-
Lifestyle changes and reduced time availability for traditional cooking are increasingly driving consumers towards pre-prepared foods directly from the retail stores[1]. At the same time, antioxidants and anti-inflammatory-rich foods are becoming a necessity for maintaining a healthy lifestyle[1−3]. Therefore, there is a need to provide consumers with nutritious food that requires less time for preparation. Ready-to-eat, minimally processed, shelf-stable, and nutritious food products are some of the alternatives to traditional cooking[1].
Besides being convenient, requiring no refrigeration, and needing only a few minutes for preparation from stove-top to plate, shelf-stable packaged foods can reduce food waste, especially in developing countries that lack adequate storage facilities. Moreover, shelf-stable foods are ideal for usage for army rations[1,4]. However, the shelf-stable foods that are currently available commercially have lower nutritional and sensorial qualities compared to fresh or refrigerated products.
Traditional retort processing is being able to produce shelf-stable foods through thermal inactivation of pathogenic bacterial spores. However, the lengthy exposure of food products to high temperature during these thermal processes can cause severe losses of heat sensitive nutrients, and negatively impacts the rheology, texture, color, aroma, and taste of the products[5−7]. Advanced food processing techniques such as the microwave-assisted thermal sterilization (MATS) process sharply reduce the processing time to achieve the required thermal lethality to target bacterial spores[4,5,8], resulting in a minimal loss of quality and nutritional value while retaining a higher level of taste, flavor, and consumer satisfaction[1,2,5]. However, since the technology is novel, there is a lack of research data pertaining to the effects of MATS processing on quality parameters like anthocyanins and encapsulated vitamins, necessitating a more detailed study.
Along with optimized processing, high barrier packaging is essential for retaining the quality attributes and extending the shelf life of thermally sterilized foods in storage at ambient temperature. MATS is incompatible with traditional metal-based high barrier flexible packaging such as aluminum foil[4]. High barrier multilayer films with a metal oxide (MO)-coated barrier layer are compatible with MATS as well as with the traditional retort processes[4,9−13]. MO coatings of aluminum and silicon oxides (AlOx, SiOx) can significantly lower oxygen and moisture permeation of multilayered polymer films used for packaging shelf-stable food products[11,12,14−17]. Along with thermal processing and packaging, light exposure can also adversely impact the shelf life of packaged foods[18,19]. Since shelf-stable products are intended to have an extended shelf life, there is a need to understand how light can affect the product quality during storage, especially in commercial grocery stores. In addition, certain shelf-stable products are fortified with vitamins before thermal processing to mitigate the heat-induced losses[8,15]. This is done by adding non-encapsulated vitamins to the packaged foods prior to processing[8,15]. Encapsulation is a promising method that may potentially enhance the retention of heat-susceptible nutrients and vitamins in packaged foods[9]. However, there has been no data available to assess the efficiency of the encapsulation process on retention of nutrients in packaged foods processed with MATS, necessitating a thorough investigation in that regard.
This research was conducted with three primary objectives: (1) to assess the barrier performance of multilayer packaging after MATS process; (2) to investigate the influence of packaging barrier properties and light exposure on quality attributes of purple mashed potatoes during prolonged storage; and (3) to understand the impact of MATS processing and storage on the encapsulated vitamin C (EVC) and nonencapsulated vitamin C (NVC) in purple mashed potatoes. To achieve the above-mentioned objectives, purple mashed potatoes were prepared, packaged with two high barrier multilayer polymer packaging, processed via a pilot-scale MATS system, with accelerated storage at 37.8 ± 0.2 °C for seven months. Samples were extracted at the end of each month of storage for measuring weight loss, color, anthocyanin, total phenolic content, and vitamin C retention.
-
The recipe used for preparing the purple mashed potatoes consisted primarily of pure dried purple potato powder (Fluxias GmbH, Germany) and potato flakes (Idahoan Original Mashed potato, Idaho Falls, ID, USA). The color of the potato flakes and potato powder were white and purple, respectively. Other ingredients, including cream cheese (Kraft, Philadelphia), non-iodized salt (Morton, IL, USA) and vegetable oil (Crisco, OH, USA), were purchased from the local Walmart store. This recipe was fortified with encapsulated (proprietary formulation designed to withstand high heating conditions) vitamin C (EVC) (Glanbia, ID, USA) and non-encapsulated vitamin C (NVC) (Now Foods Co., Bloomingdale, IL, USA). We procured Vitamin C from BioXtra (Purity >99%). Meta-phosphoric acid was supplied by Sigma-Aldrich (St. Louis, MO, USA), while potassium phosphate was manufactured by Fisher Scientific (Fair Lawn, NJ, USA). At the same time, butylated hydroxytoluene was provided by Acros Organics (Geel, Belgium), and lastly, the manufacturers for hexane, ethanol, acetone, and phosphoric acid used in the study was Avantor Performance Materials (Center Valley, PA, USA).
Preparing purple mashed potatoes
-
The formulation for purple mashed potatoes was originally designed for the US Army Natick Soldier Centre (Natick, MA, UA). Antioxidant-rich diets have been associated with a lower incidence of heart disease and certain cancers[20]. According to previous studies demonstrating the nutritional properties, purple potatoes contain 9–38 mg of anthocyanin per 100 g (FW) and 20 mg per 100 g FW of the vitamin C, both of which contribute towards increasing the antioxidant properties of purple flesh potatoes than potatoes without pigments[20]. For preparing the purple mashed potatoes for this study, at first salt was mixed thoroughly in 12 L distilled water, followed by non-encapsulated vitamin C (NVC). Both were mixed for 5 min in a Hobart mixer (Hobart GmbH, Offenburg, Germany). In the next step, purple potato powder was thoroughly mixed followed by cream cheese and oil to form a smooth slurry. Once all the above-mentioned ingredients were thoroughly blended, the potato flakes were added and mixed for 15 min. The time of mixing was determined based on the texture of the mashed potatoes, which needed to be smooth. The same process was followed to make an EVC-fortified batch of purple mashed potatoes.
Packaging materials and filling conditions
-
Two types of transparent multilayer polymer pouches were used in this study. Their structural information is provided in Table 1. The organic coating acted as the primary barrier layer in this film. An aluminum foil-based pouch (Al-Foil) (KSM Enterprises, Gig Harbor, WA, USA) was also used for packaging one set of TLMO pouches. This was done immediately after MATS processing and these pouches served as control in this study. The pouches were filled with 230 g of purple mash potatoes each. After filling, they were degassed two times at 0.6 bar for 10 s. Subsequently, the pouches were vacuum sealed (vacuum level: 99%) using Easy-Pack (UltraSource, LLC. Kansas, MO, USA) at 180–200 °C for 3–5 s.
Table 1. Details regarding the package label, structure, dimensions (length × width), and thickness of multilayer pouches studied.
Package Structure Dimensions (L × W)(cm) Total thickness (µm, n = 5) TLMO AlOx-coated PET (12 µm)//AlOx-coated PET (12 µm)//
AlOx-coated PET (12 µm)//
ONy (15 µm)//CPP (70 µm)18.5 × 14 121 ± 0.8 PAA PET (12 µm)//organic coating (1 µm) // ONy (15 µm)//CPP (60 µm) 18 × 13 95 ± 0.4 AL-Foil PET (12 µm)//aluminum (9 µm)//nylon (15 µm)//PP (80 µm) 21 × 12 120 ± 0.1 ONy and CPP indicate biaxially oriented nylon 6 and cast polypropylene, respectively. Microwave-assisted thermal sterilization (MATS)
-
The microwave assisted thermal sterilization processes were conducted by using a single-mode, pilot scale MATS system (25 kW, 915 MHz). The processing conditions were developed as described previously[3,5,8,21] and an F0 = 10.7 min was obtained. Since the MATS system is incompatible with metallic packaging, one set of TLMO pouches were processed and subsequently vacuum-packed into aluminum foil-based packaging to serve as the control.
Storage and light treatment
-
For accelerated shelf-life studies, all the pouches (TLMO, PAA, Al-Foil) were studied by placing them in a closed incubator (KB055, Darwin Chambers, Saint Louis, MO, USA) at 37.8 ± 0.2 °C for 7 months to simulate the storage of 3.5 years at room temperature (22 °C)[5,8]. To measure the effect of light on the product quality, 12–15 TLMO pouches were stored under LED lights in a flat manner in a separate incubator set as same temperature conditions. The light sources were placed at the top of the incubator. The pouches were first exposed to the light on the top surface for 15 d and then were turned so that back side of the pouches could be exposed to the same duration of light. These lights were bought from a local store (Walmart, Pullman, WA, USA) with 700 lumens and 5000 K daylight correlated color temperature. The distance between the lights and the pouches was 12–15 in. Anthocyanin content, total phenolic content, vitamin C content, and sample color were measured over the storage period.
Barrier properties
-
The oxygen and moisture transmission rates (OTRs and WVTRs) of the pouches were measured using a Mocon Ox-Tran 2/21 MH and Permatran 3/33 MG model (Modern Control, Minneapolis, MN, USA), respectively. OTRs of the pouches was measured (n = 2) at 55% RH, 23 °C, and 1 atm pressure, as per ASTM F 1927 while we measured the WVTRs at 100% RH, 38 °C and 1 atm (ASTM F 372-99)[8,12,13,22].
Weight loss
-
The physical and chemical qualities of packaged food are often influenced by weight or moisture loss through polymer packages during storage[8,23]. In this study, the initial weight of two types of packages (TLMO and PAA) was recorded immediately after MATS processing, and weight loss during storage at 37.8 ± 0.2 °C was monitored using a GK703-model balance scale (Sartorius, Gottingen, Germany) for 7 months (n = 3).
Measurements of color
-
The L*, a*, and b* of the samples were measured using a spectrophotometer (CM-5 spectrophotometer, Konica Minolta,Ramsey, NJ, USA). ΔE, the total color difference in the samples, was calculated based on equation 1[8]:
$ \Delta E=\sqrt{{\left(\Delta {L}^{*}\right)}^{2}+{\left(\Delta {a}^{*}\right)}^{2}+{\left(\Delta {b}^{*}\right)}^{2}} $ (1) Quantification of anthocyanin content
-
We measured the amount of anthocyanin present in the samples using a spectrometer at 530 nm. Three grams of sample were taken out from the packaged purple mashed potatoes and homogenized with 10 mL acidified methanol (1% HCl in methanol) using a Polytron PT 2500 E homogenizer (Kinematica, Bohemia, NY, USA) in a 50 mL tube for 2 min at 7,000 rpm. A Whatman No. 4 filter paper was used to vacuum filter the homogenate and the precipitate was further homogenized with 10 mL of solvent for 30 s. The extract from both extractions was combined, diluted to 25 mL in a volumetric flask. The extract was diluted by a factor of 1:1 using acidified methanol solution; the absorbance was measured at 530 nm and the anthocyanin content was calculated using equation 2.
$ Total \;monomeric \;anthocyanin \;content \;(mg/L) = \frac{A\times {M}_{\omega }\times DF\times 1000}{\varepsilon \times L} $ (2) where A indicates absorbance, Mw indicates molecular weight, while DF, L and ε indicate the dilution factor, path length (cm), and molar extinction coefficient, respectively. We quantified the total anthocyanin content by considering cyanidin-3-glucoside as the prominent anthocyanin (molecular weight: 449.2 g/mol, molar extinction coefficient = 26,900 L/cm/mol).
Quantification of total phenolic content (TPC)
-
Total soluble phenolic content (TPC) analysis was adapted from[24,25]. One gram of sample was homogenized with 80% methanol and water solution at 7000 rpm for 2 min. Subsequently, the sample was centrifuged at 8000 rpm for 6 min. The homogenate was vacuum filtered using filter paper (Whatman No. 1) and the supernatant was again homogenized in a mixture of 10 mL methanol and water for 30 s. Samples from both the extractions were combined and diluted into 25 mL. Meanwhile, Folin-Ciocalteu reagent was prepared using 5 mL FC, 95 mL water and 7.5 g Na2CO3 in 100 mL water. One mL supernatant was mixed with 5 mL of FC reagent solution and 4 mL Na2CO3 solution and incubated for 1 h at 25 °C. Spectrophotometric readings at 725 nm were acquired and the total phenolics were expressed as mg chlorogenic acid equivalent per 100 g dry weight basis on a standard curve[25].
Quantification of vitamin C
-
This study investigated the effects of thermal processing, packaging barrier properties, and light on the efficiency and retainability of the vitamin C content in the purple mashed potatoes. The vitamin C was quantified as described previously[15].
Data analysis
-
The data was analyzed using Tukey's HSD using Origin 2021 at α = 0.05 to determine the significant differences. Samples were extracted from different packages for analysis unless otherwise mentioned and tested in triplicate.
-
Tables 1 & 2 shows the structure, dimensions, thickness, and the OTRs, and WVTRs of the two films before and after MATS processing and 7 months of storage at 37.8 ± 0.2 °C. The TLMO films with three MO-coated PET layers did not have any detectable OTRs before and after MATS processing (Table 1). At the same time, PAA films with poly acrylic acid (PAA) barrier coating had an OTR of 0.21 cc/m2·day before processing. This value didn't change significantly (P > 0.05) after MATS processing. This reconfirmed the gentle effect of MATS processing on the flexible barrier films. After 7 months of storage, the OTRs of TLMO and PAA increased to 0.34, and 0.29 cc/m2·day, respectively (P < 0.05).
Table 2. Oxygen Transmission Rate (OTR) and Water Vapor Transmission Rate (WVTR) of polymer pouches before and after MATS processing and after storage at 37.8 ± 0.2 ºC.
Pouch type OTR
(cc/m2·day)WVTR
(g/m2.day)Before processing After processing After storage
at 38ºC/ 7 monthsBefore processing After processing After storage for
38 ºC/ 7 monthsTLMO ND ND 0.34 ± 0.00AB 0.13 ± 0.01aA 0.32 ± 0.08cB 0.23 ± 0.03bB PAA 0.21 ± 0.01ab 0.19 ± 0.03a 0.29 ± 0.12bA 5.92 ± 0.08aB 12.5 ± 0.89bA 12.6 ± 0.29bA Values are presented as Mean ± SD. Different lowercase and uppercase superscripts show significant differences (P < 0.05) between values within rows and columns, respectively. ND: Not detectable since the value is lower than the instrument’s detection limit. ONy and CPP indicate biaxially oriented nylon 6 and cast polypropylene, respectively. AlOx or SiOx coatings deposited on PET or PP substrates can reduce the oxygen permeation, helping to maintain the quality of the packaged food products[12,26,27]. However, the occurrence of cracks and pinholes upon exposure to thermal sterilization processes such as MATS can reduce ability of the coatings to successfully prevent oxygen permeation[12,21,28]. As a countermeasure, researchers have used multilayer films containing more than one barrier layer[12,21]. If one layer develops defects, the presence of other layers can effectively prevent the oxygen molecules from diffusing through the packaging into the food[12,21]. It has been observed that unless a crack or defect is present across all the coated PET layers, very limited oxygen permeation occurs through the multilayered structure[12,21]. This could be the reason that the TLMO films showed no detectable oxygen permeation in the unprocessed state as well as after MATS processing. At the same time, poly acrylic acid forms crosslinks in the presence of moisture and heat, both of which are present during the MATS processing[11,12,29]. The resulting crosslinking limits the oxygen and moisture permeation and thus reduces the impact of thermal processing on the barrier performance. The coated films can also be susceptible to mechanical stress-induced cracks and pinholes. This can be a cause of concern, since the exposure to thermal processing tends to make the polymer films brittle due to moisture absorption during processing and results in changes in the crystallinity in each of the polymer layers that constitute the multilayer structure[11]. In this study, mechanical stress-induced changes could be one of the reasons for the PAA films to have shown a lower OTR than the TLMO films after storage.
In terms of the moisture barrier properties of the films, a significantly better performance was observed in TLMO than PAA. The TLMO films showed a lower water vapor transmission rate of 0.13 g/m2·day. With exposure to the thermal processing, the films' WVTRs increased 2.5 times. Furthermore, storage for 7 months at 37.8 ± 0.2 °C after MATS processing led to a 77% increase in WVTRs of TLMO films in comparison to their pre-processing WVTRs. Concurrently, organic coated (PAA) films had a very high-water vapor transmission rate of 5.92 g/m2·day in the unprocessed condition. This increased by 1.11 times to 12.5 g/m2·day and this stayed at the same level even after the storage of 7 months at 37.8 ± 0.2 °C. WVTRs of multilayer structures with coated PET layers can be dependent on the occurrence and density of the defects in the primary coating layer as well as the interaction between the water molecules and the coating present[11,12,28,30]. Prior research has shown that multilayer films with a PAA barrier layer had a higher WVTR than the films containing inorganic coating[11,12]. This was due to the hydrophilic polyacrylic acid layer, which may have caused the PAA films to have a higher WVTR than the TLMO films. Overall, both the films provided a highly effective barrier against oxygen but TLMO films were better in preventing moisture permeation.
Weight loss
-
The weight of the samples in TLMO pouches did not change significantly (P > 0.05) during storage, while that in PAA decreased significantly (P < 0.05) (Fig. 1). The samples in PAA decreased by 60 g (25.3%) in their weights by the end of the storage at 37.8 ± 0.2 °C. In the purple mashed potatoes packaged with TLMO pouches, a weight change of 1.45 g was observed during the storage. The weight loss in polymer pouches containing shelf stable foods has been directly related to the water vapor transmission rates (WVTRs) of the respective films[3,5]. The packaging's water vapor transmission rates were directly proportional to the weight lost by the samples during storage[5,12,22,29]. TLMO films effectively protected the packaged samples against moisture loss.
Color
-
Visual color changes in purple mashed potatoes after processing, during storage, and under light treatment can be seen in Fig. 2. Before MATS processing, the color of untreated sample was L* = 27.39 ± 0.4, a* = 13.01 ± 0.14 and b* = −5.34 ± 0.14. After MATS processing, the color parameters for AL & TLMO, and PAA pouches were L* = 27.37 ± 0.57, a* = 14.05 ± 0.13, b* = −2.36 ± 0.17 and L* = 26.14 ± 0.23, a* = 12.76 ± 0.38, b* = −1.54 ± 0.20, respectively. Since the samples in aluminum foil packaging were basically TLMO pouches packaged inside the aluminum foil-based pouches, their color parameters were the same as the TLMO samples on day 0. The total color change in the purple mashed potatoes packaged in aluminum foil pouches increased significantly (P < 0.05) from a value of 3 right after the MATS process, to 15 after 7 months storage at 37.8 ± 0.2 °C (Fig. 3). Similarly, the total color change in the products packaged in TLMO pouches increased significantly (P < 0.05) from 3 to 16 while the total color change in the purple mashed potatoes packed into PAA pouches increased from 4 to 14 after storage (P < 0.05). The change in the ΔE was higher in TLMO pouches than that in the PAA pouches.

Figure 2.
Color change in the purple mashed potatoes samples with light exposure and storage at 37.8 ± 0.2 °C for 7 months packaged in the AL-Foil, PAA and TLMO pouches.
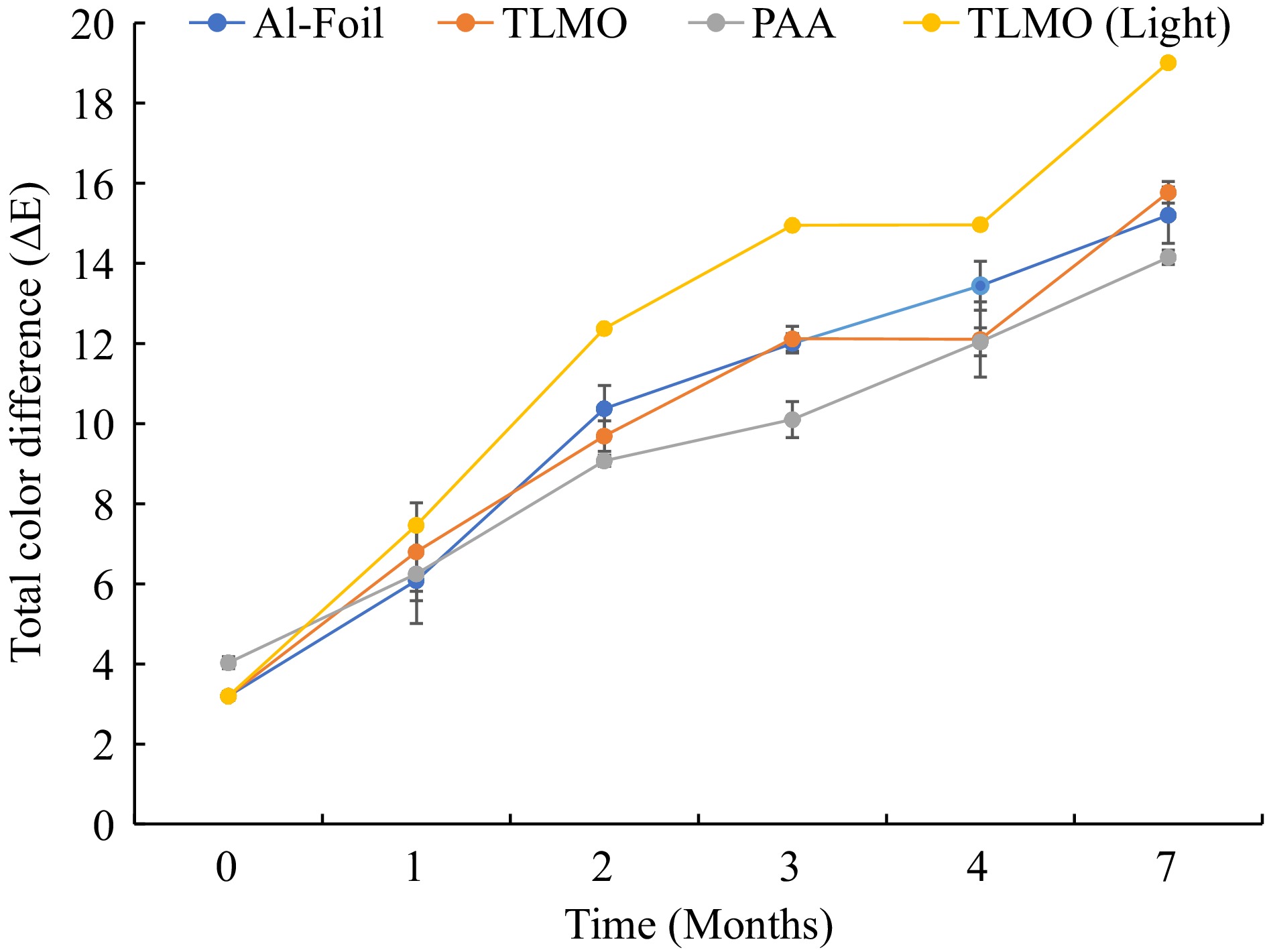
Figure 3.
Changes in the total color difference (ΔE) in the packaged purple mash potatoes with and without light exposure during storage for 7 months at 37.8 ± 0.2 °C
The purple color in the purple mashed potatoes is due to the presence of the anthocyanin in the samples, specifically the mono or diacylated peonidin and cyanidin[31]. Anthocyanin is one of those food pigments that is highly sensitive towards oxygen and light-induced deterioration[24]. In this study, it was observed that the samples packaged in TLMO films showed higher color change compared to PAA. This correlated well with the oxygen transmission data obtained after MATS processing and during storage. It was observed that the TLMO films did not have any oxygen permeation after exposure to MATS but showed OTRs of 0.34 cc/m2·day after 7 months of storage at 37.8 ± 0.2 °C. Similarly, PAA had OTRs of 0.19 and 0.21 cc/m2·day after MATS and storage, respectively. It has been observed that oxygen can significantly affect the color and other physicochemical properties of purple mashed potatoes. The oxygen permeation during storage through MATS processed polymer pouches containing purple mashed potatoes has known to increase the ΔE causing browning in the samples[8]. Hence, the lower oxygen permeation in the PAA at the end of the storage could be one of the reasons why the total color change was lower in the samples packaged into these pouches.
In previous studies, with a higher moisture loss through packaging, the processed food samples showed more lipid oxidation, resulting in a higher browning index (BI) and higher ΔE[3,32]. PAA had significantly higher WVTRs than TLMO and, resultingly, a higher weight loss. In this study, the purple mashed potatoes did not contain fat, hence the impact of BI was insignificant. Due to higher WVTR of PAA, those samples could have been more concentrated than the samples in TLMO and Al foil pouches. This could be one of the reasons why the PAA pouches showed a lesser change in the ΔE than TLMO samples. In addition, a ΔE value between 6 and 12 suggests a strong indication of color difference, while a ΔE greater than 12 indicates a significant color change[8,32]. In this study, a total color change higher than six by month one in all three samples was observed. This could be indicative of the strong influence of storage time and temperature on anthocyanin content.
The effect of light on the color change in the purple mashed potatoes was also assessed (Fig. 3). The samples that were exposed to the light treatment during the storage showed rapid deterioration in the color, resulting an overall higher ΔE. At the beginning, there was no significant difference (P > 0.05) between the samples (until month 1). However, by the second month of the storage, the packaged purple mashed potatoes that were exposed to light showed a significantly higher color change than the ones that were stored without light exposure. The pouches continued to follow a similar trend until the end of the storage period.
As discussed in the previous sections, light can cause and accelerate lipid oxidation and degrade riboflavin and anthocyanins[18−20,33,34]. In our case, anthocyanin degradation was also higher in the purple mashed potatoes packaged with TLMO films and exposed to light due to photoinitiated chemical decomposition of the anthocyanin. Additionally, light may also have contributed towards lipid oxidation and browning[8,22], resulting in a higher ΔE change in the light exposed samples than that in the samples that were not exposed to the light during storage.
Anthocyanin content
-
Anthocyanin-rich purple potatoes have anti-inflammatory, anti-viral, and antioxidant properties. Hence, it is pertinent to understand the changes in its properties in the purple mashed potatoes after MATS processing and during the storage. Figure 4 illustrates the changes in the anthocyanin levels in the purple mashed potatoes packaged into TLMO and PAA pouches before and after processing and during storage.
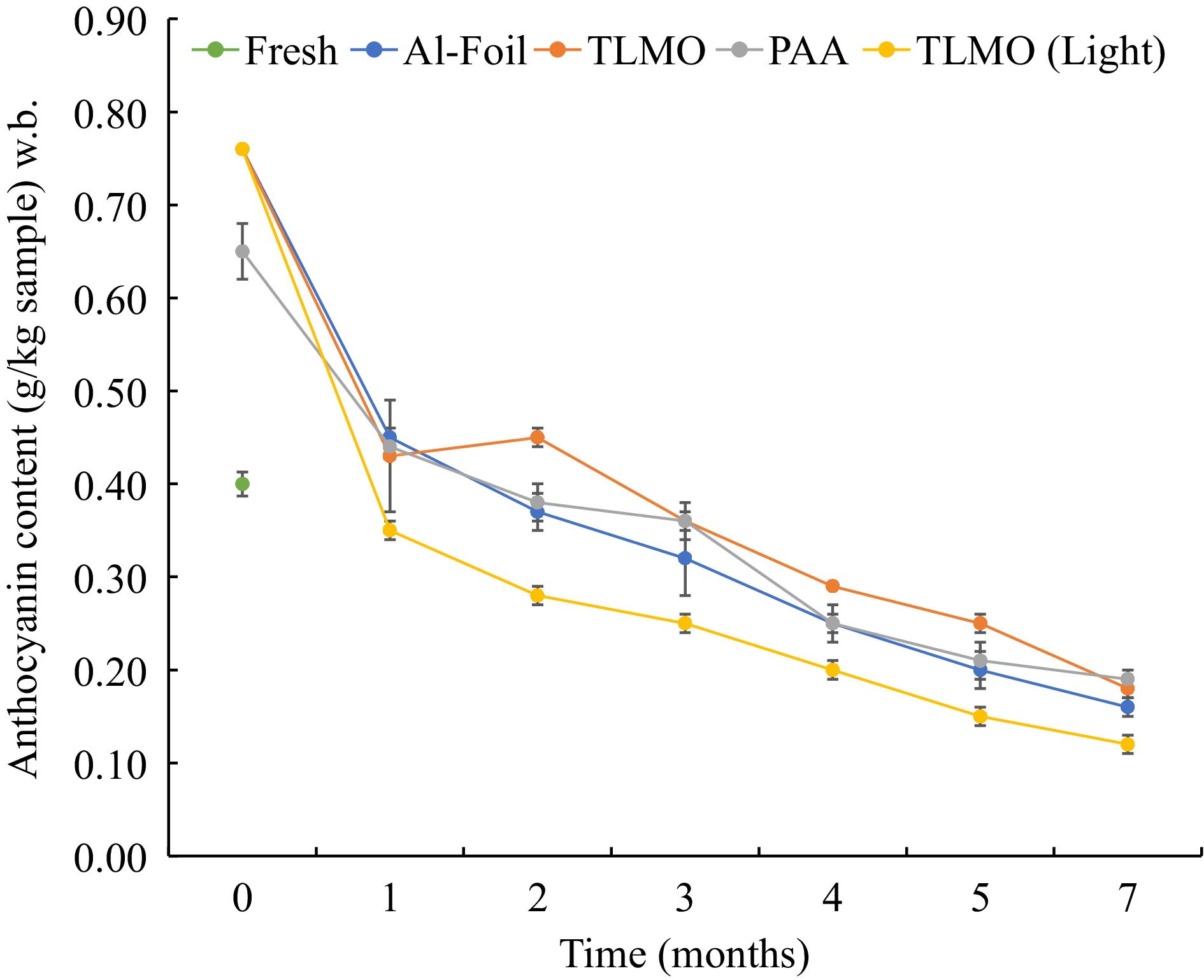
Figure 4.
Anthocyanin content in purple mash potato in all the pouches during storage at 37.8 ± 0.2 °C for 7 months.
The anthocyanin present in the purple mashed potatoes is responsible for the purple color. Fresh samples of purple mashed potato recipe, unexposed to thermal sterilization, had a 0.4 g/kg of anthocyanin content. After the MATS processing, the anthocyanin amount increased significantly (P < 0.05) to 0.76 g/kg in the TLMO and 0.65 g/kg in PAA pouches. Previously, researchers have observed that the anthocyanin content in fresh foods can increase after exposure to steam[31]. This can happen due to a lowered enzymatic degradation resulting from the steam treatment. In the present study, higher anthocyanin content in the MATS processed purple mashed potatoes can be attributed to the thermal treatment which may have caused cell disruption, resulting in a higher extractability of anthocyanin in the MATS processed pouches. A lower anthocyanin content in PAA pouches may have been due to a higher heating during MATS processing, as the presence of organic coating can increase the dielectric loss factor of these pouches, causing a higher heating during the MATS process.
Secondly, the anthocyanin content in the MATS-processed purple mashed potatoes decreased significantly (P < 0.05) during storage. In the case of PAA, anthocyanin content decreased from 0.65 to 0.19 g/kg from month 0 to 7. At the same time, samples packaged with the TLMO pouches showed a decrease from 0.76 to 0.18 g/kg during the same storage period. Although the retention in anthocyanin at months one and four was not significantly different (P > 0.05) between different packaging, TLMO pouches overall had a significantly higher (P < 0.05) retention than the PAA samples. This could be attributed to the lower OTR of the TLMO pouches than that of the PAA pouches, which may have reduced the extent of deterioration of anthocyanin[31]. In foil samples, there was no significant difference (P > 0.05) compared to the TLMO packaged samples after 7 months of storage.
Anthocyanin can be highly oxygen and light sensitive[31]. Samples packaged into the TLMO pouches and exposed to the light showed significantly higher anthocyanin degradation (P < 0.05) than other pouches. Additionally, light-exposed samples packaged with TLMO films showed a significant deterioration during storage as well (P < 0.05). In this study, light had a detrimental effect on the anthocyanin present in the purple mashed potatoes during the storage. Photodegradation of phytochemicals such as anthocyanin and riboflavin has been observed in milk and betacyanin in Basella alba[19,33]. Light tends to induce bond cleavage in anthocyanin, leading to deterioration in the color compounds. Since the TLMO poches did not show any oxygen transmission after MATS processing and had a very low WVTR, the results signified the detrimental effect of light on the nutritional content of the samples packaged in transparent pouches.
Total phenolic count (TPC)
-
Phenolic compounds are rich in antioxidant, antimicrobial, and anti-inflammatory properties. In this study, the total phenolic count of the unprocessed purple mashed potatoes (5.08 mg/g) was higher than the MATS processed samples (2.21 mg/g) (Fig. 5). The phenolic compounds tend to be heat sensitive. Resultingly, the MATS processed samples showed a lower retention in the phenolic compounds than the unprocessed samples. In addition, the MATS processed samples in PAA were lower in TPC retention. This could be related to the heat absorption during MATS processing, as explained previously.

Figure 5.
Total phenolic content in purple mash potato in all the pouches during storage at 37.8 ± 0.2 ºC for 7 months. The mean values were compared for significant differences for determining the pouch effect (Superscripts: A−D), storage effects (I−II) and processing effects (a−b), respectively.
With storage, no significant differences (P < 0.05) between the pouches at different storage times was observed, aside from the just processed samples which changed significantly (P < 0.05) by the end of the first month of the storage. The samples packaged with the TLMO films had a slightly better retention in the total phenolic counts than PAA for the same storage conditions. Interestingly, no significant impact of the exposure to light in the samples packaged with the TLMO films was observed. This can lead to deducing that the total phenolics were more sensitive towards thermal processing and less towards the oxidative and light-induced degradations in the samples during the storage period[19,31,35]. At the same time, it can also be due to the protective effects provided by the synergistic deterioration in the anthocyanins and the ascorbic acid because of light exposure, oxygen ingress, and thermal processing.
Vitamin C
-
This study assessed the effects of MATS processing, storage, light exposure, and encapsulation on vitamin C retention in purple mashed potatoes. The Vit C content (EVC) in prepared fresh purple mash potato was 50.62 ± 10.8 mg/100 g (db) and non encapsulated vitamin C (NVC) was 53.73 ± 4.8 mg/100g (db) before processing. Prior research has shown that vitamin C can be highly heat and oxidation sensitive[8]. Hence, shelf-stable products are generally fortified with added vitamin C[5,15]. The results from this study were encouraging, as MATS processing did not result in any significant vitamin C loss. This could be attributed to the lower thermal exposure time and volumetric heating conditions of the MATS processing[3,4,36].
During storage, the vitamin C content in the purple mashed potatoes steadily deteriorated (P < 0.05). Figure 6 shows the changes in non-encapsulated vitamin C concentrations in the purple mashed potatoes packaged in aluminum foil, TLMO (with and without light), and PAA pouches during storage period of 7 months. After one month of storage at 37.8 °C, there was a significant deterioration of ~36% in vitamin C content in all the pouches, including control Al-Foil. This could be due to residual air in the headspace leading to oxidation and due to higher storage temperature. At the end of the storage period of 7 months, there was an average loss of 56% of non-encapsulated vitamin C in TLMO, 52% loss in PAA and 50% loss in AL-Foil pouch. The most degradation, 65%, occurred in the TLMO pouches exposed to light for 7 months storage (TLMO-Light). This indicates the adverse effects of light on the vitamin C content in purple mashed potatoes. Light exposure can induce photosensitization in the vitamin C, can cause photooxidation and lastly, can lead to autooxidation of the ascorbic acid. All the above-mentioned reactions required both oxygen and light, hence high barrier packaging can reduce the impact of light induced destruction of vitamin C[33,37]. It is recommended to have opaque packaging for low acid shelf stable foods fortified with vitamin C to have the maximum shelf life at ambient conditions. Vitamin C deterioration during the storage period varied based on the OTRs of the pouches as well. As described in Table 1, it is evident that the PAA films had a lower OTR at the end of storage compared to the TLMO films. This reflected the respective deterioration in the packaged purple mashed potatoes in these films.
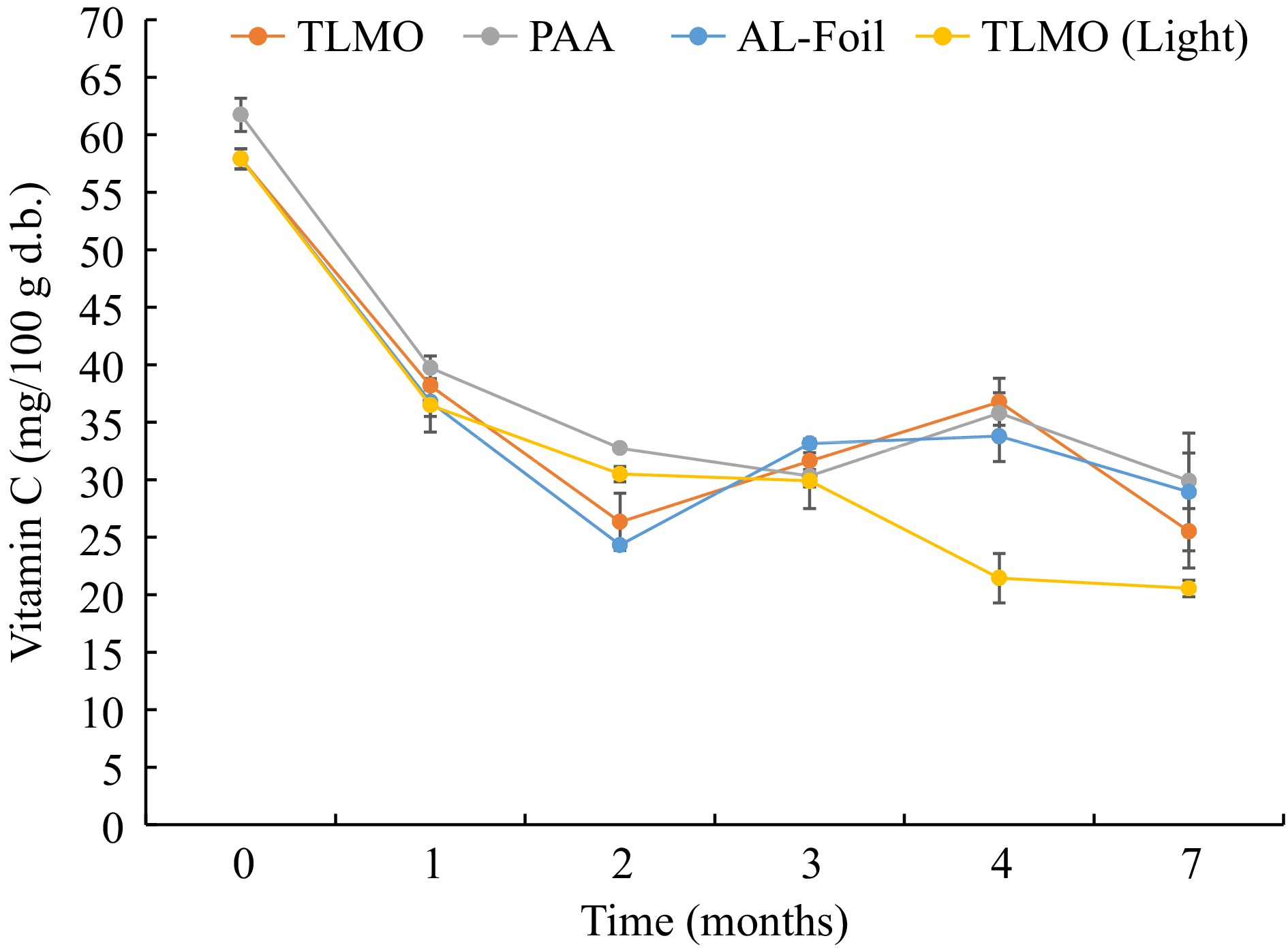
Figure 6.
Non-encapsulated Vitamin C content in purple mash potatoes in all the pouches including light exposure pouch during storage at 37.8 ± 0.2 ºC for 7 months.
Figure 7 shows the performance difference between NVC and EVC samples in TLMO and PAA pouches. The encapsulated samples packaged with the PAA films showed slightly better retention within 2 months of storage than the NVC samples in PAA. This could be due to the combined effects of the low oxygen transmission rates of the PAA films and the effect of encapsulation in successfully reducing the impact of the oxidative degradation and the storage on the vitamin C deterioration. Previously, authors have observed encapsulation to have provided a better protection than the non-encapsulated vitamin C samples[15,38]. But this is the first time the encapsulation effect is being studied in low acid shelf stable food processed via MATS.

Figure 7.
Non-encapsulated Vitamin C (NVC) and encapsulated Vitamin C (EVC) content in purple mash potatoes in TLMO & PAA pouches during storage at 37.8 ± 0.2 ºC for 7 months. The mean values were compared for significant differences for determining the effect of packaging used (lowercase superscripts), storage (uppercase superscripts) and MATS processing (roman superscripts), respectively.
At the end of the storage, it was observed that there was no significant difference in the encapsulated vitamin C content in the samples in TLMO and PAA pouches. Encapsulation provided effective protection to heat labile vitamin C during thermal processing and during short term storage of 2 months at 37.8 °C. A more detailed study will be needed where the effects of thermal processing and storage conditions on the retention and applicability of the different types of encapsulated vitamins can be assessed.
-
Purple mashed potatoes packaged in TLMO pouches and exposed to light during the storage study showed a higher deterioration in the pigments and vitamin C. The overall color of the samples deteriorated with storage, as was the case with the pigments and vitamin C. PAA pouches with organic coating showed a higher retention of encapsulated and non-encapsulated vitamin C until 2 months. However, the high WVTR of the PAA films reduced their applicability due to a significantly higher weight loss (P < 0.05) than the TLMO samples. Overall, this study was the first of its kind to assess the effects of packaging, storage, and encapsulated vitamins on the retention of pigments, vitamins, and product quality. This study can assist the industry in developing high barrier packaging more tailored to MATS processing. In addition, this study can encourage the food industry to use encapsulated vitamins with the shelf-stable foods to enhance their nutritional value.
-
Microwave assisted thermal sterilization (MATS), high barrier packaging, and encapsulated vitamins can better preserve the quality and nutritional elements of shelf-stable food products and increase their shelf life.
Funding for this study was provided by USDA National Institute of Food and Agriculture Research grants 2016-67017‐24597, 2016-68003-24840 and Hatch project #1016366. The authors would like to convey their appreciation and gratitude to the polymer companies for supplying the film pouches.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Patel J, Parhi A, Tang Z, Tang J, Sablani SS. 2023. Storage stability of vitamin C fortified purple mashed potatoes processed with microwave-assisted thermal sterilization system. Food Innovation and Advances 2(2):106−114 doi: 10.48130/FIA-2023-0013
Storage stability of vitamin C fortified purple mashed potatoes processed with microwave-assisted thermal sterilization system
- Received: 13 February 2023
- Accepted: 19 April 2023
- Published online: 28 May 2023
Abstract: Quality changes in ready-to-eat, shelf-stable foods, during storage can be influenced by many factors, such as processing, storage conditions, and the barrier properties of the packaging. This research investigated retention of vitamin C and anthocyanin in purple mashed potatoes as influenced by packaging barrier properties and encapsulation during storage after microwave assisted thermal sterilization. Purple mashed potatoes fortified with encapsulated (EVC) or non-encapsulated vitamin C (NVC) were packaged in two high-barrier polymer pouches (TLMO and PAA), processed with a pilot-scale microwave assisted thermal sterilization (MATS) system (F0 = 10.7 min), and stored at 37.8 °C for 7 months. MATS processing caused a significant increase (P< 0.05) in the oxygen transmission rates (OTRs) of PAA pouches but did not affect the barrier properties of TLMO pouches. PAA film also had a significantly higher (P< 0.05) water vapor transmission rate (WVTRs) than TLMO films, which resulted in a significantly higher (P< 0.05) weight loss in the samples packaged in PAA pouches than TLMO pouches. Purple mashed potatoes containing encapsulated vitamin C in both TLMO and PAA pouches showed the highest retention over 2 months of storage at 37.8 °C than non-encapsulated vitamin C. Additionally, purple mashed potatoes exposed to 700 lumens light showed a significantly higher (P< 0.05) deterioration in the anthocyanin, total phenolic content, color, and vitamin C. Overall, MATS processed purple mashed potatoes in high barrier polymeric packaging can minimize the quality changes when stored in dark conditions during storage and have an extended shelf life.
-
Key words:
- flexible packaging /
- shelf life /
- thermal processing /
- antioxidant /
- encapsulation













