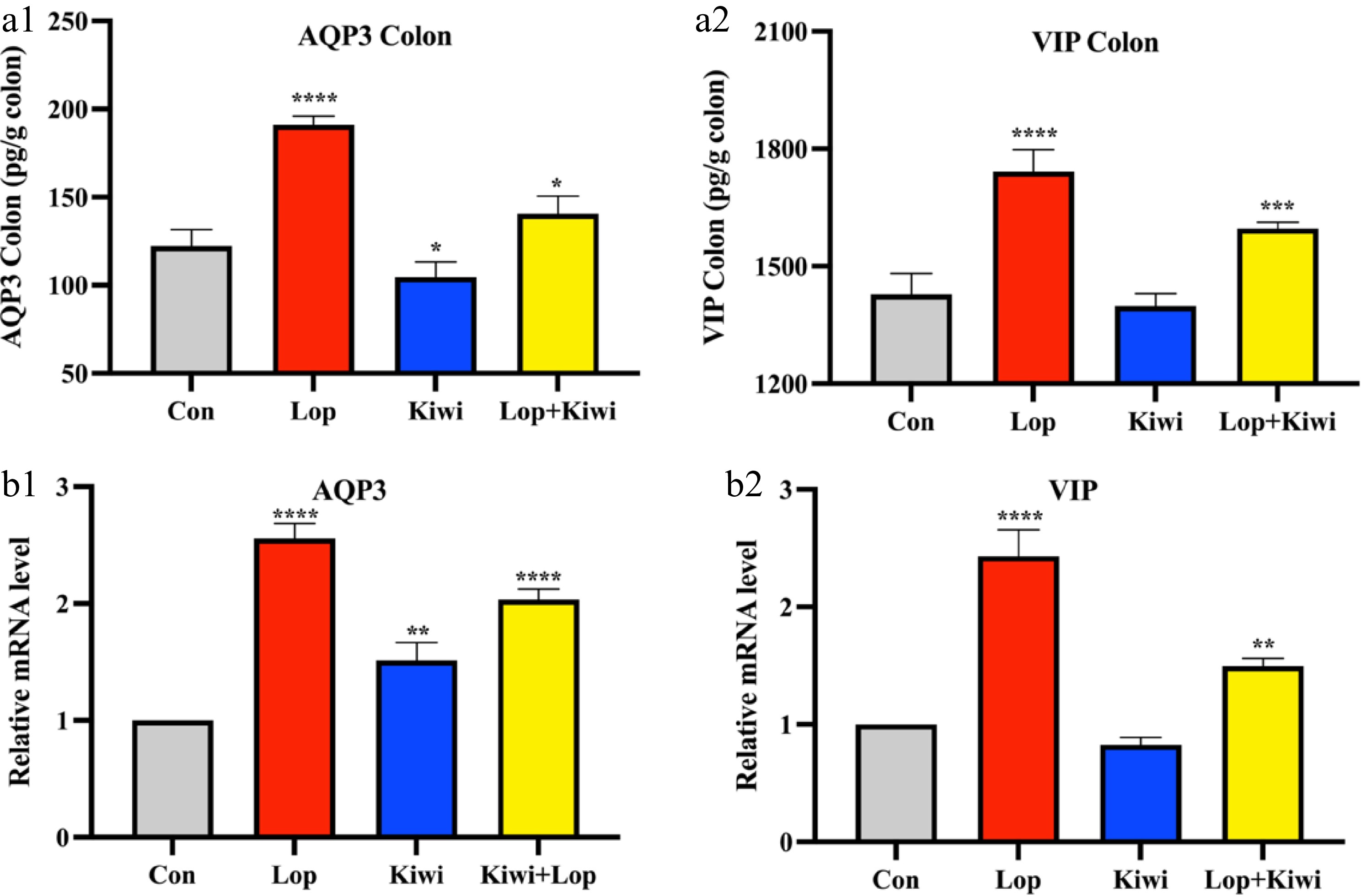
Figure 4.
Effect of kiwi berry on (a1) aquaporin-3 (AQP3) and (a2) vasoactive intestinal peptide (VIP) levels in colon tissue. Relative mRNA levels of (b1) AQP3 and (b2) VIP. Data were represented as mean ± SD. **** P < 0.0001, 0.0001 ≤ *** P < 0.001, 0.001 ≤ ** P < 0.01, 0.01 ≤ * P < 0.05, vs Con group.
Kiwi berry relieved constipation in an experiment conducted by the ICC
-
The findings of the ICC experiment showed that the consumption of kiwi berry reduced the amount of AQP3 (Fig. 5a) and VIP (Fig. 5b) in the body, hence relieving symptoms of constipation. During the ICC experiment, it was shown that the mRNA level of AQP3 in the kiwi berry group was considerably decreased compared to the control group in all three-time intervals (3, 6, and 12 h), but the level of AQP3 in the lop group was discovered to be raised. At the same time, the mRNA level of VIP in the kiwi berry group was considerably lower in 3, 6, and 12 h compared to the normal mice (P < 0.05). This was observed in all three time points (Fig. 5b).
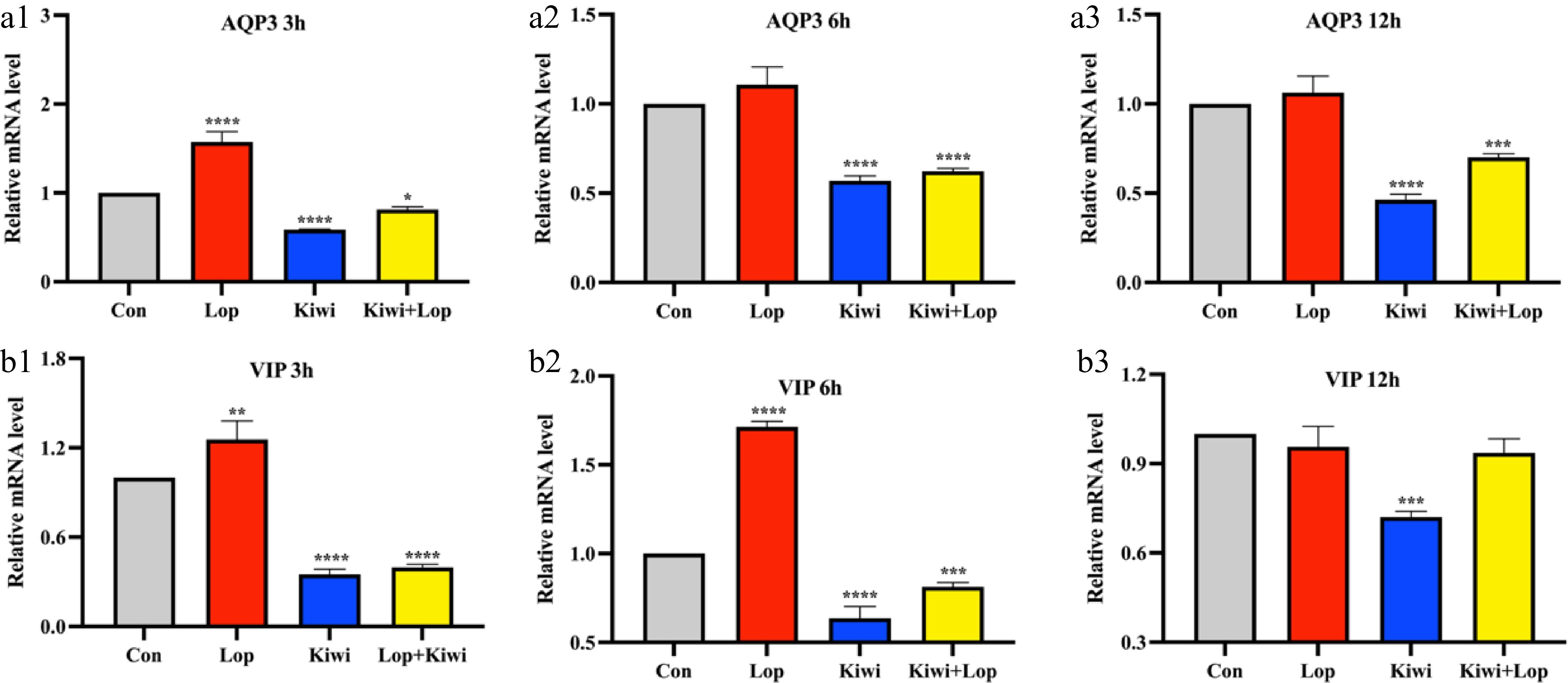
Figure 5.
Kiwi berry alleviated constipation by reducing AQP3 and VIP content. Relative mRNA level of (a) AQP3 and (b) VIP in interstitial cells of Cajal (ICC) experiments. Data were represented as mean ± SD. **** P < 0.0001, 0.0001 ≤ *** P < 0.001, 0.001 ≤ ** P < 0.01, 0.01 ≤ * P < 0.05, vs Con group.
Neurotransmitters and indicators of constipation are correlated
-
Performing research on how neurotransmitters and symptoms of constipation are connected. The estimated values of the Pearson correlation coefficient are displayed in Fig. 6. The levels of excitatory neurotransmitters in the serum and colon were found to have a positive correlation with bowel movements. It was shown that a negative correlation existed between defecation parameters and AQP3 and VIP in the colon.
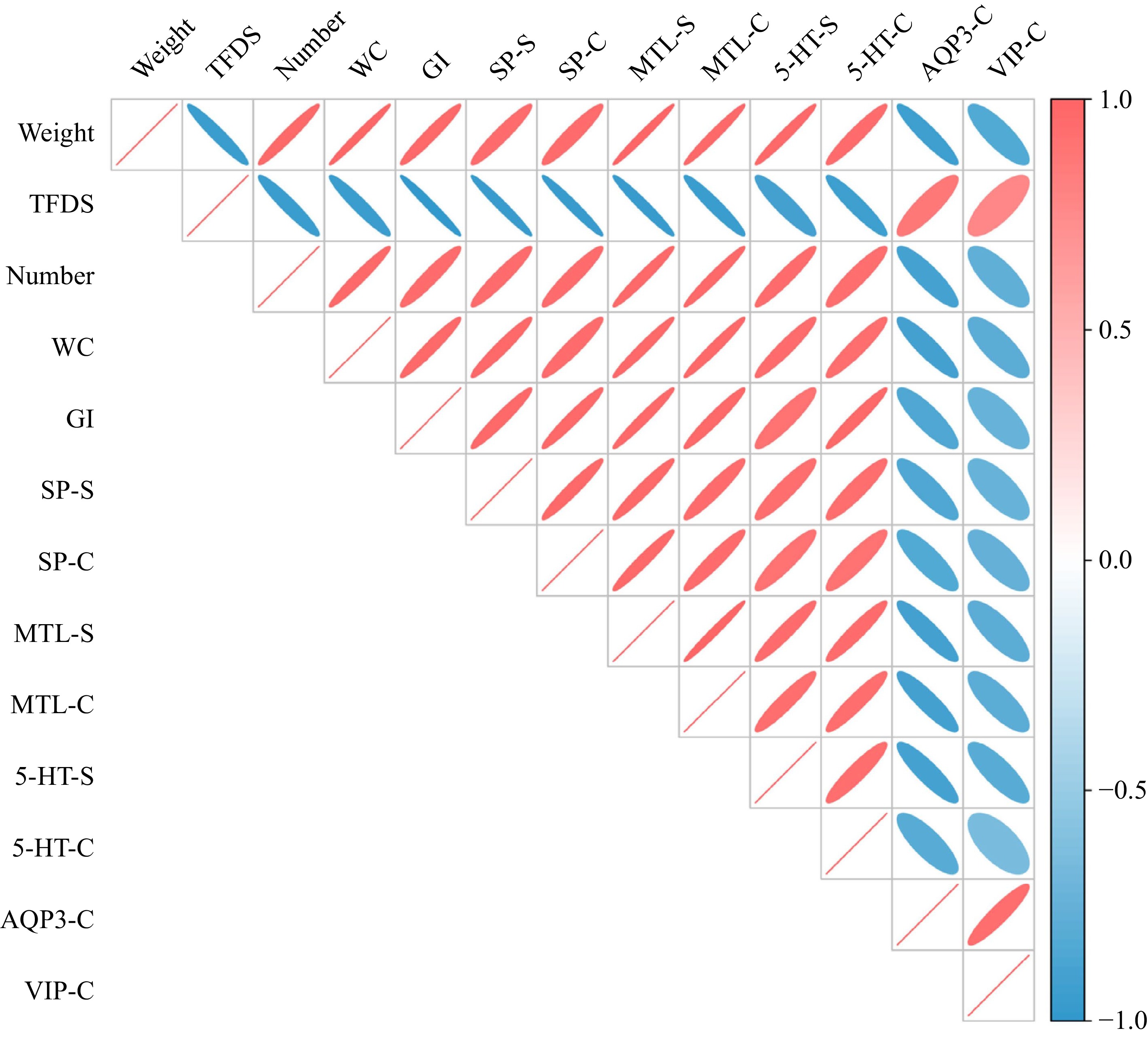
Figure 6.
Correlation between constipation-related indicators and neurotransmitters in animal experiment. (TFDS is time to the first dark stool, WC is water content of feces, GI is gastrointestinal transit rate, SP-S is SP level in the serum, SP-C is SP level in the colon). The pink and blue colors represent the positive and negative correlation between two indicators.
-
Constipated patients have slower bowel and stool movements, causing the water in the stool to be absorbed and subsequent hardening and drying of the feces[24]. Therefore, defecation time, defecation volume, and stool water content can all be used as the standard to measure constipation. GI rate is a significant index of intestinal action, which helps judge the degree of constipation in mice[17,25]. A higher GI rate presents a lower level of constipation. The study found that kiwi berries raised the weight, number, and water content of feces, meanwhile shortening first-time defecation compared with those of untreated mice. The consumption of kiwi berry, which is abundant in nutrients, has been demonstrated to stimulate growth as well as a large rise in body weight in mice[9]. At the same time, it substantially improves the fecal state and low intestinal propulsion rate to achieve a pleasing effect in relieving constipation.
Research has revealed that polyphenols have great qualities against inflammation and cancer, and the total polyphenol content of kiwi berries can reach up to 360 mg per 100 g of fresh weight[7]. Free-form phenolic compounds make up the vast majority of those found in fruits[26]. It is possible to lessen the amount of oxidative stress that happens within the body's cells and stop the onset of chronic disease through the fast release and uptake of free phenolics in the small intestine[27]. Kiwi berry, on the other hand, includes not only significant quantities of free phenolics but also bound phenolics in its composition. It has been demonstrated that the retention of these phenolics in the intestinal tract and stomach after absorption and their subsequent release into the colon as a result of bacterial fermentation has a positive impact on the regulation of gastrointestinal health as well as the prevention of gastrointestinal cancer[28]. In the research by Wang et al., it was found that providing a diet consisting of 0.75 g kg−1 L-arabinose to mice resulted in increased fecal weight as well as an increase in the number of fecal particles, in addition to boosting intestinal motility[29]. Apoptosis caused by oxidative stress can be prevented by using the treatment cyanidin-3-O-glucoside, which is beneficial for human health[30]. The findings of the current study are in line with the general direction that has been found in prior research, where it has been demonstrated that kiwi berries may considerably decrease the death of intestinal cells caused by loperamide and can restore intestinal health.
In recent years, there has been much research on the effect of neurotransmitters on constipation[31]. Currently, the most studied enteric neurotransmitters include SP, MTL, and 5-HT[32, 33]. Some investigations showed that the reductive expression of SP in the colon might lead to colonic relaxation and affect its normal peristalsis, thereby leading to constipation[34, 35]. 5-HT is a neurotransmitter and signal transduction factor that regulates gastrointestinal motility[36]. The concentrations of neurotransmission that were identified in the serum of healthy individuals were revealed to be substantially higher than those that were found in patients who were suffering from constipation, which is in keeping with the findings of past studies[37, 38]. Based on previous studies, the content of neurotransmitters in colon tissue was also measured. In a similar study, mice with constipation were fed different amounts of konjac mannooligosaccharides, and blood levels of MTL and SP were shown to be higher in these mice. However, there was no discernible difference in VIP levels between the two groups of mice[39].
For the analysis of inhibitory neurotransmitter VIP, both animal and ICC experiments were performed. There have also been many studies on VIP and AQP3 with similar results to this study[40, 41]. Adding 10% and 15% High Specific Volume Polysaccharide to the basal diet can significantly reduce the protein expressions of VIP in the constipation model group[40]. Lactulose reverses loperamide-induced increase in AQP3[41]. Allium mongolicum Regel and its flavonoids can down-regulate AQP3 in constipated mice and have a positive effect on regulating intestinal water metabolism[42]. It has been reported that epithelial cells are an essential part of the colon, and their apoptosis contributes to colon health and integrity to a reasonable extent[43,44]. In disease states, the normal processes of apoptosis and growth of epithelial cells are disrupted. As a consequence, colonic propulsion is reduced, and insufficient stem cell components are secreted; this makes the barrier to colonic propulsive action even more formidable[45].
The term 'brain-gut axis' primarily refers to the connection that exists between neurology and the gastrointestinal system. This connection is currently receiving a lot of attention in the field of research on chronic disorders[46, 47]. This work was motivated by the previous research on the 'brain-gut axis,' and it built a novel 'animal-ICC cell community' axis method to evaluate the effects of preventing and relieving constipation in mice. Previous research on constipation mainly concentrates on relief after the disease. However, the best way to avoid constipation is prevention, so this study also studied kiwi berry's prevention effects on constipation. Therefore, it is significant to study the mechanism of preventing and relieving constipation by kiwi berry, which has biological activity and no side effects. These results can prove that the kiwi berry can be used as an alternative strategy to regulate intestinal health and promote the development of characteristic functional berries.
-
This study showed that kiwi berries have both preventative and relieving benefits on rats with constipation. Using kiwi berries can help improve the health of the mice's feces and can also assist with the peristalsis of the intestinal tract. The excitatory neurotransmitter concentrations in the serum and colon of the kiwi berry-treated animals were substantially greater than those observed in control mice. On the other hand, the levels of VIP and AQP3 showed the opposite trend in the mRNA assays. In addition, kiwi berries can control the apoptosis of intestinal epithelial cells, which is good for the health of the intestines. All of these findings demonstrate that kiwi berries have positive preventive and relieving effects on constipated mice and that they can be utilized as a dietary supplement to improve intestinal function as well as a promising dietary treatment for constipation.
-
All experimental procedures were conducted according to the guidelines provided by the Animal Care Committee. The Ethical Committee approved all experiments for the Experimental Use of Animals at Shenyang Agricultural University (LACUC Issue No: 2022030315, Approved Date: 2022.03.03).
The First Batch of Liaoning 'Unveiling Leader' Scientific and Technological Projects (2021JH1/10400036); China Agriculture Research System of MOF and MARA(CARS-29); Liaoning Province food processing chief science and technology special commissioner (2022JH5/10400125)
-
The authors declare that they have no conflict of interest. Bin Li is the Editorial Board member of Food Innovation and Advances. He is blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and his research groups.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhang J, Li D, Tian Q, Ding Y, Jiang H, et al. 2023. The effect of kiwi berry (Actinidia arguta) on preventing and alleviating loperamide-induced constipation. Food Innovation and Advances 2(1):1−8 doi: 10.48130/FIA-2023-0001
The effect of kiwi berry (Actinidia arguta) on preventing and alleviating loperamide-induced constipation
- Received: 25 August 2022
- Accepted: 24 November 2022
- Published online: 31 January 2023
Abstract: This research aimed to study the preventive and relieving outcomes of kiwi berry on constipation. The administration of kiwi berries to mice resulted in a significant increase in body weight gain of 148.2% compared to mice that were constipated. The number of stools and the water content of stools both increased by 138.5% and 106.5%, respectively. The gastrointestinal transit rate increased by 45.3%, and the time it took for the first dark stool to form decreased by 57.5%. The levels of the excitability neurotransmitters were found to be higher in the group that had been given kiwi berries in comparison to the group that had been given loperamide. The opposite results were produced by vasoactive intestinal peptide (VIP) and aquaporin-3 (AQP3). In addition, kiwi berry consumption may lessen epithelial cell apoptosis and promote colon health. All the results point to kiwi berries as an extremely promising food supplement for the prevention and relief of constipation in the future since they successfully prevent and alleviate constipation brought on by loperamide.
-
Key words:
- Kiwi berry /
- Constipation /
- Gastrointestinal transit /
- AQP3 /
- VIP /
- Neurotransmitter












