-
Huanglongbing (HLB) is a highly destructive and fast-spreading bacterial disease in the citrus industry. For more than half a century, it has seriously restricted the development of the citrus industry worldwide[1, 2]. HLB is caused by 'Candidatus Liberibacter spp.', which has hardly been cultured. The pathogen of HLB in China and most countries is the relatively heat-resistant Asiaticus species ('Ca. L. asiaticus', CLas)[3]. CLas bacterium in host plants is unevenly distributed[4−6], mainly concentrated in bark tissue, leaf midribs, roots, flowers and fruits[7, 8]. The concentration of CLas in fruits is much higher compared with that in leaves. Considering different parts of fruits, CLas in the piths of tangerines was the highest[9].
HLB is a graft-transmissible, infectious disease, and could also be transmitted by dodder (Cuscuta campestris), and natural vectors of psyllids (Diaphorina citri or Trioza erytreae)[10]. As one of the fruit reproduction methods, grafting or graftage is a horticultural technique whereby tissues of the scions and rootstocks are joined and fused so as to continue their growth together. Stem grafting and bud grafting are two common asexual propagation methods of commercially grown citrus. The complex interactions between scion and rootstock can regulate the development of plants and their structure[11]. Fine fusion of the vascular cambium tissues between the stock plants and scions contributes to successful grafting. Grafting is also widely used in transmitting nonculturable pathogens such as CLas[12]. The graft-inoculation promotes horizontal exchange of genetic material between stem and spike cells, including the transmission of pathogens[13]. Transmission of CLas through grafting is more effective, direct, simple and controllable than transmission through vector insects, which greatly shortens the trial period[14, 15]. Consequently, grafting provides the basis of studies on the distribution and proliferation of CLas.
Traditional grafting methods for spreading plant diseases involve the insertion of pathogen-infected branches, leaves, petioles or bark fragments into the cut stem of the recipient plants[16, 17]. There are usually side grafting, top grafting, skin grafting, and 'T' grafting methods in citrus graft-inoculation[18, 19]. The disease sources used could be from branches[20], leaves[21] and branch bark[17]. Experimentally, the age and number of scions, the concentration of CLas, the combination status of scions and rootstocks and other factors can significantly affect the transmission rate of HLB[22, 23]. With higher concentration of CLas in the scions, a higher transmission efficiency would occur[24]. In addition to disease transmission studies, grafting techniques have also been used to assist the improvement of citrus genetic materials and provide graft-transmissible epigenetic modifications[25, 26]and evaluate the effects of antibiotics on inhibiting pathogenic bacteria[27]. The breeding process can be accelerated and resistance or quality can be improved by using a suitable combination of rootstock and scion or grafting method[18, 28].
Several methods of grafting for disease transmission have been reported, but no study has been reported to evaluate the effects of the different grafting methods comprehensively and systematically. Therefore, by studying the comparison of different grafting methods and different numbers of grafting buds on CLas transmission, this study screened out the grafting combination with the highest efficiency on CLas transmission. The results provide a reference for the plant materials acquirement through grafting. In citrus production, based on ensuring the propagation of grafting buds, grafting methods with high transmission efficiency should be avoided to reduce the incidence of HLB.
-
Firstly, the relationship between the weight of plant samples and the purity and concentration of DNA extracted from them was assessed. There was no significant difference in A260/A280 and A260/A230 values when the weight of leaf midrib and branch bark used for DNA extraction ranged from 0.0063 g to 0.1 g. Specifically, A260/A280 values were from 2.04 to 2.11 for the leaf midrib samples and from 2.08 to 2.11 for bark samples, while A260/A230 values were 2.24−2.48 and 2.19−2.73 accordingly for the two types of samples. DNA concentration also showed an increasing trend with the multiple increases of sample weight from 0.0063 g to 0.1 g (Fig. 1). Intriguingly, CLas titers did not increase with the weight of sample.

Figure 1.
Associations of the DNA quantity or 'Candidatus Liberiabcter asiaticus' titers with the weight of (a) leaf midrib or (b) bark tissue from sour tangerine (Citrus sunki Hort. ex Tanaka) seedlings.
Analysis of the relationship between sample weight and the concentration of CLas in them showed that the CLas could be detected by RT-qPCR in the leaf midrib or branch bark samples weighted from 0.0063 g to 0.1 g (Table 1). Although the qualitative diagnosis of HLB could be guaranteed, the accuracy of quantitative detection remains to be evaluated. Comparatively, the detection results of CLas titers were significantly affected by the lowest weight (0.0063 g) of samples. When the weight of leaf midrib or branch bark samples was 0.0125 g, CLas titers were the highest in every gram of fresh samples. Thus, enough DNA can be extracted from only 0.0125 g leaf midrib or bark samples for accurate and efficient detection of CLas. Leaf midrib samples weighted from 0.0125 g to 0.1 g and bark samples ranging from 0.0063 g to 0.0125 g were more suitable for CLas detection than the other sample types.
Table 1. DNA quantity and 'Candidatus Liberibcter asiaticus titer' titers of the samples from leaf midrib or bark tissues of sour tangerine (Citrus sunki Hort. Ex Tanaka) seedlings.
Sample weight (g) DNA concentration
(ng/µL)Ct No of CLas per ng DNA No of CLas per g sample and per ng DNA 0.1000 346.80 ± 8.55a 20.59 ± 0.27 437.99 ± 0.18a 4379.88 ± 1.78c 0.0500 302.32 ± 32.65a 20.81 ± 0.18 327.38 ± 14.86b 6547.54 ± 297.13c 0.0250 209.01 ± 9.05b 20.99 ± 0.09 455.11 ± 11.93a 18204.47 ± 477.17b 0.0125 93.24 ± 13.42c 21.98 ± 0.23 443.26 ± 28.64a 35460.98 ± 2291.10a 0.0063 67.27 ± 4.58c 24.88 ± 1.25 41.52 ± 11.59c 6591.01 ± 1839.16c 0.1000 569.70 ± 4.63a 25.52 ± 0.71 4.79 ± 1.55d 47.89 ± 15.48c 0.0500 410.17 ± 24.84b 21.51 ± 0.04 161.35 ± 6.90c 3227.05 ± 138.04b 0.0250 255.51 ± 17.53c 21.76 ± 0.15 218.51 ± 13.48b 8740.35 ± 539.30b 0.0125 133.12 ± 6.73d 22.66 ± 0.69 360.91 ± 9.14a 28873.06 ± 731.36a 0.0063 66.78 ± 2.89e 24.21 ± 0.16 154.34 ± 21.75c 24498.65 ± 3452.89a Data in the table are showed as mean ± standard error (SE). Data of the first five lines are for leaf midrib samples, while the later five lines are for the shoot bark samples. The statistic analysis was carried out separately between sample weight and each of the six groups of relative index separately. Relationship between growth status of grafting buds and incidence of HLB
-
The growth status of buds used as donors for graft-inoculation was classified into three categories. Seedlings marked as 'a-bud', 'b-bud' and 'c-bud' indicate the buds grew vigorously, moderately and poorly respectively after 6 month-after-graft-inoculation (MAG) (Fig. 2a), with 30, 36, and 26 plants in each group respectively.
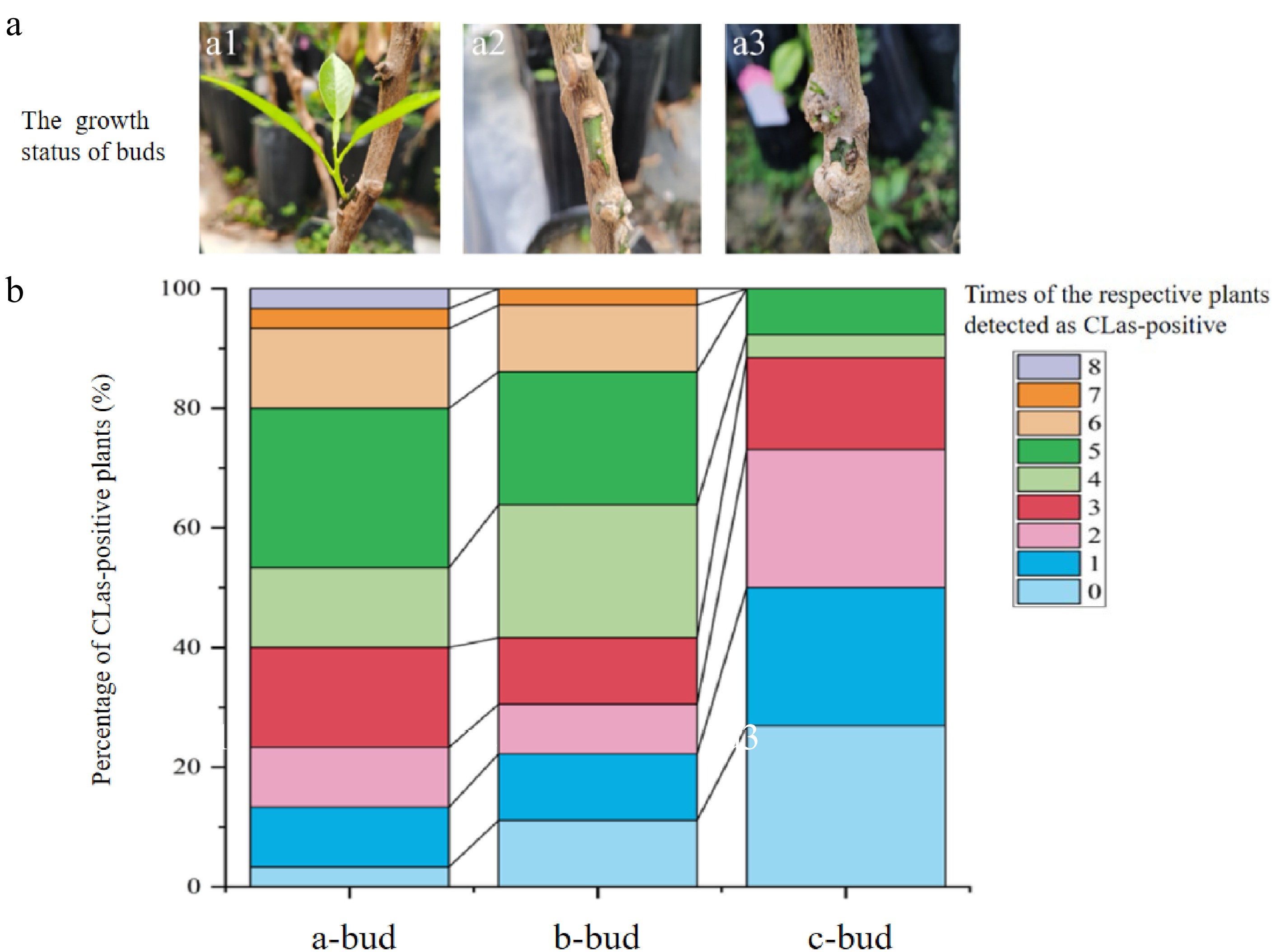
Figure 2.
Different growth status of buds carrying 'Candidatus Liberibacter asiaticus' used for graft inoculation (a) and percentage stacking of the number of months that plants with different buds were positive for CLas (b). (a1) The 'Candidatus Liberibacter asiaticus' (CLas)-infected donor buds were in vigorous growth after grafting. A total of 30 stock plants were with 'a-bud' . (a2) The buds were in moderate growth condition after grafting. A total of 36 acceptor plants were with 'b-bud'. (a3) The buds with poor growth status on stock seedlings after grafting. A total of 26 stock seedlings were with 'c-bud'.
The relationship between the status of grafting buds and the CLas transmission results in the stock plants was analyzed. The success possibility of HLB transmission is directly related to the vitality of buds. Only one 'a-bud' seedling was HLB-negative even after 13 MAG, while CLas was not detected throughout in as many as seven seedlings (accounting for 26.92%) for the 'c-bud' group (Fig. 2b). In the first three assays (from 6 MAG to 8 MAG), 16.67% and 11.11% of plants with a-type buds and b-type buds were found successfully infected. However, CLas in all plants with c-type buds was failed to be detected until 9 MAG. Most 'a-bud' (70%) and 'b-bud' (66.67%) stock seedlings were CLas-positive for three to six times' detection. However, the detectable CLas titers for most c-type-bud seedlings (88.46%) were only observed in less than three times' assays.
Effect of different graft-inoculation methods
-
After graft-inoculation with CLas-infected buds, the CLas-positive rates increased until about 6 MAG and then stabilizes over time (Table 2). Statistical analysis showed that different grafting methods had a significant influence on the infection densities (P < 0.01). In all five time tests, the success rates of top grafting ('T' grafting and 'V' grafting, as shown inFig. 3b, c) were higher than those of side grafting (Fig. 3a), wherein 'T' grafting has the highest disease transmission efficiency which was significantly higher than those of side grafting and 'V' grafting (P < 0.001) in the four tests from 5 MAG to 8 MAG. However, there was no significant difference in the success rates of disease transmission between side grafting and 'V' grafting (P = 0.097). For the CLas titers in the samples of successfully infected plants, no significant difference was detected among three different grafting methods (P = 0.232), indicating that different grafting methods had little effect on CLas titers in the leaf midrib of successfully infected plants. The plants used as control were not detected to carry CLas at all detection time points.
Table 2. The successful transmission rate and effect of different grafting methods.
Time Grafting method Side grafting 'T' grafting 'V' grafting 4 MAG Success rate 16.7%ab 41.7%a 16.7%ab CLas concentration* 35.71 ± 25.83 269.40 ± 97.35 295.61 ± 168.07 Average Ct 25.65 ± 0.96 23.21 ± 0.39 22.78 ± 0.88 5 MAG Success rate 25.0%bc 83.3%a 41.7%b CLas concentration* 171.51 ± 138.89 139.30 ± 28.85 135.07 ± 29.65 Average Ct 25.03 ± 0.95 23.00 ± 0.23 22.76 ± 0.43 6 MAG Success rate 33.3%c 83.3%a 33.3%c CLas concentration* 78.29 ± 18.67 213.69 ± 74.35 232.99 ± 78.33 Average Ct 23.67 ± 0.39 24.66 ± 0.64 23.31 ± 0.22 7 MAG Success rate 33.3%bc 83.3%a 41.7%b CLas concentration* 107.38 ± 28.00 164.86 ± 29.64 266.92 ± 76.64 Average Ct 23.72 ± 0.53 23.78 ± 0.55 24.21 ± 0.53 8 MAG Success rate 33.3%bc 83.3%a 41.7%b CLas concentration* 204.82 ± 63.54 111.26 ± 18.72 83.67 ± 28.61 Average Ct 25.53 ± 1.48 25.25 ± 0.38 26.18 ± 0.54 MAG, Month-after-grafting. Statistic analysis was done horizontally in the table, indicating the differences among different grafting methods.
* The unit of CLas concentration is copy number per ng DNA. The significance analysis was performed with letter markers such as a, b, and c. The difference is not significant if the marked letters are the same, and vice versa.Effect of grafting bud numbers on infection densities and pathogen titers
-
With different numbers of diseased buds, the success rate of disease transmission increased with time and tended to be stable at the later stages (Table 3). The efficiency of CLas transmission to citrus plants was significantly influenced by the number of grafted diseased buds (P = 0.001). By contrast, the CLas transmission rates of two and three bud-grafting inoculations were significantly higher than that of one bud-grafting (P = 0.004 and P = 0.002, respectively). Wherein, the disease transmission rate of two-bud-grafting was significantly higher than that of three-bud-inoculation (P = 0.025). The two-bud and three-bud graft-inoculation have relatively higher efficiency in HLB transmission than one-bud-inoculation, although no significant difference was detected at 4 MAG. At 5 and 6 MAG, the success ratio of two-bud-grafting was higher than that of one-bud-grafting and three-bud-grafting. At 7 and 8 MAG, the success ratio of two-bud-grafting was significantly higher than that of three-bud-grafting, which in turn was significantly higher than that of one-bud-grafting. Similarly, there was no significant difference (P = 1.000) between the number of grafting buds and the CLas titers in the successfully graft-inoculated plants except for the one-bud-grafting at 8 MAG. In conclusion, the number of grafted buds had little effect on the titers of CLas in the affected plants, but had a very significant effect on the success rate of disease transmission, with two-bud-grafting had the highest efficiency. No CLas was detected in the control plants at all detection time points.
Table 3. The successful transmission rate and effect of the numbers of grafting buds on HLB transmission by grafting.
Time Numbers of buds One bud Two buds Three buds 4 MAG Success rate 13.3%ab 33.3%a 33.3%a CLas concentration* 35.71 ± 25.83 45.81 ± 14.51 231.58 ± 147.16 Average Ct 25.65 ± 0.96 25.19 ± 0.59 25.29 ± 1.74 5 MAG Success rate 20.0%b 80.0%a 66.7%a CLas concentration* 171.51 ± 138.89 153.61 ± 39.57 93.99 ± 16.63 Average Ct 25.03 ± 0.95 24.30 ± 0.48 24.39 ± 0.53 6 MAG Success rate 26.7%bc 73.3%a 53.3%ab CLas concentration* 78.29 ± 18.67 206.20 ± 37.25 158.53 ± 38.07 Average Ct 23.67 ± 0.39 24.62 ± 0.26 25.91 ± 0.69 7 MAG Success rate 26.7%c 93.3%a 66.7%b CLas concentration* 107.38 ± 28.00 180.34 ± 36.64 61.94 ± 12.32 Average Ct 23.72 ± 0.53 23.25 ± 0.51 24.19 ± 0.36 8 MAG Success rate 26.7%c 93.3%a 66.7%b CLas concentration* 204.82 ± 63.54 68.42 ± 14.07 55.99 ± 18.69 Average Ct 25.53 ± 1.48 26.52 ± 0.38 26.46 ± 0.51 MAG, Month-after-grafting. Statistic analysis was carried out horizontally in the table, indicating the differences among different number of buds.
* The unit of CLas concentration is copy number per ng DNA. -
Based on the influence of sample weight on the detection accuracy of CLas, the propagation direction of CLas in the tree canopy was discussed. We analyzed the different grafting methods of transmission efficiency and the number of grafting buds to further understand the effects of grafting on HLB transmission. In order to optimize the experiment results, samples of similar growth stage and apropos weight should be used for DNA extraction to detect CLas in them. A published study established two sets of CLas micro detection methods to accurately detect CLas based on 0.01 g leaf midrib samples from the perspective of the concentration and purity of extracted DNA[29], but only qualitative detection of CLas was performed. The concentration and purity of DNA extracted from 0.0125 g citrus leaf midrib and branch bark samples in this study were comparable to the established microextraction of the second and third method by Zou et al.[29]. Besides, this study also proved that samples with a weight of 0.0125 g were the most suitable for the detection of CLas (with significantly higher relative CLas concentration) through accurate quantitative analysis of RT-qPCR. These findings provide a valuable suggestion for the sampling of materials and thereafter detection of unculturable pathogens within them.
CLas is unevenly distributed in host plants[4-6], with the CLas detection rates the highest in older leaves with mottled symptoms[30], though the titers were usually lower than those of fully developed yellowing and zinc-deficient younger leaves on the same diseased branches[31]. In our study, two kinds of tissues were also used to detect CLas, and we found that CLas content in the same amount of branch bark or leaf midrib were quite different. The direction of CLas propagation in phloem is a valuable reference for the epidemiological research of HLB. One point, proposed by Johnson et al, is that CLas follows the phloem sap flow rule[32], which mainly moves from mature source tissues to active growing banks, such as roots, root tips, and new shoots[33]. Braswell et al.[34]had proved experimentally that CLas was detected earlier and more evenly in citrus roots than in tree crowns after infection on 4 or 5-yr-old field trees. In addition, the underground part is suitable for early detection of HLB[35]. Ibanez & Stelinski[36]also experimentally demonstrated the transmission of CLas from roots to mature leaves through phloem. Thus, all the leaves used for CLas detection in this experiment were fully expanded in new flush.
As the transmission ability of single bud segments was better than that of non-bud segments[37], all grafting bud segments used in this experiment were each with single bud. The disease transmission effect was labile before 4 MAG because of the slowly propagated bacterium[38]. Our continuous monitoring of the transmission efficiency suggests that CLas titers and infection rates tended to be stable after 6 months of grafting onto two-year-old seedlings. In terms of the grafting method, side grafting is one of the most commonly used methods for disease transmission. However, it had proved that side grafting had a low survival rate and also healed slowly after grafting[39]. Although survival or growth of diseased buds is not an essential factor for successful transmission of the CLas to receptor plants, this study demonstrated a significant correlation between diseased bud growth status and successful transmission of CLas. Collectively, the success rate of 'T' grafting was significantly higher than that of side grafting, which may be related to the difference in healing performance of cut areas and the apical growth advantage of buds. According to the practical experience of our laboratory, removing the top after side-grafting can accelerate the uniform distribution of CLas in citrus canopy, this can also explain the above conclusion. Therefore, top grafting was beneficial to bud growth and pathogen transmission, which was speculated to be related to the top dominance of plants. Similarly, the established single leaf grafting method that has a high success rate (78% of rootstocks were infected with diseased leaves) would depend on its slight wounding area and high healing rate[17].
Different numbers of CLas-infected buds have a great influence on the success rate of HLB transmission. As early as the 1980s, scientists proposed that an appropriate increase in the number of diseased buds could improve the transmission rate of HLB[40]. We found that the success rate of disease transmission by grafting two buds and three buds was significantly higher than that of grafting one bud. This is partly in accordance with Lin's view that revealed advantages of two CLas-positive-bud infections compared to using only one diseased bud[41]. Intriguingly, our results also proved that the number of buds used was not positively related to the effectiveness of infection. Collectively, the two-bud grafting inoculation was optimal in both grafting efficiency and disease transmission rate, which may be related to the total damaged area of stock plants. In citrus production, the number of grafting buds should also be considered to minimize the possibility of disease transmission.
-
Based on the analysis of the transmission direction and detection efficiency of CLas, this experiment compared the effects of different grafting methods and the number of grafted buds on the success rate of CLas, and obtained the following conclusions. Leaf midrib samples are more suitable for detection of CLas than branches, in which only 0.0125 g of diseased leaf midrib samples can be extracted from enough DNA for efficient detection of CLas. The growth of scion is closely related to the success rate of disease transmission. The methods of 'T' grafting and two-bud grafting were proved to be efficient for HLB transmission.
-
Three grafting methods, including side grafting, 'T' grafting and 'V' grafting were used to insert CLas-infected scions onto the healthy stock seedlings. Side grafting (Fig. 3a) is performed on the lateral side of the stem of the stock plants. For side grafting, one, two or three scions were grafted on each seedling. 'T' grafting (Fig. 3b) is T-shaped grafting on the top of the seedling with only one scion on each (Fig. 3c).
Plant material and sample collection
-
The CLas-infected buds used as scions for grafting were collected from sweet orange (Citrus sinensis) plants in Huizhou, Guangdong, China (latitude 23°48'10" N, longitude 114°45'73" W). The grafted recipient seedlings were one-year-old upright sour tangerine (Citrus sunki Hort. ex Tanaka) seedlings with consistent good growth conditions. The seedlings were confirmed to be CLas-negative by qPCR. By contrast, the scions carried high concentration of CLas, with detected average Ct values of 21 ± 0.24.
The leaves of HLB-affected seedlings at 6 MAG were collected, and groups of the leaf midrib and branch bark samples with the weights of 0.1 g, 0.05 g, 0.025 g, 0.0125 g and 0.0063 g were weighed respectively. Each group had three replicates. DNA of each sample was extracted for pathogen content detection to explore the influence of sample quantity on DNA extraction efficiency and PCR detection of CLas.
All grafted plants with well grown buds were selected to compare the difference caused by grafting methods (side grafting, 'T' grafting and 'V' grafting) or bud numbers (one bud, two buds and three buds). In the first assay, the relationship between the growth status of donor buds after grafting and HLB transmission results of the receptor plants was evaluated at 6 MAG and thereafter monthly using 92 one-bud-side-grafted plants. For the second assay, the effects of different grafting methods on the success ratio of disease transmission and the concentration of CLas were investigated using 12 plants for each method. Leaves from each tree were sampled at 4, 5, 6, 7 and 8 MAG. Additionally, four groups of tangerine seedlings, with 15 plants in each group, were selected to explore the influence of the number of diseased scions on the disease transmission effect. One, two or three diseased scions or healthy scions (as control) were side-grafted onto the seedlings of each group. The sampling time for this experiment was the same as previous. In the control groups, scion without CLas was grafted on seedlings by the corresponding methods.
DNA extraction and qPCR detection
-
A proper amount of leaf midrib or bark tissues were cut and further used for DNA extraction. DNA extraction was performed using the EZNATM High Performance Plant DNA Kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer's instructions. All extracted DNA samples were quantified with the Qubit® 2.0 Fluorometer (Life Technologies, CA, USA) and stored at −20 °C for further use. For each sample, 100 ng DNA was used for quantitative real-time PCR (qPCR) using primers as described by Chen et al.[31]. The primers used for qPCR detection of CLas were HLB-4G (5'-AGTCGAGCGCGTATGCGA-3') /HLBr (5'-GCGTTATCCCGTAGAAAAAGCTAG-3')[42].
Statistical analysis
-
The standard curve equation (Y(Ct) = 3.31 lgx + 37.463) was used to calculate the relative titer of CLas (x) for each sample. Data are expressed as mean ± standard error (SE). When required, the data were subjected to statistical analysis by one-way analysis of variance (ANOVA) followed by Duncan's new multiple range test using SPSS 13.0 software. Statistical significance was defined as P < 0.05.
The authors thank Ye Hu from Zhejiang Normal University for her linguistic assistance during the preparation of this manuscript. This research was supported by the Natural Science Foundation of Guangdong Province (2022A1515010889) and China Agriculture Research System of MOF and MARA.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Cui X, Zhang J, Liu Y, Luo X, Deng X, et al. 2022. Comparison of different grafting methods on the effect of 'Candidatus Liberibacter asiaticus' transmission. Fruit Research 2:15 doi: 10.48130/FruRes-2022-0015
Comparison of different grafting methods on the effect of 'Candidatus Liberibacter asiaticus' transmission
- Received: 20 May 2022
- Accepted: 18 August 2022
- Published online: 29 September 2022
Abstract: Grafting is a commonly used method for citrus propagation and transmitting 'Candidatus Liberibacter asiaticus' (CLas), the putative causing agent of citrus Huanglongbing (HLB). Optimization of the grafting inoculation methods facilitates the material preparation in HLB research. Citrus buds with CLas were grafted onto healthy sour tangerine (Citrus sunki Hort. ex Tanaka) seedlings by different methods such as top grafting ('T' grafting and 'V' grafting) and side grafting (abdominal grafting). Along with the symptom observation, titers of CLas in the leaves were detected by RT-qPCR monthly. The correlation between the growth status of buds or different grafting methods and the success rate of HLB transmission were analyzed. Our results suggest that sufficient DNA could be extracted to accurately detect the CLas from even only 0.0125 g leaf midrib or branch bark. The probability of CLas transmission was higher in plants inoculated with buds in better growth conditions. The success rate of 'T' grafting was significantly higher than that of side grafting and 'V' grafting. Additionally, in terms of HLB transmission efficiency, the two-bud grafting scheme was superior to the single-bud and three-bud grafting schemes. In conclusion, the grafting combinations with the highest HLB transmission efficiency were screened to provide a methodological reference for the practice or research of grafting to obtain plant material.
-
Key words:
- citrus /
- grafting /
- Huanglongbing /
- inoculation /
- transmission













